Abstract
Mood stabilizers that are approved for treating bipolar disorder (BD), when given chronically to rats, decrease expression of markers of the brain arachidonic metabolic cascade, and reduce excitotoxicity and neuroinflammation-induced upregulation of these markers. These observations, plus evidence for neuroinflammation and excitotoxicity in BD, suggest that AA cascade markers are upregulated in the BD brain. To test this hypothesis, these markers were measured in postmortem frontal cortex from 10 BD patients and 10 age-matched controls. Mean protein and mRNA levels of AA-selective cytosolic phospholipase A2 IVA (cPLA2), secretory (s)PLA2 IIA, cyclooxygenase (COX)-2, and membrane prostaglandin E synthase (mPGES) were significantly elevated in the BD cortex. Levels of COX-1 and cytosolic PGES (cPGES) were significantly reduced in BD cortex relative to controls, whereas levels of Ca2+-independent iPLA2VIA, 5-, 12-, and 15-lipoxygenase, thromboxane synthase and cytochrome p450 epoxygenase protein and mRNA levels were not significantly different. These results confirm that the brain AA cascade is disturbed in BD, and that certain enzymes associated with AA release from membrane phospholipid and with its downstream metabolism are upregulated. Since mood stabilizers downregulate many of these brain enzymes in animal models, their clinical efficacy may depend on suppressing a pathologically upregulated cascade in BD. An upregulated brain AA cascade should be considered as a target for future drug development and for neuroimaging in BD.
Keywords: PLA2, inflammation, mood stabilizers, COX, PGES, excitotoxicity
Introduction
Bipolar disorder (BD) is characterized by recurrent depressive and manic episodes. It afflicts about 1.5% of the United States population (1), increases the risk of suicide by approximately 5–17 fold (2), and has multiple risk alleles consistent with a polygenic inheritance (3). Recent studies suggest progressive brain atrophy and neuronal loss in BD patients, with increased brain levels of proinflammatory cytokines, and evidence of increased glutamatergic function and excitotoxicity (4–6). Some of these features are also found in psychiatric and neurodegenerative diseases including schizophrenia (SZ) and Alzheimer disease (AD). However, patients with BD have many more features that overlap with those of SZ patients (7, 8), than with AD patients (9, 10), such as an early onset, genetic association and drug therapy.
Inflammation and excitotoxicity can activate many brain signaling pathways, including the arachidonic acid (AA, 20:4n-6) metabolic cascade (11–13). For example, activation of the cytokine interleukin (IL)-1 receptor cascade can increase expression of AA metabolizing enzymes, including AA-selective cytosolic phospholipase A2 (cPLA2) (14-16), secretory sPLA2 (16), and cyclooxygenase (COX)-2 (17), as well as transcription factors that regulate gene transcription of these enzymes, particularly activator protein (AP)-2 and/or nuclear kappa B (NF-κB). With regard to excitotoxicity, rats chronically administered a subconvulsant dose of NMDAshowed an increase in brain AA turnover, protein and mRNA levels of cPLA2 IVA, AP-2 DNA binding activity, AP-2α and AP-2β protein, and cytokine levels (13, 18).
AA is a nutritionally essential polyunsaturated fatty acid (PUFA) found mainly in the stereospecifically numbered (sn)-2 position of membrane phospholipids, from which it can be hydrolyzed by cPLA2 or sPLA2 (19). A portion of the AA released can be metabolized into bioactive prostaglandin H2 (PGH2) by COX-1 or COX-2, to cytoprotective epoxyeicosatrienoic acids by cytochrome p450 epoxygenase, or to cytotoxic leukotrienes by lipoxygenase subtypes 5,12 and 15 (20). Bioactive PGH2 is converted to prostaglandin E2 (PGE2) by membrane prostaglandin synthase-1 (mPGES-1) or cytosolic prostaglandin synthase (cPGES). PGH2 can also be converted to thromboxane A2 (TXA2) by thromboxane synthase (TXS) (21) (Figure 1). Of the two COX isoenzymes, COX-1 is constitutively expressed, whereas COX-2 is inducible (22, 23). cPGES uses PGH2 produced by COX-1, whereas mPGES-1 uses COX-2-derived endoperoxide (24). AA and its metabolites can modulate signal transduction, transcription, neuronal activity, apoptosis, and many other processes within the brain (25–27).
Figure 1.
Schematic diagram of arachidonic acid cascade.
Lithium, valproic acid, carbamazepine and lamotrigine are approved by the FDA as “mood stabilizers” for treating BD. Each of these agents, when given chronically to rats to produce a therapeutically relevant plasma concentration, downregulate parts of the brain AA cascade, including AA turnover in brain phospholipids (lithium, valproic acid, carbamazepine), cPLA2 IVA and its transcription factor AP-2 (lithium and carbamazepine), acyl-CoA synthetase (valproic acid), COX-1 (valproate), COX-2 (all four), and NF-κB (valproate) (28–33). Chronic lithium and carbamazepine also prevent elevations of brain AA cascade markers in rat models of neuroinflammation and excitotoxicity (34, 35).
In view of the evidence linking excitotoxicity and neuroinflammation to BD (see above) (11), and the inhibition rat brain AA metabolism by mood stabilizers, we hypothesized that the AA cascade is upregulated in the BD brain. To test this hypothesis, protein and mRNA levels of AA cascade enzymes (see above) were compared between postmortem frontal cortex from 10 BD patients and 10 unaffected controls. We also compared expression of Ca2+-independent iPLA2, which is selective for docosahexaenoic acid (DHA, 22:6n-3) in membrane phospholipid (36), and of neuron-specific enolase (NSE), a marker of postmortem tissue integrity in the absence of acute injury (37, 38). The frontal cortex (Brodmann area 9) was chosen for this study because functional and structural abnormalities have been reported in this region in BD patients (5), and because relevant data on this region have been published previously (11, 38). Preliminary data on the subjects have been published in abstract form (39).
Materials and Methods
Post-mortem brain samples
The protocol was approved by the Institutional Review Board of McLean Hospital, and by the Office of Human Subjects Research (OHSR) of the NIH (# 4380). Frozen postmortem human frontal cortex from 10 BD patients and 10 age-matched controls was provided by the Harvard Brain Tissue Resource Center (McLean Hospital, Belmont, MA) under PHS grant number R24MH068855 to JS Rao. Age (years, control: 43 ± 3.5 vs. BD: 49 ± 7.2), postmortem interval (hours, control: 27 ± 1.5 vs. BD: 21 ± 3.0), and brain pH (control: 6.6 ± 0.16 vs. BD: 6.7 ± 0.09) did not differ significantly between the two groups, whereas the BD patients were exposed to various psychotropic medications as reported previously (Table 1) (38).
Table 1.
Characteristics of control and bipolar disorder subjects
| Group | Age, (yr) | Sex | PMI, (h) | Cause of death | Medications |
|---|---|---|---|---|---|
| Control | 32 | F | 29 | Cardiopulmonary attack | Antibiotics |
| Control | 46 | M | 30 | Cardiopulmonary attack | Insulin |
| Control | 54 | M | 24 | Cardiopulmonary attack | Insulin |
| Control | 36 | M | 21 | Electrocution | Vitamins |
| Control | 41 | M | 30 | Cardiopulmonary attack | None |
| Control | 49 | M | 27 | Cardiopulmonary attack | Vitamins |
| Control | 35 | M | 20 | Cardiac arrest | Not available |
| Control | 35 | M | 26 | unknown | Not available |
| Control | 45 | M | 24 | unknown | Not available |
| Control | 25 | M | 15 | Myocardial Infarction | Not available |
| BD | 29 | M | 20 | Suicide | Paxil |
| BD | 74 | M | 7 | Pneumonia | Neurontin |
| BD | 51 | F | 35 | Ischemic heart disease | Ambien |
| BD | 47 | F | 16 | Major system failure | Lithium carbonate |
| BD | 40 | M | 30 | Suicide | Risperidone |
| BD | 75 | M | 20 | Myocardial infarction | Prozac, Avandia |
| BD | 90 | F | 19 | Ventricular tachycardia | Lithium carbonate, |
| BD | 27 | M | 20 | Suicide | Lithium carbonate |
| BD | 25 | F | 11 | Suicide | Not available |
| BD | 35 | M | 42 | Suicide | Lithium |
PMI, postmortem interval; F, Female; M, Male
Preparation of cytosolic and membrane fraction
Cytosolic and membrane extracts were prepared from postmortem frontal cortex of BD and control subjects as previously reported (40). Frontal cortex tissue was homogenized in a homogenizing buffer containing 20 mM Tris-HCl (pH 7.4), 2 mM EGTA, 5 mM EDTA,1.5 mM pepstatin, 2 mM leupeptin, 0.5 mM phenylmethylsulfonyl fluoride, 0.2 U/ml aprotinin, and 2 mM dithiothreitol, using a Teflon homogenizer. The homogenate was centrifuged at 100,000g for 60 minat 4°C. The resulting supernatant-1 (S1) was the cytosolic fraction, andthe pellet was resuspended in the homogenizing buffer containing 0.2% (w/v) Triton X-100. The suspension was kept at 4°C for 60min with occasional stirring and then centrifuged at 100,000g for 60 min at 4°C. The resulting supernatant-2 (S2) was the membrane fraction. Protein concentrations in membrane and cytosolic fractions were determined with Bio-Rad Protein Reagent (Bio-Rad, Hercules, CA). The membrane and cytosolic fractions were confirmed using the specific markers, cadherin and tubulin, respectively.
Western blot analysis
Proteins (50 μg) from the cytoplasmic and membrane extracts were separated on 4–20% SDS-polyacrylamide gels (PAGE) (Bio-Rad). Following electrophoresis, the proteins were transferred to a PVDF membrane (Bio-Rad). Cytoplasmic protein blots were incubated overnight in Tris-buffered-Saline buffer, containing 5% nonfat dried milk and 0.1% Tween-20, with specific primary antibodies (1:200 dilution) for the group IVA cPLA2,, group IIA sPLA2, group VIA iPLA2, COX-1 (1:1000), COX-2 (1:500), cytochrome P450 epoxygenase, TXS, 5-, 12-, and 15-LOX (Cell Signaling, Beverly, MA) and NSE (1:10,000) (Abcam, Cambridge, MA). mPGES-1 was determined using a specific (1:200) primary antibody (Abcam). Cytoplasmic and membrane protein blots were incubated with appropriate HRP-conjugated secondary antibodies (Bio-Rad) and visualized (Kodak, Rochester, NY). Optical densities of immunoblot bands were measured using Alpha Innotech Software (Alpha Innotech, San Leandro, CA) and were normalized to β–actin (Sigma-Aldrich, St. Louis, MO) to correct for unequal loading. All experiments were carried out twice with 10 controls and 10 post-mortem brain samples from BD patients. Values were expressed as percent of control.
Total RNA isolation and real time RT-PCR
Total RNA was isolated from the frontal cortex using an RNeasy mini kit (Qiagen, Valencia, CA). RNA integrity number (RIN) was measured using Bioanalyzer (Agilent 2100 Bioanalyzer, Santa Clara, CA). RIN values for control and BD were 6.9 ± 0.4 and 7.1 ± 0.5, respectively (Mean + SEM). Complementary DNA (cDNA) was prepared from total RNA using a high-capacity cDNA Archive kit (Applied Biosystems, Foster City, CA). mRNA levels of cPLA2, sPLA2, iPLA2, COX-1, COX-2, mPGES-1, cPGES, LOX-5, 12, 15, TXS, cytochrome P450 epoxygenase and NSE were measured by quantitative RT-PCR, using an ABI PRISM 7000 sequence detection system (Applied Biosystems). Specific primers and probes for cPLA2, sPLA2, iPLA2, COX-1, COX-2, mPGES-1, cPGES, LOX-5, 12, 15, TXS, and cytochrome P450 epoxygenase were purchased from TaqManR gene expression assays (Applied Biosystems), and consisted of a 20X mix of unlabeled PCR primers and Taqman minor groove binder (MGB) probe (FAM dye-labeled). The fold-change in gene expression was determined by the ΔΔCT method (41). Data were expressed as the relative level of the target gene (cPLA2, sPLA2, iPLA2, COX-1, COX-2, mPGES-1, cPGES, LOX-5, 12, 15, TXS, cytochrome P450 epoxygenase and NSE) in the post-mortem BD patients normalized to the endogenous control (β-globulin) and relative to the control (calibrator), as previously described (42). All experiments were carried out twice in triplicates with 10 controls and 10 post-mortem brain samples from BD patients. The data were expressed as relative expression of control.
Statistical Analysis
The data are presented as mean ± SEM. Statistical significance of means was calculated using a two-tailed unpaired t-test. Power analysis was carried out according to Mitulsky (1995). We have set α, the threshold for significance for two-tailed distribution, to 0.05 and β, the power index to 20%. Pearson correlations were made between age, post-mortem interval and pH of the frontal cortex, and mRNA levels of cPLAB2B, sPLAB2B, iPLAB2B, COX-1, COX-2, mPGES-1 and cPGES in post-mortem brain from controls and BD patients combined. A subgroup statistical comparison was performed on control, all BD subjects and BD subjects that were on lithium medication using Bonferroni’s multiple comparison test, to assess the effects of lithium on the molecular markers analyzed. A separate Bonferroni’s multiple comparison test was made between control, all BD and BD subjects who died by suicide, to determine whether suicide was a factor affecting gene and protein expression. Statistical significance was set at p < 0.05.
Results
Upregulated protein and mRNA levels of cPLA 2, sPLA2 and COX-2
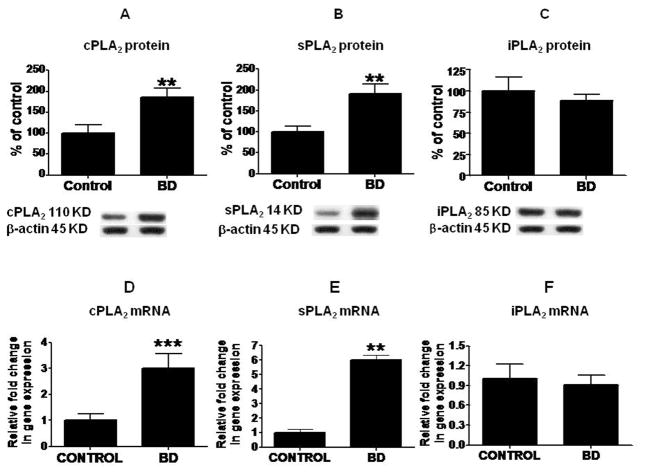
Mean protein levels of cPLA2 IVA and sPLA2 IIA were increased significantly (p < 0.01), by 87% and 92% respectively (Fig. 2A and 2B), in BD compared with control frontal cortex, whereas the mean iPLA2 protein level did not differ significantly between the groups (Fig. 2C). Mean mRNA levels of cPLA2 and sPLA2 were increased significantly in BD compared with control brain by three-fold (p < 0.001) and six-fold (p < 0.01), respectively (Figs. 2D and E), but iPLA2 mRNA was not significantly different (Fig. 2F). COX-2 protein and mRNA levels were increased significantly by 82% (Fig. 3A, p < 0.01) and 3.4-fold (Fig. 3B, p < 0.01), respectively, whereas COX-1 protein and mRNA were significantly decreased in the BD cortex by 40% (p < 0.01, Fig. 3C) and 0.6 fold (p < 0.05, Fig. 3D), respectively.
Figure 2. Protein and mRNA levels of PLA2 enzymes.
Mean cPLA2 (A), sPLA2 (B) and iPLA2 (C) protein (with representative immunoblots) as percent of control in frontal cortex, from control (n = 10) and BD (n = 10) subjects. Data are optical densities relative to that of β-actin. Mean mRNA as percent of control of cPLA2 (D), sPLA2 (E) and iPLA2 (F) in frontal cortex from control (n = 10) and BD (n =10) subjects, measured using RT-PCR. Data are normalized to the endogenous control (β-globulin) and expressed relative to the control (calibrator), using the ΔΔCT method. Mean ± SEM, ** p < 0.01, ***p < 0.001.
Figure 3. Protein and mRNA levels of COX enzymes.
Mean COX-2 (A) and COX-1 (C) protein (with representative immunoblots) as percent of control in frontal cortex, from control (n = 10) and BD (n = 10) subjects. Data are optical densities relative to that of β-actin. COX-2 (B) and COX-1 (D) mRNA levels in the frontal cortex from controls (n = 10) and BD patients (n = 10), measured using RT-PCR. Data are normalized to the endogenous control (β-globulin) and expressed relative to the control (calibrator), using the ΔΔCT method. Mean ± SEM, * p < 0.05, **p < 0.01.
Increased protein and mRNA levels of mPGES-1
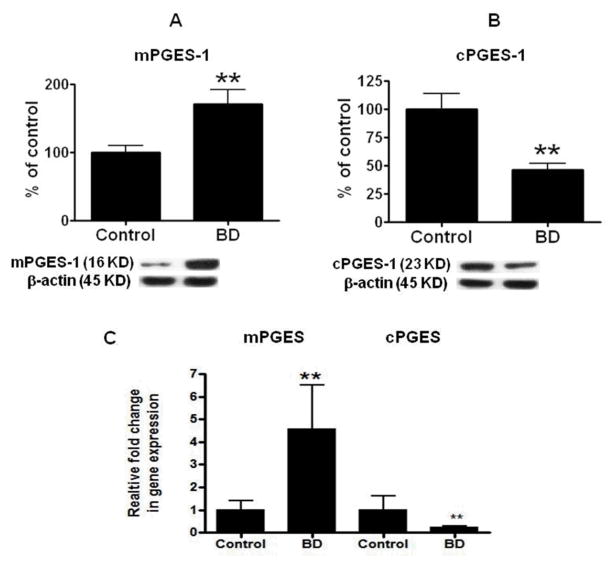
Statistically significant increases were found in mPGES-1 protein (by 71%, p < 0.01, Fig. 4A) and mRNA (by 3.6 fold, p < 0.01, Fig. 4C) in samples from BD patients relative to controls. cPGES was significantly decreased with regard to the levels of its protein (by 54%, p < 0.01, Fig. 4B) and mRNA (by 0.76 fold, p < 0.01, Fig. 4C). There was no significant difference in either the protein (Fig. 5A, B and C) or mRNA (Fig. 5D) level for LOX 5, 12, 15, TXS (Fig. 6A and D), or cytochrome P450 (Fig. 6B and E) between groups.
Figure 4. Protein and mRNA levels of PGES enzymes.
Mean mPGES-1 (A) and cPGES- 2 (B) protein (with representative immunoblots) in control (n = 10) and BD (n = 10) frontal cortex. Data are optical densities of PGES protein to β-actin, expressed as percent of control. mRNA levels of mPGES-1 and cPGES-2 (C) in postmortem control (n = 10) and BD (n =10) frontal cortex, measured using RT-PCR. Data are levels of PGES in the BD patients normalized to the endogenous control (β-globulin) and relative to control level (calibrator), using the ΔΔCT method. Mean ± SEM, ** p < 0.01.
Figure 5. Protein and mRNA levels of lipoxygenases.
Mean 5 LOX (A), 12 LOX (B) and 15 LOX (C) protein levels (with representative immunoblots) in frontal cortex from control (n = 10) and BD (n = 10) subjects. Bar graphs are ratios of optical densities of LOXs to that of β-actin, expressed as percent of control. LOX mRNA (D) in postmortem frontal cortex from the control (n = 10) and BD (n = 10) subjects, measured using RT-PCR. Data are levels of LOXs in BD normalized to the endogenous control (β-globulin) and relative to the control (calibrator), using the ΔΔCT method. Mean ± SEM.
Figure 6. Protein and mRNA levels of thromboxane synthase, P450 epoxygenase and neuron specific enolase.
Mean TXS (A), P450 epoxygenase (B) and neuronal specific enolase (NSE) (C) protein in postmortem frontal cortex from control and BD subjects. Bar graph is ratio of optical density of each protein to that of β-actin, expressed as percent of control. TXS (D), P450 epoxygenase (E) and NSE mRNA (F) in postmortem frontal cortex from control (n = 10) and BD (n = 10) subjects, measured using RT-PCR. Data are level in the BD brain normalized to the endogenous control (β-globulin) and relative to control (calibrator), using the ΔΔCT method. Mean ± SEM.
Mean protein and mRNA levels of NSE did not differ significantly between BD and control brains (Fig. 6C and E).
Power analysis and correlations with brain variables
Power analysis revealed that a sample size of 10 in each group is sufficient to detect a difference of 20% between the two groups, based on our estimated mean and SD values (as described in the material and methods). Pearson correlations between variables (age, PMI and pH) and the mRNA levels from across all 20 brain samples (control and BD patients combined) were not statistically significant (Table 2).
Table 2.
Probabilities and Pearson correlation r squared between brain mRNA levels and subject age, postmortem interval and brain pH.
| N=20 | cPLA2 | sPLA2 | iPLA2 | COX-1 | COX-2 | mPGES | cPGES | |
|---|---|---|---|---|---|---|---|---|
| Age, (yr) | P | 0.45 | 0.16 | 0.23 | 0.15 | 0.32 | 0.82 | 0.81 |
| r2 | 0.03 | 0.10 | 0.07 | 0.10 | 0.05 | 0.00 | 0.00 | |
| PMI, (hr) | P | 0.62 | 0.96 | 0.19 | 0.69 | 0.45 | 0.13 | 0.31 |
| r2 | 0.01 | 0.00 | 0.09 | 0.00 | 0.03 | 0.12 | 0.05 | |
| pH | P | 0.46 | 0.82 | 0.62 | 0.11 | 0.77 | 0.82 | 0.53 |
| r2 | 0.04 | 0.24 | 0.30 | 0.37 | 0.098 | 0.08 | 0.04 |
PMI, postmortem interval
Bonferroni’s multiple comparison tests showed a significant decrease in AA cascade markers (protein and mRNA) between controls and BD subjects, and controls and BD subjects on lithium medication (p < 0.05). However, no significant changes in AA cascade markers were observed between all BD subjects and the subgroup of BD subjects treated with lithium medication. Similarly, both BD subjects and BD subjects who committed suicide showed reduced AA cascade markers (protein and mRNA) relative to controls (p < 0.05). No significant differences were found between all BD subjects and the subgroup of BD subjects who committed suicide, in AA cascade markers levels.
Discussion
In this study, mean protein and mRNA levels of cPLA2 IVA, sPLA2 IIA, COX-2, and mPGES were significantly elevated in postmortem frontal cortex of BD patients compared with controls. Protein and mRNA levels of COX-1 and of cPGES were significantly reduced, whereas protein and mRNA levels of iPLA2, 5-, 12-, and 15-lipoxygenase, thromboxane synthase, cytochrome p450 epoxygenase were not significantly altered. These results are consistent with the hypothesis that the brain AA cascade is disturbed in BD. The hypothesis is based on the observation that each of the four mood stabilizers approved for treating BD, when given chronically to rats, downregulate AA turnover in brain phospholipids and other markers of brain AA metabolism, and on evidence of neuroinflammation and excitotoxicity associated with disease progression in BD, including brain atrophy and cell loss, cognitive decline and symptom worsening (5, 43–46), in BD.
An upregulated AA cascade may contribute to disease progression in BD in many ways (47). For example, excess unesterified AA and lysophospholipids formed following AA hydrolysis can induce apoptosis by damaging mitochondria (48), activating caspases-3 and -9, releasing cytochrome C (49), decreasing expression of brain derived neurotrophic factor (BDNF) (50), and reducing neuronal viability (51).
The increased expression of cPLA2 IVA, sPLA2 IIA and COX-2 in the BD brain may be related to underlying excitotoxicity and/or neuroinflammation. An elevated brain glutamate/glutamine ratio, increased glutamate concentration, and decreased levels of the NMDA receptor subunits NR1, NR2A and NR3A, have been reported in the BD brain (41, 42, 52, 53). In this regard, studies have demonstrated that chronic subconvulsive NMDA administration to rats reduced brain levels of NR1 and NR3A, increased AA turnover in brain membrane phospholipids and increased protein and mRNA levels of cPLA2 IVA and sPLA2 IIA in the brain (42). Increased Ca2+ entry into a cell via the glutamatergic NMDA receptor may directly activate Ca2+-dependent AA-selective cPLA2 to release AA from membrane phospholipids (34, 54); chronic lithium, carbamazepine or valproate can inhibit this process (34, 35).
Neuroinflammation has been reported to activate AA cascade markers. For instance, the exposure of rat astrocytes to bacterial lipopolysaccharide (LPS) was reported to increase cPLA2 transcription via an AP-2 and NF-κB dependent manner (41, 55). A rat model of inflammation, caused by chronic LPS infusion into the cerebroventricular system showed an increase AA incorporation and turnover within brain phospholipids, elevated concentrations of unesterified AA, PGE2 and other AA metabolites, and increased cPLA2 and sPLA2 activities (35, 56). LPS infusion was also shown to increase IL-1β, TNFα, and beta-amyloid precursor protein in activated microglia and astrocytes, resulting in degeneration of hippocampal CA3 pyramidal neurons, and altered behavior (56–58). Cytokines formed during inflammation have been reported to activate both cPLA2 and sPLA2 at astrocytic cytokine receptors (19, 59–61).
Excitotoxicity and neuroinflammation have been associated with upregulation of mPGES-1, which is functionally coupled to COX-2 (24, 62). Coupling is consistent with our finding of increased expression of both mPGES-1 and COX-2 in the BD frontal cortex. On the other hand, cPGES is coupled to COX-1, and the expression of both these enzymes was significantly reduced in the BD brain. This is consistent with evidence showing that products of COX-1 are selectively metabolized by cPGES (24, 62). Decreased expression of COX-1 and cPGES might be a compensatory response for increased expression of COX-2 and mPGES.
Consistent with an elevated AA metabolism in BD, studies have reported increased hydrolysis of serum phospholipids (63–65) and increased levels of AA-derived prostaglandins in saliva (66), cerebrospinal fluid (67) and serum (64) from BD patients. An increase in AA cascade markers, including cPLA2 IVA, sPLA2 IIA and COX-2 protein and mRNA were elevated in frontal cortex of n-3 PUFA deprived rats (42), which were shown to exhibit BD-like behavioral symptoms (68). Expression of BDNF and cyclic AMP response element binding (CREB) protein was also reduced in the n-3 PUFA deprived animals (42).
The absence of a significant difference in iPLA2 expression in the frontal cortex between BD patients and controls is consistent with unaltered iPLA2 activity in BD serum (69, 70). iPLA2 is thought to hydrolyze DHA from membrane phospholipids (71), and its expression was not elevated in rat brain following either chronic NMDA administration or cerebroventricular LPS infusion (35, 42, 56). There was no significant difference in other AA and prostaglandin metabolism enzymes, such as P450 expoxygenease, 5-, 12-, and 15-LOX, as well as TXS, between BD and control frontal cortex. These results suggest that increased AA signaling is channeled into prostanoid synthesis, and is selective only to parts of the brain AA cascade.
In parallel with BD, studies in schizophrenic patients have indicated an increase in brain calcium dependent and independent PLA2 activity, as well as PLA2 IVA protein level in red blood cells (72, 73). Similar AA cascade changes also have been reported in postmortem brains from Alzheimer disease (AD) patients, where excitotoxicity and neuroinflammation are considered to play a role (74, 75). In AD post mortem brain tissue, cPLA2 (76), sPLA2 (59), and COX-2 expression (77) are upregulated. Reduced cPLA2 expression ameliorated cognitive deficits in a mouse model of Alzheimer disease (78). Thus, the changes noted here may not be specific to BD, but may be generally related to excitotoxic and inflammatory processes that occur in multiple chronic and progressive neurodegenerative and neuropsychiatric disorders, including Alzheimer disease, Parkinson disease, schizophrenia and unipolar depression (52, 69, 79).
Many but not all of the differences between the BD and control brain were in an opposite direction to brain changes in rats chronically administered mood stabilizers. For example, chronic lithium and carbamazepine was shown to decrease mRNA and protein levels of cPLA2 IVA in rat brain while this enzyme’s expression was upregulated in the BD brain. sPLA2 expression also was upregulated in the BD brain; chronic lithium did not reduce sPLA2 expression in the normal rat brain (18), but prevented the upregulation that was caused by cerebroventricular LPS infusion (Basselin et al., unpublished results). Increased expression of sPLA2 IIA in BD is consistent with reports of increased risk associated with alleles for pancreatic PLA2 (80, 81) and for the sPLA2 receptor in BD (3). However, COX-1 was reduced in the BD brain as well as in the brain of rats given chronic valproate (32), whereas COX-2 was elevated in the BD brain but reduced by lithium, carbamazepine, valproate and lamotrigine (18). Opposite changes in AA cascade markers in the BD brain compared with the brain of rats treated with mood stabilizers may be the basis, in part, for their efficacy in BD.
Levels of mRNA in either BD or control brains did not correlate significantly with postmortem interval, brain pH, or subject age, and mean values of these parameters did not differ significantly between the two groups. Nevertheless, the BD patients were exposed to a variety of drugs not taken by the control subjects, which may have affected the results, since antipsychotics and mood stabilizers can have neurotoxic effects when given chronically (82, 83). No statistical differences were found in all AA cascade genes studied in the present study when the BD subjects were compared to the subgroup of BD subjects treated with lithium. Also, no statistical significance was found when the BD subjects were compared to BD subjects that died by suicide. This suggests that lithium or suicide do not have profound effects on the studied AA cascade markers.
The limitation of the present study is non-availability of medical diagnosis at the time of death, whether patients are in manic or depressive conditions. However, since several BD patients died by suicide, they may have been in a depressed phase of their illness. Future studies should examine AA cascade markers in brains from patients with schizophrenia (to control for comparable drug exposure), or with unipolar (primary major) depression or Alzheimer disease to test for disease specificity (84)
In conclusion, many markers of the AA cascade were significantly upregulated in postmortem frontal cortex from BD patients. These changes may reflect neuroinflammation and excitotoxicity, associated with cell death or drug exposure, or may be intrinsic to the disease independent of these pathological processes. Some of these AA cascade markers were downregulated in rat brain by chronically administered mood stabilizers, which may account for their efficacy in BD. Accordingly, new agents that are shown to downregulate the brain AA cascade in animal models could be considered for treating BD.
The results suggest that brain AA metabolism is elevated in BD, and this could be tested directly with the help of positron emission tomography and [1-11AA] as a radioligand (85). If correct, an increased AA image would be a biological marker of disease progression and could be used to evaluate therapeutic efficacy. Increased brain AA metabolism has been imaged in patients with Alzheimer disease using positron emission tomography (86), in which cPLA2, sPLA2 and COX-2 were also found to be elevated in post-mortem tissue (59, 76, 77).
Acknowledgments
We thank the Harvard Brain Bank, Boston, MA for providing the postmortem brain samples under PHS grant number R24MH068855. This research was entirely supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Bethesda, MD 20892. We also thank the Fellows’ Editorial Board at NIH for reviewing the manuscript.
Abbreviations
- AA
Arachidonic acid
- AP-2
activator protein-2
- BD
bipolar disorder
- BDNF
brain derived neurotrophic factor
- cPLA2
cytosolic phospholipase A2
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- iPLA2
calcium-independent phospholipase A2
- LPS
lipopolysaccharide
- NF-κB
nuclear factor kappa B
- sPLA2
secretory phospholipase A2
- NMDA
N-methyl-D-aspartate
- PGE2
prostaglandin E2
- TXS
thromboxane synthase
- LOX
lipoxygenase
- NSE
neuron-specific enolase
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord. 2003 Jan;73(1–2):123–131. doi: 10.1016/s0165-0327(02)00332-4. [DOI] [PubMed] [Google Scholar]
- 2.Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry. 2000 Dec;157(12):1925–1932. doi: 10.1176/appi.ajp.157.12.1925. [DOI] [PubMed] [Google Scholar]
- 3.Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008 Feb;13(2):197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007 Dec 1;62(11):1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006 Feb;8(1):65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 6.Rao JS, Harry J, Rapoport SI, Kim H-W. Increased excitotoxicity markers and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Molecular Psychiatry. 2009 doi: 10.1038/mp.2009.47. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskvina V, Craddock N, Holmans P, Nikolov I, Pahwa JS, Green E, et al. Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Mol Psychiatry. 2009 Mar;14(3):252–260. doi: 10.1038/mp.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ledda MG, Fratta AL, Pintor M, Zuddas A, Cianchetti C. Early-onset psychoses: comparison of clinical features and adult outcome in 3 diagnostic groups. Child Psychiatry Hum Dev. 2009 Sep;40(3):421–437. doi: 10.1007/s10578-009-0134-0. [DOI] [PubMed] [Google Scholar]
- 9.Lyketsos CG, Corazzini K, Steele C. Mania in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1995 Summer;7(3):350–352. doi: 10.1176/jnp.7.3.350. [DOI] [PubMed] [Google Scholar]
- 10.Ankenman R. Treatment of psychotic symptoms in Alzheimer’s patients. J Clin Psychiatry. 1990 Oct;51(10):437–438. [PubMed] [Google Scholar]
- 11.Basselin M, Villacreses NE, Lee HJ, Bell JM, Rapoport SI. Chronic lithium administration attenuates up-regulated brain arachidonic acid metabolism in a rat model of neuroinflammation. J Neurochem. 2007 Aug;102(3):761–772. doi: 10.1111/j.1471-4159.2007.04593.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Rao JS, Chang L, Rapoport SI, Bazinet RP. Chronic N-methyl-D-aspartate administration increases the turnover of arachidonic acid within brain phospholipids of the unanesthetized rat. J Lipid Res. 2008 Jan;49(1):162–168. doi: 10.1194/jlr.M700406-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Rao JS, Ertley RN, Rapoport SI, Bazinet RP, Lee HJ. Chronic NMDA administration to rats up-regulates frontal cortex cytosolic phospholipase A2 and its transcription factor, activator protein-2. J Neurochem. 2007 Sep;102(6):1918–1927. doi: 10.1111/j.1471-4159.2007.04648.x. [DOI] [PubMed] [Google Scholar]
- 14.Cao Z, Henzel WJ, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996 Feb 23;271(5252):1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Gao X, Li S, Cao Z. Recruitment of IRAK to the interleukin 1 receptor complex requires interleukin 1 receptor accessory protein. Proc Natl Acad Sci U S A. 1997 Nov 25;94(24):12829–12832. doi: 10.1073/pnas.94.24.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997 Dec;7(6):837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 17.Bauer MK, Lieb K, Schulze-Osthoff K, Berger M, Gebicke-Haerter PJ, Bauer J, et al. Expression and regulation of cyclooxygenase-2 in rat microglia. Eur J Biochem. 1997 Feb 1;243(3):726–731. doi: 10.1111/j.1432-1033.1997.00726.x. [DOI] [PubMed] [Google Scholar]
- 18.Chang YC, Kim HW, Rapoport SI, Rao JS. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: cross-talk between excitotoxicity and neuroinflammation. Neurochem Res. 2008 Nov;33(11):2318–2323. doi: 10.1007/s11064-008-9731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oka A, Itoh M, Takashima S. The early induction of cyclooxygenase 2 associated with neurofibrillary degeneration in brains of patients with Fukuyama-type congenital muscular dystrophy. Neuropediatrics. 1999 Feb;30(1):34–37. doi: 10.1055/s-2007-973454. [DOI] [PubMed] [Google Scholar]
- 20.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001 Nov 30;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 21.Needleman P, Minkes M, Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science. 1976 Jul 9;193(4248):163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- 22.Seibert K, Masferrer J, Zhang Y, Gregory S, Olson G, Hauser S, et al. Mediation of inflammation by cyclooxygenase-2. Agents Actions Suppl. 1995;46:41–50. doi: 10.1007/978-3-0348-7276-8_5. [DOI] [PubMed] [Google Scholar]
- 23.Pepicelli O, Fedele E, Bonanno G, Raiteri M, Ajmone-Cat MA, Greco A, et al. In vivo activation of N-methyl-D-aspartate receptors in the rat hippocampus increases prostaglandin E(2) extracellular levels and triggers lipid peroxidation through cyclooxygenase-mediated mechanisms. J Neurochem. 2002 Jun;81(5):1028–1034. doi: 10.1046/j.1471-4159.2002.00897.x. [DOI] [PubMed] [Google Scholar]
- 24.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007 Sep;59(3):207–224. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 25.Kam PC, See AU. Cyclo-oxygenase isoenzymes: physiological and pharmacological role. Anaesthesia. 2000 May;55(5):442–449. doi: 10.1046/j.1365-2044.2000.01271.x. [DOI] [PubMed] [Google Scholar]
- 26.Leslie JB, Watkins WD. Eicosanoids in the central nervous system. J Neurosurg. 1985 Nov;63(5):659–668. doi: 10.3171/jns.1985.63.5.0659. [DOI] [PubMed] [Google Scholar]
- 27.O’Banion MK. Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology. Crit Rev Neurobiol. 1999;13(1):45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- 28.Rao JS, Bazinet RP, Rapoport SI, Lee HJ. Chronic treatment of rats with sodium valproate downregulates frontal cortex NF-kappaB DNA binding activity and COX-2 mRNA. Bipolar Disord. 2007 Aug;9(5):513–520. doi: 10.1111/j.1399-5618.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- 29.Rao JS, Bazinet RP, Rapoport SI, Lee HJ. Chronic administration of carbamazepine down-regulates AP-2 DNA-binding activity and AP-2alpha protein expression in rat frontal cortex. Biol Psychiatry. 2007 Jan 15;61(2):154–161. doi: 10.1016/j.biopsych.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Rao JS, Rapoport SI, Bosetti F. Decrease in the AP-2 DNA-binding activity and in the protein expression of AP-2 alpha and AP-2 beta in frontal cortex of rats treated with lithium for 6 weeks. Neuropsychopharmacology. 2005 Nov;30(11):2006–2013. doi: 10.1038/sj.npp.1300740. [DOI] [PubMed] [Google Scholar]
- 31.Chang MC, Contreras MA, Rosenberger TA, Rintala JJ, Bell JM, Rapoport SI. Chronic valproate treatment decreases the in vivo turnover of arachidonic acid in brain phospholipids: a possible common effect of mood stabilizers. J Neurochem. 2001 May;77(3):796–803. doi: 10.1046/j.1471-4159.2001.00311.x. [DOI] [PubMed] [Google Scholar]
- 32.Bosetti F, Weerasinghe GR, Rosenberger TA, Rapoport SI. Valproic acid down-regulates the conversion of arachidonic acid to eicosanoids via cyclooxygenase-1 and -2 in rat brain. J Neurochem. 2003 May;85(3):690–696. doi: 10.1046/j.1471-4159.2003.01701.x. [DOI] [PubMed] [Google Scholar]
- 33.Bazinet RP, Weis MT, Rapoport SI, Rosenberger TA. Valproic acid selectively inhibits conversion of arachidonic acid to arachidonoyl-CoA by brain microsomal long-chain fatty acyl-CoA synthetases: relevance to bipolar disorder. Psychopharmacology (Berl) 2006 Jan;184(1):122–129. doi: 10.1007/s00213-005-0272-4. [DOI] [PubMed] [Google Scholar]
- 34.Basselin M, Chang L, Bell JM, Rapoport SI. Chronic lithium chloride administration attenuates brain NMDA receptor-initiated signaling via arachidonic acid in unanesthetized rats. Neuropsychopharmacology. 2006 Aug;31(8):1659–1674. doi: 10.1038/sj.npp.1300920. [DOI] [PubMed] [Google Scholar]
- 35.Basselin M, Villacreses NE, Chen M, Bell JM, Rapoport SI. Chronic carbamazepine administration reduces N-methyl-D-aspartate receptor-initiated signaling via arachidonic acid in rat brain. Biol Psychiatry. 2007 Oct 15;62(8):934–943. doi: 10.1016/j.biopsych.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strokin M, Sergeeva M, Reiser G. Role of Ca2+-independent phospholipase A2 and n-3 polyunsaturated fatty acid docosahexaenoic acid in prostanoid production in brain: perspectives for protection in neuroinflammation. Int J Dev Neurosci. 2004 Nov;22(7):551–557. doi: 10.1016/j.ijdevneu.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Nogami M, Takatsu A, Endo N, Ishiyama I. Immunohistochemistry of neuron-specific enolase in neurons of the medulla oblongata from human autopsies. Acta Histochem. 1998 Nov;100(4):371–382. doi: 10.1016/S0065-1281(98)80034-2. [DOI] [PubMed] [Google Scholar]
- 38.Rao JS, Rapoport SI, Kim H-W. Decreased GRK3 but not GRK2 expression in frontal cortex from bipolar disorder patients. International Journal of Neuropharmacology. 2009 doi: 10.1017/S146114570900025X. In press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Rao JS, Kim H-W, Lee H-J, Rapoport SI. Up-regulated arachidonic acid cascade enzymes and their transcription factors in post-mortem frontal cortex from bipolar disorder patients. Abstr Soc Neurosci. 2007;37:707.705. [Google Scholar]
- 40.Dwivedi Y, Rizavi HS, Rao JS, Pandey GN. Modifications in the phosphoinositide signaling pathway by adrenal glucocorticoids in rat brain: focus on phosphoinositide-specific phospholipase C and inositol 1,4,5-trisphosphate. The Journal of Pharmacology and Experimental Therapeutics. 2000 Oct;295(1):244–254. [PubMed] [Google Scholar]
- 41.Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic carbamazepine decreases the incorporation rate and turnover of arachidonic acid but not docosahexaenoic acid in brain phospholipids of the unanesthetized rat: relevance to bipolar disorder. Biol Psychiatry. 2006 Mar 1;59(5):401–407. doi: 10.1016/j.biopsych.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Kim H-W, Lee HJ, Rapoport SI, Rao JS. Hyperglutamatergic state in postmortem frontal cortex of bipolar disorder patients. Soc Neurosci Abstr. 2007:707.704/Z703. [Google Scholar]
- 43.Coyle TR, Kochunov P, Patel RD, Nery FG, Lancaster JL, Mangin JF, et al. Cortical sulci and bipolar disorder. Neuroreport. 2006 Nov 6;17(16):1739–1742. doi: 10.1097/01.wnr.0000239957.53072.f0. [DOI] [PubMed] [Google Scholar]
- 44.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000 Oct 15;48(8):766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 45.Post RM. Sensitization and kindling perspectives for the course of affective illness: toward a new treatment with the anticonvulsant carbamazepine. Pharmacopsychiatry. 1990 Jan;23(1):3–17. doi: 10.1055/s-2007-1014476. [DOI] [PubMed] [Google Scholar]
- 46.Osuji IJ, Cullum CM. Cognition in bipolar disorder. Psychiatr Clin North Am. 2005 Jun;28(2):427–441. doi: 10.1016/j.psc.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Kolko M, de Turco EB, Diemer NH, Bazan NG. Secretory phospholipase A2-mediated neuronal cell death involves glutamate ionotropic receptors. Neuroreport. 2002 Oct 28;13(15):1963–1966. doi: 10.1097/00001756-200210280-00026. [DOI] [PubMed] [Google Scholar]
- 48.Saitoh M, Nagai K, Yaguchi T, Fujikawa Y, Ikejiri K, Yamamoto S, et al. Arachidonic acid peroxides induce apoptotic Neuro-2A cell death in association with intracellular Ca(2+) rise and mitochondrial damage independently of caspase-3 activation. Brain Res. 2003 Nov 21;991(1–2):187–194. doi: 10.1016/j.brainres.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 49.Garrido R, Mattson MP, Hennig B, Toborek M. Nicotine protects against arachidonic-acid-induced caspase activation, cytochrome c release and apoptosis of cultured spinal cord neurons. J Neurochem. 2001 Mar;76(5):1395–1403. doi: 10.1046/j.1471-4159.2001.00135.x. [DOI] [PubMed] [Google Scholar]
- 50.Garrido R, Springer JE, Hennig B, Toborek M. Apoptosis of spinal cord neurons by preventing depletion nicotine attenuates arachidonic acid-induced of neurotrophic factors. J Neurotrauma. 2003 Nov;20(11):1201–1213. doi: 10.1089/089771503322584628. [DOI] [PubMed] [Google Scholar]
- 51.Toborek M, Malecki A, Garrido R, Mattson MP, Hennig B, Young B. Arachidonic acid-induced oxidative injury to cultured spinal cord neurons. J Neurochem. 1999 Aug;73(2):684–692. doi: 10.1046/j.1471-4159.1999.0730684.x. [DOI] [PubMed] [Google Scholar]
- 52.Mueller HT, Meador-Woodruff JH. NR3A NMDA receptor subunit mRNA expression in schizophrenia, depression and bipolar disorder. Schizophr Res. 2004 Dec 1;71(2–3):361–370. doi: 10.1016/j.schres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 53.Mundo E, Tharmalingham S, Neves-Pereira M, Dalton EJ, Macciardi F, Parikh SV, et al. Evidence that the N-methyl-D-aspartate subunit 1 receptor gene (GRIN1) confers susceptibility to bipolar disorder. Mol Psychiatry. 2003 Feb;8(2):241–245. doi: 10.1038/sj.mp.4001218. [DOI] [PubMed] [Google Scholar]
- 54.Weichel O, Hilgert M, Chatterjee SS, Lehr M, Klein J. Bilobalide, a constituent of Ginkgo biloba, inhibits NMDA-induced phospholipase A2 activation and phospholipid breakdown in rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1999 Dec;360(6):609–615. doi: 10.1007/s002109900131. [DOI] [PubMed] [Google Scholar]
- 55.Zhou HR, Islam Z, Pestka JJ. Kinetics of lipopolysaccharide-induced transcription factor activation/inactivation and relation to proinflammatory gene expression in the murine spleen. Toxicol Appl Pharmacol. 2003 Mar 15;187(3):147–161. doi: 10.1016/s0041-008x(02)00077-7. [DOI] [PubMed] [Google Scholar]
- 56.Baum L, Lam LC, Kwok T, Lee J, Chiu HF, Mok VC, et al. Apolipoprotein E epsilon4 allele is associated with vascular dementia. Dement Geriatr Cogn Disord. 2006;22(4):301–305. doi: 10.1159/000095246. [DOI] [PubMed] [Google Scholar]
- 57.Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998 Jan 12;780(2):294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- 58.Richardson RL, Kim EM, Gardiner T, O’Hare E. Chronic intracerebroventricular infusion of lipopolysaccharide: effects of ibuprofen treatment and behavioural and histopathological correlates. Behav Pharmacol. 2005 Nov;16(7):531–541. doi: 10.1097/01.fbp.0000179278.03868.96. [DOI] [PubMed] [Google Scholar]
- 59.Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004 Feb;45(2):205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Luschen S, Adam D, Ussat S, Kreder D, Schneider-Brachert W, Kronke M, et al. Activation of ERK1/2 and cPLA(2) by the p55 TNF receptor occurs independently of FAN. Biochem Biophys Res Commun. 2000 Aug 2;274(2):506–512. doi: 10.1006/bbrc.2000.3173. [DOI] [PubMed] [Google Scholar]
- 61.Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002 Sep-Oct;20(5 Suppl 27):S1–13. [PubMed] [Google Scholar]
- 62.Takemiya T, Matsumura K, Yamagata K. Roles of prostaglandin synthesis in excitotoxic brain diseases. Neurochem Int. 2007 Jul-Sep;51(2–4):112–120. doi: 10.1016/j.neuint.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 63.Hibbeln JR, Palmer JW, Davis JM. Are disturbances in lipid-protein interactions by phospholipase-A2 a predisposing factor in affective illness? Biol Psychiatry. 1989 Apr 1;25(7):945–961. doi: 10.1016/0006-3223(89)90274-6. [DOI] [PubMed] [Google Scholar]
- 64.Lieb J, Karmali R, Horrobin D. Elevated levels of prostaglandin E2 and thromboxane B2 in depression. Prostaglandins Leukot Med. 1983 Apr;10(4):361–367. doi: 10.1016/0262-1746(83)90048-3. [DOI] [PubMed] [Google Scholar]
- 65.Sublette ME, Russ MJ, Smith GS. Evidence for a role of the arachidonic acid cascade in affective disorders: a review. Bipolar Disord. 2004 Apr;6(2):95–105. doi: 10.1046/j.1399-5618.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 66.Nishino S, Ueno R, Ohishi K, Sakai T, Hayaishi O. Salivary prostaglandin concentrations: possible state indicators for major depression. Am J Psychiatry. 1989 Mar;146(3):365–368. doi: 10.1176/ajp.146.3.365. [DOI] [PubMed] [Google Scholar]
- 67.Linnoila M, Whorton AR, Rubinow DR, Cowdry RW, Ninan PT, Waters RN. CSF prostaglandin levels in depressed and schizophrenic patients. Arch Gen Psychiatry. 1983 Apr;40(4):405–406. doi: 10.1001/archpsyc.1983.01790040059008. [DOI] [PubMed] [Google Scholar]
- 68.DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006 Jan;47(1):172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 69.Maida ME, Hurley SD, Daeschner JA, Moore AH, O’Banion MK. Cytosolic prostaglandin E2 synthase (cPGES) expression is decreased in discrete cortical regions in psychiatric disease. Brain Res. 2006 Aug 4;1103(1):164–172. doi: 10.1016/j.brainres.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 70.Thommesen L, Sjursen W, Gasvik K, Hanssen W, Brekke OL, Skattebol L, et al. Selective inhibitors of cytosolic or secretory phospholipase A2 block TNF-induced activation of transcription factor nuclear factor-kappa B and expression of ICAM-1. J Immunol. 1998 Oct 1;161(7):3421–3430. [PubMed] [Google Scholar]
- 71.Strokin M, Chechneva O, Reymann KG, Reiser G. Neuroprotection of rat hippocampal slices exposed to oxygen-glucose deprivation by enrichment with docosahexaenoic acid and by inhibition of hydrolysis of docosahexaenoic acid-containing phospholipids by calcium independent phospholipase A2. Neuroscience. 2006 Jun 30;140(2):547–553. doi: 10.1016/j.neuroscience.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 72.Noponen M, Sanfilipo M, Samanich K, Ryer H, Ko G, Angrist B, et al. Elevated PLA2 activity in schizophrenics and other psychiatric patients. Biol Psychiatry. 1993 Nov 1;34(9):641–649. doi: 10.1016/0006-3223(93)90157-9. [DOI] [PubMed] [Google Scholar]
- 73.Ross BM, Turenne S, Moszczynska A, Warsh JJ, Kish SJ. Differential alteration of phospholipase A2 activities in brain of patients with schizophrenia. Brain Res. 1999 Mar 13;821(2):407–413. doi: 10.1016/s0006-8993(99)01123-3. [DOI] [PubMed] [Google Scholar]
- 74.Greenamyre JT, Young AB. Excitatory amino acids and Alzheimer’s disease. Neurobiol Aging. 1989 Sep-Oct;10(5):593–602. doi: 10.1016/0197-4580(89)90143-7. [DOI] [PubMed] [Google Scholar]
- 75.McGeer EG, McGeer PL. Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003 Aug;27(5):741–749. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 76.Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer’s disease brain. Neurobiol Dis. 1996 Feb;3(1):51–63. doi: 10.1006/nbdi.1996.0005. [DOI] [PubMed] [Google Scholar]
- 77.Pasinetti GM, Aisen PS. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience. 1998 Nov;87(2):319–324. doi: 10.1016/s0306-4522(98)00218-8. [DOI] [PubMed] [Google Scholar]
- 78.Sanchez-Mejia RO, Newman JW, Toh S, Yu GQ, Zhou Y, Halabisky B, et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat Neurosci. 2008 Nov;11(11):1311–1318. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith AW, Dougall AL, Posluszny DM, Somers TJ, Rubinstein WS, Baum A. Psychological distress and quality of life associated with genetic testing for breast cancer risk. Psychooncology. 2008 Aug;17(8):767–773. doi: 10.1002/pon.1291. [DOI] [PubMed] [Google Scholar]
- 80.Dawson E, Gill M, Curtis D, Castle D, Hunt N, Murray R, et al. Genetic association between alleles of pancreatic phospholipase A2 gene and bipolar affective disorder. Psychiatr Genet. 1995 Winter;5(4):177–180. doi: 10.1097/00041444-199524000-00005. [DOI] [PubMed] [Google Scholar]
- 81.Jacobsen N, Daniels J, Moorhead S, Harrison D, Feldman E, McGuffin P, et al. \ Association study of bipolar disorder at the phospholipase A2 gene (PLA2A) in the Darier’s disease (DAR) region of chromosome 12q23-q24.1. Psychiatr Genet. 1996 Winter;6(4):195–199. doi: 10.1097/00041444-199624000-00005. [DOI] [PubMed] [Google Scholar]
- 82.Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, et al. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008 Apr 15;63(8):759–765. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gomez-Sintes R, Hernandez F, Bortolozzi A, Artigas F, Avila J, Zaratin P, et al. Neuronal apoptosis and reversible motor deficit in dominant-negative GSK-3 conditional transgenic mice. Embo J. 2007 Jun 6;26(11):2743–2754. doi: 10.1038/sj.emboj.7601725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benes FM. Searching for unique endophenotypes for schizophrenia and bipolar disorder within neural circuits and their molecular regulatory mechanisms. Schizophr Bull. 2007 Jul;33(4):932–936. doi: 10.1093/schbul/sbm064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giovacchini G, Lerner A, Toczek MT, Fraser C, Ma K, DeMar JC, et al. Brain incorporation of [11C]arachidonic acid, blood volume, and blood flow in healthy aging: a study with partial-volume correction. J Nucl Med. 2004 Sep;45(9):1471–1479. [PubMed] [Google Scholar]
- 86.Esposito G, Giovacchini G, Liow JS, Bhattacharjee AK, Greenstein D, Schapiro M, et al. Imaging neuroinflammation in Alzheimer’s disease with radiolabeled arachidonic acid and PET. J Nucl Med. 2008 Sep;49(9):1414–1421. doi: 10.2967/jnumed.107.049619. [DOI] [PMC free article] [PubMed] [Google Scholar]