Abstract
Although treatments for breast cancer have improved and long-term survival after diagnosis is now common, prevention of the disease is the ultimate goal. Weight loss or weight maintenance is one approach that has been recommended to reduce the risk of breast cancer, particularly for peri/postmenopausal women. This approach is supported by decades of data indicating that calorie restriction prevents spontaneous and chemically induced mammary tumor development in rodents. In most cases, calorie restriction was implemented by a consistent daily reduction of calories, i.e. chronic calorie restriction (CCR). There have also been several studies where periods of reduced caloric intake were followed by periods of refeeding, i.e. intermittent calorie restriction (ICR), resulting in the prevention of spontaneous mammary tumorigenesis. In most of the early studies, there were no direct comparisons of CCR to ICR. One study using moderate calorie restriction in a chemically induced breast cancer rat model found a slight increase in mammary tumor incidence compared with ad libitum fed and CCR rats. However, recently, it has been demonstrated in several transgenic mouse models of breast cancer that ICR consistently provided a greater degree of protection than CCR. This review will provide a detailed comparison of ICR and CCR for breast cancer prevention. It will also examine potential mechanisms of action that may include periods of reduced IGF-I and leptin as well as an increase in the adiponectin:leptin ratio. Application of this approach to at-risk women may provide an approach to lower the risk of breast cancer in overweight/obese women.
Keywords: Animal models, breast cancer, calorie restriction, intermittent calorie restriction
INTRODUCTION
The past several decades have seen significant advances in the treatment of breast cancer, with long-term survival now an expected outcome for most women.[1] Clearly, however, the ultimate goal is the prevention of this disease. The complete etiology of breast cancer is multifactorial and remains poorly understood. Some of the factors are genetics,[2] exposure to increased levels of certain hormones,[3] inflammation,[4] radiation exposure[5] and exposure to carcinogens.[6] In order to better understand the role of these factors in breast cancer etiology, a number of different animal models have been developed. Two different types of animal models are commonly utilized. In the first model, the tumors arise spontaneously due to transgenic modifications leading to overexpression of the genes related to inflammation[7] or growth factors and their receptors.[8] In the second model, mammary tumors are induced by exposure to high doses of carcinogens.[9] Both types of models can be useful tools for investigating a disease as complex as breast cancer provided they are utilized properly and their limitations are taken into account.
The risk factors for breast cancer are better understood than the etiology, and can be utilized to identify at-risk women based on specified criteria. Effective preventative approaches, including medications and/or lifestyle interventions, can then be determined based on an individual's risk. The Gail Model has been used as one means to identify women at increased risk for breast cancer. This approach uses a number of factors, including age, number of pregnancies and family history. Women considered at high risk can be offered chemoprevention with tamoxifen or raloxifene.[10] However, these interventions carry significant risks and additional options to reduce breast cancer risk are desired by the women affected and their advocates.
Tamoxifen acts by directly binding to the estrogen receptor (ER), inhibiting proliferation of ER-positive cells in the breast.[11] Several large, long-term studies have shown that tamoxifen significantly prevents breast cancer relapse and is now a first-line adjuvant therapy for postmenopausal women with ER-positive breast cancer.[12] In addition, tamoxifen treatment has been shown to prevent the initial occurrence of ER-positive tumors in high-risk individuals.[13] However, the actual use of this compound by at-risk women has been low due to the potential side–effects.[14] Raloxifene has been more recently approved for prevention, but the long-term acceptance rates are not yet clear. Low acceptance rates for tamoxifen appear to be a result of a variety of factors, including hot flushes as well as potentially life–threatening events such as increased risk of endometrial cancer, venous thromboembolism, hepatic steatosis and stroke.[11,15]
Aromatase inhibitors (AIs) block the synthesis of estrogens from androgens by inhibiting the cytochrome P450 enzyme aromatase.[16] Recently, AIs have been introduced as another treatment option for breast cancer and may also have a potential for chemoprevention. However, the side–effects of AIs, particularly osteoporosis and a higher number of fractures compared with control groups, may limit their use for the chemoprevention of breast cancer.[17]
Elevated body mass index [BMI = weight (kg)÷height (m2)] was not originally included as part of the Gail Model. However, data obtained during the past 20 years have shown that body weight increase and overweight/obesity can now be considered as additional risk indicators of breast cancer for peri/postmenopausal women.[18] This subject has been reviewed periodically.[19–23] Recently, there has been increasing support for either weight maintenance or loss in women approaching menopause as strategies to reduce postmenopausal breast cancer risk.
Calorie restriction in humans
Although few in number, there are studies in humans which support that voluntary weight loss through calorie restriction will lower the risk of postmenopausal breast cancer diagnosis.[24–26] These three studies utilized data collected through interview and body weight information primarily based on recall. Using data from the cancer registries from several states, Trentham-Dietz et al.[26] reported that women between the ages of 50 and 79 years, with a weight loss greater than 15 kg in adulthood reduced breast cancer risk by 20% compared with the reference group that gained 5–9.9 kg or women whose weight change was between loss of 5 kg to 4.9 kg gained. Analysis of data obtained from the Iowa Women's Health Study indicated the lowest rates of breast cancer were found for women who either maintained or lost weight in adulthood.[25] In a Japanese cohort, women who lost more than 5 kg in adulthood had a hazard ratio for development of breast cancer of 0.35 when compared with women who lost or gained less than 2 kg.[24] It has also been reported that weight loss following gastric bypass surgery reduced the risk of developing breast cancer.[27] However, this is an extreme measure and not likely to be widely implemented. Interest has also focused on data from Okinawa, where individuals who eat in the traditional manner until they are 80% full have very low incidence rates of breast cancer relative to other Japanese.[28]
Several short-term studies have evaluated the consequences of weight loss on estrogen metabolites as possible explanations for how reduced calorie intake would affect factors that may impact breast cancer development.[29,30] These have tended to use premenopausal women and combined exercise with reduced caloric intake, making it unclear what was actually responsible for the hormonal changes. Also, these were fairly short-term studies. In one study, it was found that although overall there was no effect on either 2-hydroxyestrone, 16α-hydroxyestrone or their ratio, if the subjects were divided by tertiles of starting levels, the ratio was improved in women who initially had a low level.[29] In the second study, estrogen and progesterone exposure was reduced by exercise plus calorie restriction compared with women who did only light conditioning.[30]
One report has presented data on overweight/obese premenopausal women who lost weight over 1 month consuming 864 kcal/day.[31] These women lost weight (7 kg) and had significant reductions in serum insulin, leptin, cholesterol and triacylglycerol levels. Analyses of breast tissue biopsies before and after the intervention indicated changes in genes associated with glycolysis and lipid synthesis.
Chronic calorie restriction in rodents
Calorie restriction (also termed energy restriction and/or food/diet restriction) has consistently been reported to prevent mammary tumor development in spontaneous and chemically induced breast cancer rodent models.[32–39] With respect to the development of spontaneous mammary tumors in mice in relation to calorie restriction, Dirx, et al. performed a meta-analysis of 14 studies published between 1942 and 1994.[40] Calorie intake was reduced by 23–50% compared with control mice and the pooled estimate of reduction in mammary tumors was 55%.
Previous work has illustrated several important points concerning mammary tumor inhibition by calorie restriction. In general, the approach was to provide the same reduction in calories on a daily basis, i.e. chronic calorie restriction (CCR). In addition, in most cases, the intervention was initiated in fairly young animals and then maintained throughout the course of the experiment. The results obtained over many decades clearly illustrate that inhibition of mammary tumorigenesis by CCR is possible. Even when initiated in older animals, a protective effect of calorie restriction has been documented. For example, over 70 years ago, Tannenbaum reported that severe calorie restriction (50–67% reduction) beginning at 23 weeks of age reduced spontaneous mammary tumor development from 40% in ad libitum fed mice to 2% in the calorie-reduced group.[41] This age is well beyond the timing of reaching sexual maturity, which, for mice, is 6 weeks of age. In most cases within a specific study, investigators applied only one level of calorie restriction. However, a few reports utilizing two or more levels of restriction in carcinogen-induced mammary tumorigenesis suggested that as the degree of restriction increases, so does the degree of prevention.[32,33]
Despite the overwhelming evidence that CCR can prevent and/or delay the development of mammary tumors, it has not been embraced for the prevention of breast cancer in humans. This may be partially due to that fact that although it is easy to implement this intervention in controlled preclinical studies, application to human subjects may have limited long-term compliance. Further, weight loss, if it does occur in humans, is frequently followed by weight regain. This raises the question, what is the impact of regaining this weight on breast cancer initiation and progression?
Intermittent calorie restriction studies
As indicated above, the usual approach when implementing calorie restriction has been to do so in a chronic fashion providing the same reduced intake daily. However, several investigators reported that periods of intermittent calorie restriction (ICR) followed by periods of refeeding also reduced the incidence of spontaneous mammary tumors in rodents.[42–46] A summary of these five studies is presented in Table 1. It should be mentioned that in several of these papers it is only clear upon reading the papers that the calorie restriction intervention was implemented in an intermittent fashion. This is because they provided the mice with food only twice a week, which results in the mice consuming all their available food in the first day or two and then having little or no food for the remaining time.
Table 1.
Summary of intermittent calorie restriction on spontaneous mammary tumor development
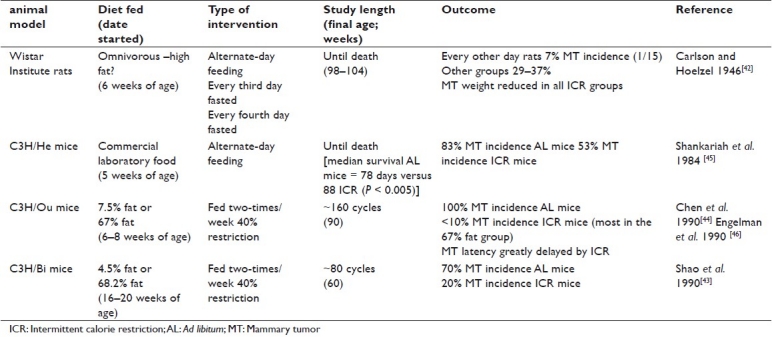
In contrast to the findings presented in Table 1 showing consistently that spontaneous mammary tumors are reduced by calorie restriction, carcinogen administration-induced mammary tumor studies found that mammary tumors were only reduced under certain circumstances by calorie restriction. It is important therefore to carefully examine the studies using carcinogens to induce mammary tumors to identify those that are most relevant to human breast cancer. The carcinogen was generally given for short periods of time at a relatively young age. In several cases, calorie restriction/refeeding was initiated at the time of carcinogen administration at 8 weeks of age.[47–49] The amounts of carcinogen administered were considerably higher than most humans would receive unless they were exposed to some type of industrial accident. The high dose of carcinogen and early age of exposure led to mammary tumor development at a relatively early age compared with the age that humans would normally be expected to develop carcinogen-induced breast cancer.
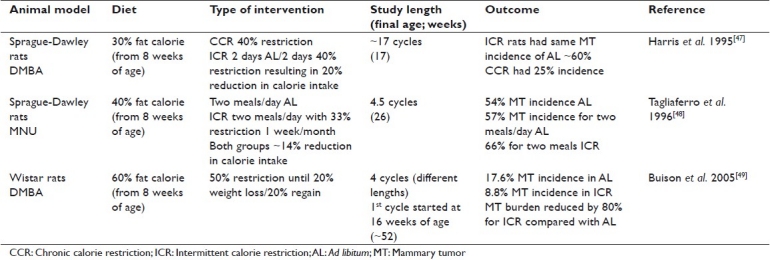
Specific examples of the effect of ICR in carcinogen-induced mammary tumors include two studies during which high-fat diets were fed and body weight levels were maintained but not decreased in the ICR groups. The ad libitum fed rats gained weight during the experiments. In the first study, calorie restriction of 40% alternated with two days of ad libitum feeding resulted in a 20% reduction in calorie intake.[47] There was no effect on mammary tumor incidence of these ICR rats compared with ad libitum fed rats as a result of this intervention. In the second study, ICR was implemented 1 week a month for 4.5 months.[48] In this case, mammary tumor incidence was slightly and significantly higher in the ICR rats compared with those fed ad libitum.
In a more recent study when four periods of 50% calorie restriction (maintained until rats lost 20% body weight) were introduced 2 months after carcinogen administration,[49] mammary tumor incidence was 8.8% compared with 17.6% for the ad libitum fed rats.[49] In addition, mammary tumor burden was reduced by 80% in the ICR rats. This suggests that loss of weight during ICR may be required for inhibition of mammary tumorigenesis. A comparison of these three studies is shown in Table 2. The different results obtained may also be due to timing with respect to the administration of the carcinogen in relationship to the calorie restriction intervention. For example, it was reported that when rats were fasted for 3 days starting 1 week after carcinogen administration, decreased mammary tumor latency was reported as well as a 100% tumor incidence, while nonfasted rats had a 80% incidence.[50] In another study of rats, three periods of fasting/refeeding following carcinogen administration increased mammary tumor number compared with rats either fed ad libitum or subjected to only one period of fasting/refeeding.[51] We speculate that the periods of refeeding at the time of high-level carcinogen administration may enhance tumor development due to the release of many growth factors as well as the hormonal milieu present in young rats as they become sexually mature. The relevance of this to the etiology of most human cancers is not known.
Table 2.
Summary of intermittent calorie restriction cycles on carcinogen-induced mammary tumor development
Comparison of chronic versus intermittent calorie restriction
Only one of the above studies directly compared CCR with ICR. In that case, rats injected with NMU were calorie restricted for 1 week a month for 4.5 months, resulting in a reduced calorie intake of 14%.[48] Mammary tumor incidence was 66% for the ICR rats compared with 54% in the control and 57% in the CCR rats. To further investigate how ICR affected mammary tumors, we conducted a study directly comparing CCR and ICR using the MMTV-TGF-α transgenic mouse strain. This mouse strain overexpresses human TGF-α. These mice develop tumors later in life,[52] which makes them highly relevant as a model of postmenopausal breast cancer. The interventions were initiated at 10 weeks of age. A control group, ad libitum fed, had free access to AIN-93M diet. The ICR group was calorie restricted at 50% of ad libitum for 3-week intervals with a diet formulated to provide the same nutrients as consumed by ad libitum fed mice except for restriction of carbohydrates. Each restriction period was followed by 3 weeks of free access to the AIN-93M diet. A third group, CCR, was calorie restricted by daily pair-feeding to age-matched ICR mice for each 6-week cycle. Mice were followed until 80 weeks of age (1 week of refeeding in the 12th cycle). Overall, the reduction in calorie intake for the ICR and CCR mice was approximately 21%. As expected, mammary tumor incidence was reduced to 44% by calorie restriction in the CCR mice compared with 77% for ad libitum fed mice. In addition, the ICR protocol resulted in a mammary tumor incidence of only 3%.[53] This was quite a spectacular development with only one tumor in one ICR mouse discovered upon necropsy at the termination of the study. This single tumor weighed only 0.063 g compared with an average tumor weight of over 1.000 g for those obtained from ad libitum fed mice and 0.688 g for CCR mice. Overall, these results illustrate a large degree of mammary tumor inhibition by ICR as compared with CCR.
In a second experiment, Study 2, also using MMTV-TGF-α mice, the degree of protection from ICR was not quite as great as that described above for Study 1. Mammary tumor incidence for ICR mice of 15% was obtained[54] compared with 3% in Study 1. We hypothesized that a possible explanation was that during refeeding in Study 2, the ICR mice consumed more calories than did ad libitum fed age-matched mice. This resulted in only an 11% reduction in calorie intake compared with the ad libitum fed mice. Perhaps this overeating during the refeeding period contributed to a greater tumor cell proliferation allowing some recovery? This led us to conduct a third experiment where the ICR mice were carefully matched to the food intake of ad libitum fed mice during the refeeding periods, preventing the opportunity for overeating. As seen in Figure 1, this resulted in a mammary tumor incidence rate of 9%, which was intermediate between the values found in the two earlier experiments.[55]
Figure 1.
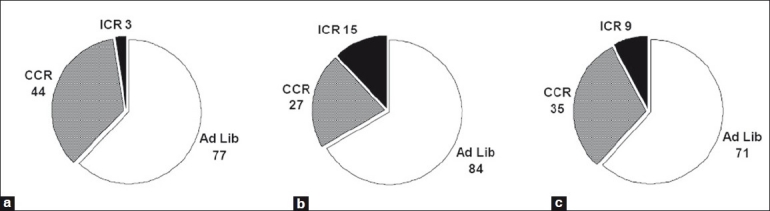
Comparison of ad libitum feeding versus chronic calorie restriction (CCR) feeding versus intermittent calorie restriction (ICR) feeding on mammary tumor incidence (%) in MMTV-TGF-α mice. (a) Study 1: intervention started at 10 weeks of age. ICR mice calorie restricted by 50% for 3 weeks followed by 3 weeks of ad libitum refeeding until 80 weeks of age (1 week of refeeding). Calorie intake for ICR and CCR mice reduced ~20%.[41] (b) Study 2: intervention started at 10 weeks of age. ICR mice calorie restricted by 50% for 3 weeks followed by 3 weeks of ad libitum refeeding until either 79 (after final restriction period) or 80 weeks of age (1 week of refeeding). Calorie intake for ICR and CCR mice reduced 11% and 14%, respectively.[42] (c) Study 3: intervention started at 10 weeks of age. ICR mice calorie restricted at 50% for 3 weeks followed by 3 weeks of controlled refeeding (i.e., matched to ad libitum fed mice's intake) until 79 (after final restriction period) or 82 weeks of age (after the final 3 weeks of refeeding). Calorie intake for ICR and CCR reduced ~25%.[43] For each study, the values are all different by Chi square analyses
In all three experiments (Studies 1–3, Figure 1), when calorie intake was chronically reduced ~20–25%, mammary tumor incidence of CCR mice was significantly lower compared with ad libitum fed mice.[53–55] However, when the same degree of restriction was implemented using 3 weeks of 50% restriction followed by 3 weeks of refeeding, this ICR protocol provided a greater degree of prevention than the CCR regimen. The mammary tumor incidence for ICR mice was reduced by 82–96% compared with a 41–68% reduction for CCR mice in comparison with ad libitum fed mice. A cross-sectional study where mice in the three diet groups were euthanized at different time points further supported the findings of a greater protective approach for ICR versus CCR.[56] Overall, these experiments provided similar results, indicating that greater protection was provided by ICR versus CCR.
In addition to studies using MMTV-TGF-α mice, we have conducted experiments in a second transgenic mouse model of breast cancer, MMTV-neu. These mice develop estrogen receptor-negative mammary tumors that overexpress HER2/neu. In the first study, CCR heterozygous MMTV-neu mice exhibited no reduction in mammary tumor incidence while the same ICR protocol described above reduced mammary tumor development by 50% compared with the ad libitum fed mice.[57] In a recent experiment, mice homozygous for the neu gene were utilized. There was a higher overall incidence of mammary tumors as compared with mice heterozygous for the neu gene (86.7% at 60 weeks versus 37.5% at 80 weeks of age), suggesting more aggressive tumor formation (ME Grossmann and MP Cleary, unpublished). There was significant mammary tumor inhibition by CCR versus ad libitum fed (47.2% versus 86.7%) mice. ICR also had a significant effect verses ad libitum (59.6% versus 86.7%), but did not show a significant difference versus CCR, suggesting that in very aggressive mammary tumor models such as homozygous neu mice and the early life carcinogen-induced rat models, the effects of ICR and CCR may be similar. This remains to be studied in greater detail in the future.
Effects of intermittent calorie restriction on other cancers and diseases
Other types of cancer have been shown to be affected by various ICR protocols. For example, ICR implemented in the TRAMP mouse model of prostate cancer indicated that this intervention delayed prostate tumor detection and death while there was little effect of CCR in comparison with ad libitum fed TRAMP mice.[58,59] A similar protocol was utilized as described above for the MMTV-TGF-α and MMTV-Her2/neu mice, but the ICR restriction phases for the TRAMP mice were for 2 weeks followed by 2 weeks of refeeding. This was done because of the shorter time span for disease development in the TRAMP mouse. In another approach, several different ICR regimens were evaluated in mice inoculated with a human prostate cancer cell line.[60] Survival of mice was improved by either 1 or 2 days of fasting per week with controlled refeeding for the remainder of the week. In several different lymphoma models, ICR reduced disease incidence even when the intervention was initiated in older animals.[61,62]
Other diseases have also been shown to be responsive to the protective effects of ICR. This includes reports that various ICR protocols have cardioprotective and antidiabetic effects in rodents and humans.[63–68] Body fat distribution was reported to be improved and adiponectin levels increased by fasting/refeeding of female mice.[69] Asthma-related symptoms were reported to improve following a fasting/refeeding regimen, which also resulted in significant weight loss in overweight human subjects.[70]
Potential mechanisms of action of intermittent calorie restriction
One way that calorie restriction may impact tumor development is through changes in body weight/fat, which in turn alters the production and secretion of adipokines. Adiponectin is of particular interest as in vitro studies have shown that the addition of adiponectin inhibits human breast cancer cell proliferation.[71–73] In addition, reduced serum adiponectin levels have been associated with the diagnosis of breast cancer.[74,75] However, in female MMTV-TGF-α mice, adiponectin levels were not altered by either CCR or ICR nor were their levels related to the presence of mammary tumors.[76,77] Further, serum adiponectin levels were not predictive of later development of mammary tumors when samples were obtained in a longitudinal protocol.[77] Other studies have not reported consistent changes in serum adiponectin levels of rodents in response to different dietary interventions.[58,59,69,78–80] However, with respect to humans with breast cancer, reduced serum adiponectin levels have been reported for postmenopausal women with breast cancer.[74,75,81]
Leptin is another adipokine that has been considered to potentially play a role in breast cancer development. In vitro studies have indicated that addition of leptin enhances the proliferation of human breast cancer cell lines.[82–84] In vivo studies found that MMTV-TGF-α mice crossed with either leptin-deficient or leptin receptor-deficient mice did not develop mammary tumors in contrast to their sisters without these homozygous genetic defects.[85,86] In the MMTV-TGF-α mice, we have recently found that serum leptin levels were reduced 30% by CCR compared with ad libitum fed mice while after 3 weeks of ICR intervention, the reduction was 80%.[77] Interestingly, after 3 weeks of refeeding, the ICR values were still 65% lower than ad libitum fed mice and still lower than those of CCR mice. However, for individual mice, there was no association of serum leptin levels with the presence of mammary tumors nor were leptin levels at a younger age predictive of the eventual development of mammary tumors. Measuring leptin levels alone in association with breast cancer diagnosis has given inconsistent findings in humans. Some studies have shown leptin to be increased in women with breast cancer, but other studies have found leptin to be decreased or unchanged.[87,88] This suggests that the role of leptin in breast cancer tumorigenesis is complex and not fully understood.
The ratio of adiponectin to leptin may be the key to understanding the role these two adipokines play in breast cancer initiation and progression. This is because these two adipokines share a unique relationship, whereby leptin rises with increasing body weight and in humans, serum adiponectin decreases.[89] Thus, as body weight increases, the ratio of these two factors changes drastically. Interestingly, in women, a low adiponectin:leptin ratio was associated with breast cancer, and in vitro studies have shown this ratio to impact breast cancer cell proliferation.[90] Calculation of the adiponectin:leptin ratio in relationship to the protective effect of ICR indicates that following restriction periods of 50%, there is a marked increase in this ratio compared with ad libitum fed, CCR or ICR mice after refeeding.[77] However, there was no association with individual mice and their mammary tumor status. In humans, a study of breast cancer patients found that a decreased adiponectin:leptin ratio appeared to indicate the presence of aggressive breast cancer independent of the effect of BMI.[91]
Insulin-like growth factor I (IGF-I) is another growth factor that has been associated with the development of various cancers, and calorie restriction has been reported to reduce the circulating levels of this protein. In particular, the reduction in mammary tumor incidence following 40% CCR in an NMU model was reported to be associated with lower IGF-I levels.[92] In CCR rats that were allowed to refeed, the IGF-I levels were rapidly restored to the levels of control rats. At the termination of the study, mammary tumor incidence for CCR-refed rats was similar to that of the controls. In MMTV-TGF-α mice following 20–25% calorie reduction by CCR protocols, the IGF-I levels were reduced as were levels in ICR mice following 3 weeks of 50% calorie restriction.[54,55] Refeeding for either 1 or 3 weeks resulted in IGF-I levels approaching the range of the ad libitum fed mice.[53–55]
Evidence for the role of IGF-I in human breast cancer remains elusive. The apparent conflict between animal models and human studies may in part be related to the effects of IGF-I on breast cancer initiation and development. This further illustrates that development of breast cancer may depend on a complex interplay between a number of different growth factors that remain to be fully elucidated. In addition, what happens to the levels of various receptors and how intracellular signaling is effected remains largely unexplored. A recent report provided additional data of other ways that ICR may affect inflammation.[93] Serum was collected from rats subjected to alternate-day fasting. The serum was added to rat C6 glioma cells and a variety of measurements were made. Notable effects included significant reduction of interleukin-6 secretion and matrix metalloproteinase-2 activity as well an increase in TGF-β1 secretion in cells exposed to the ICR serum versus ad libitum fed serum, indicating that the serum contained anti-inflammatory factors. This suggests that additional pathways and growth factors may be important at the cell level.
Humans and intermittent calorie restriction
Whether ICR impacts human breast cancer development has not been prospectively studied. A retrospective study of postmenopausal women with a history of weight cycling found no association with breast cancer development.[26] However, several studies suggest that women who have suffered from anorexia nervosa and presumably experienced periods of weight loss and regain were at reduced risk for breast cancer.[94,95] In addition, women who have experienced weight loss through gastric bypass surgery,[27] which leads to significant calorie restriction, were found to have a reduced incidence of breast cancer. Whether these women had a history of weight-cycling is not known, but one could speculate that they had as previous attempts at weight loss is frequently a criterion before surgery is implemented.[96] This illustrates the potential for ICR to reduce the breast cancer incidence in the human population.
A recent study was designed to directly compare the physiological impact of ICR versus CCR in overweight women considered to be at high risk for breast cancer. A 6-month overall 25% restriction was utilized with a number of outcomes assessed.[97] Compliance to the interventions was good and similar in both study arms. In addition, weight reduction was similar between the groups, as were most of the measurements made. One highly significant finding was that the homeostatic model assessment (HOMA) results that quantify insulin resistance and beta-cell function were reduced to a much greater extent compared with starting values in the ICR versus CCR group. This indicates that the ICR group had a reduction in insulin resistance compared with the CCR group. A slight decrease in the leptin:adiponetin ratio was also found. In another report using modified alternate-day fasting in human subjects, successful weight loss was obtained.[68] Thus, this intervention is feasible to apply to humans and in fact may be physiologically relevant from the stand point of what the human body was accustomed to up until recently, i.e. periods of calorie restriction interspersed with periods of adequate food.
CONCLUSIONS
Although the mechanism(s) of action for CCR and ICR are not known, understanding and identifying critical time points when the intervention can be implemented and be successful will provide a more focused approach toward application of this intervention to at-risk women. On a practical note, it may be easier for human subjects to tolerate short discrete periods of caloric restriction than a lifetime of chronic restriction. Here, we have presented data of the potential contributing roles of several growth factors that may mediate the effect of ICR. However, there are still many additional potential pathways and mechanisms to evaluate. Effects on apoptosis, production of reactive oxygen species and autophagy may also be involved, but have not been investigated to any degree.
AUTHOR’S PROFILE
Prof. Margot P Cleary, University of Minnesota Hormel Institute
Prof. Michael E Grossmann, University of Minnesota Hormel Institute
ACKNOWLEDGMENTS
Support for studies presented in this review was obtained from DAMD17-97-7055 (MPC), CA101858 (MPC), Komen for the Cure (MEG), The Breast Cancer Research Foundation (MPC) and The Hormel Foundation.
REFERENCES
- 1.Kiely BE, Soon YY, Tattersall MH, Stockler MR. How long have I got? Estimating typical, best-case, and worst-case scenarios for patients starting first-line chemotherapy for metastatic breast cancer: a systematic review of recent randomized trials. J Clin Oncol. 2011;29:456–63. doi: 10.1200/JCO.2010.30.2174. [DOI] [PubMed] [Google Scholar]
- 2.Gulati AP, Domchek SM. The clinical management of BRCA1 and BRCA2 mutation carriers. Curr Oncol Rep. 2008;10:47–53. doi: 10.1007/s11912-008-0008-9. [DOI] [PubMed] [Google Scholar]
- 3.Tirona MT, Sehgal R, Ballester O. Prevention of breast cancer (part I): epidemiology, risk factors, and risk assessment tools. Cancer Invest. 2010;28:743–50. doi: 10.3109/07357907.2010.494321. [DOI] [PubMed] [Google Scholar]
- 4.DeNardo DG, Coussens LM. Inflammation and breast cancer.Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Gelder R, Draisma G, Heijnsdijk EA, de Koning HJ. Population-based mammography screening below age 50: balancing radiation-induced vs prevented breast cancer deaths. Br J Cancer. 2011;104:1214–20. doi: 10.1038/bjc.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667–711. doi: 10.1002/cncr.22655. [DOI] [PubMed] [Google Scholar]
- 7.Matsui Y, Halter SA, Holt JT, Hogan BL, Coffey RJ. Development of mammary hyperplasia and neoplasia in MMTV-TGF alpha transgenic mice. Cell. 1990;61:1147–55. doi: 10.1016/0092-8674(90)90077-r. [DOI] [PubMed] [Google Scholar]
- 8.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578–82. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kritchevsky D, Weber MM, Klurfeld DM. Dietary fat versus caloric content in initiation and promotion of 7,12-dimethylbenz(a)anthracene-induced mammary tumorigenesis in rats. Cancer Res. 1984;44:3174–7. [PubMed] [Google Scholar]
- 10.Gravitz L. Chemoprevention: First line of defence. Nature. 2011;471:S5–7. doi: 10.1038/471S5a. [DOI] [PubMed] [Google Scholar]
- 11.Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527–37. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 12.Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1992;339:71–85. [PubMed] [Google Scholar]
- 13.Martino S, Costantino J, McNabb M, Mershon J, Bryant K, Powles T, et al. The role of selective estrogen receptor modulators in the prevention of breast cancer: comparison of the clinical trials. Oncologist. 2004;9:116–25. doi: 10.1634/theoncologist.9-2-116. [DOI] [PubMed] [Google Scholar]
- 14.Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev. 2010;19:443–6. doi: 10.1158/1055-9965.EPI-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murata Y, Ogawa Y, Saibara T, Nishioka A, Fujiwara Y, Fukumoto M, et al. Unrecognized hepatic steatosis and non-alcoholic steatohepatitis in adjuvant tamoxifen for breast cancer patients. Oncol Rep. 2000;7:1299–304. doi: 10.3892/or.7.6.1299. [DOI] [PubMed] [Google Scholar]
- 16.Cuzick J. Aromatase inhibitors for breast cancer prevention. J Clin Oncol. 2005;23:1636–43. doi: 10.1200/JCO.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone. 2003;32:468–73. doi: 10.1016/s8756-3282(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 18.Santen RJ, Boyd NF, Chlebowski RT, Cummings S, Cuzick J, Dowsett M, et al. Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. Endocr Relat Cancer. 2007;14:169–87. doi: 10.1677/ERC-06-0045. [DOI] [PubMed] [Google Scholar]
- 19.Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast. 2004;13:85–92. doi: 10.1016/j.breast.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Pischon T, Nothlings U, Boeing H. Obesity and cancer. T Proc Nutr Soc. 2008;67:128–45. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 21.Le Marchand L, Kolonel LN, Earle ME, Mi MP. Body size at different periods of life and breast cancer risk. Am J Epidemiol. 1988;128:137–52. doi: 10.1093/oxfordjournals.aje.a114936. [DOI] [PubMed] [Google Scholar]
- 22.Helmrich SP, Shapiro S, Rosenberg L, Kaufman DW, Slone D, Bain C, et al. Risk factors for breast cancer. Am J Epidemiol. 1983;117:35–45. doi: 10.1093/oxfordjournals.aje.a113513. [DOI] [PubMed] [Google Scholar]
- 23.Cleary MP, Maihle NJ. The role of body mass index in the relative risk of developing premenopausal versus postmenopausal breast cancer. Proc Soc Exp Biol Med. 1997;216:28–43. doi: 10.3181/00379727-216-44153b. [DOI] [PubMed] [Google Scholar]
- 24.Harvie M, Howell A, Vierkant RA, Kumar N, Cerhan JR, Kelemen LE, et al. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women's health study. Cancer Epidemiol Biomarkers Prev. 2005;14:656–61. doi: 10.1158/1055-9965.EPI-04-0001. [DOI] [PubMed] [Google Scholar]
- 25.Kawai M, Minami Y, Kuriyama S, Kakizaki M, Kakugawa Y, Nishino Y, et al. Adiposity, adult weight change and breast cancer risk in postmenopausal Japanese women: the Miyagi Cohort Study. Br J Cancer. 2010;103:1443–7. doi: 10.1038/sj.bjc.6605885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trentham-Dietz A, Newcomb PA, Egan KM, Titus-Ernstoff L, Baron JA, Storer BE, et al. Weight change and risk of postmenopausal breast cancer (United States) Cancer Causes Control. 2000;11:533–42. doi: 10.1023/a:1008961931534. [DOI] [PubMed] [Google Scholar]
- 27.Christou NV, Lieberman M, Sampalis F, Sampalis JS. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis. 2008;4:691–5. doi: 10.1016/j.soard.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A, Yano K, He Q, et al. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world's longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci. 2007;1114:434–55. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- 29.Westerlind KC, Williams NI. Effect of energy deficiency on estrogen metabolism in premenopausal women. Med Sci Sports Exerc. 2007;39:1090–7. doi: 10.1097/mss.0b013e3180485727. [DOI] [PubMed] [Google Scholar]
- 30.Williams NI, Reed JL, Leidy HJ, Legro RS, De Souza MJ. Estrogen and progesterone exposure is reduced in response to energy deficiency in women aged 25-40 years. Hum Reprod. 2010;25:2328–39. doi: 10.1093/humrep/deq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong KR, Sims AH, Harvie M, Chapman M, Dunn WB, Broadhurst D, et al. Biomarkers of dietary energy restriction in women at increased risk of breast cancer. Cancer Prev Res (Phila) 2009;2:720–31. doi: 10.1158/1940-6207.CAPR-09-0008. [DOI] [PubMed] [Google Scholar]
- 32.Smith-McCune K, Zhu YH, Hanahan D, Arbeit J. Cross-species comparison of angiogenesis during the premalignant stages of squamous carcinogenesis in the human cervix and K14-HPV16 transgenic mice. Cancer Res. 1997;57:1294–300. [PubMed] [Google Scholar]
- 33.Ruggeri BA, Klurfeld DM, Kritchevsky D, Furlanetto RW. Caloric restriction and 7,12-dimethylbenz(a)anthracene-induced mammary tumor growth in rats: alterations in circulating insulin, insulin-like growth factors I and II, and epidermal growth factor. Cancer Res. 1989;49:4130–4. [PubMed] [Google Scholar]
- 34.Bunk B, Zhu P, Klinga K, Berger MR, Schmähl D. I Influence of reducing luxury calories in the treatment of experimental mammary carcinoma. Br J Cancer. 1992;65:845–51. doi: 10.1038/bjc.1992.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beth M, Berger MR, Aksoy M, Schmähl D. Comparison between the effects of dietary fat level and of calorie intake on methylnitrosourea-induced mammary carcinogenesis in female SD rats. Int J Cancer. 1987;39:737–44. doi: 10.1002/ijc.2910390614. [DOI] [PubMed] [Google Scholar]
- 36.Gillette CA, Zhu Z, Westerlind KC, Melby CL, Wolfe P, Thompson HJ. Energy availability and mammary carcinogenesis: effects of calorie restriction and exercise. Carcinogenesis. 1997;18:1183–8. doi: 10.1093/carcin/18.6.1183. [DOI] [PubMed] [Google Scholar]
- 37.Engelman RW, Day NK, Good RA. Calorie intake during mammary development influences cancer risk: lasting inhibition of C3H/HeOu mammary tumorigenesis by peripubertal calorie restriction. Cancer Res. 1994;54:5724–30. [PubMed] [Google Scholar]
- 38.Tucker MJ. The effect of long-term food restriction on tumours in rodents. Int J Cancer. 1979;23:803–7. doi: 10.1002/ijc.2910230611. [DOI] [PubMed] [Google Scholar]
- 39.Sheldon WG, Bucci TJ, Hart RW, Turturro A. Age-related neoplasia in a lifetime study of ad libitum-fed and food-restricted B6C3F1 mice. Toxicol Pathol. 1995;23:458–76. doi: 10.1177/019262339502300403. [DOI] [PubMed] [Google Scholar]
- 40.Dirx MJ, Zeegers MP, Dagnelie PC, van den Bogaard T, van den Brandt PA. Energy restriction and the risk of spontaneous mammary tumors in mice: a meta-analysis. Int J Cancer. 2003;106:766–70. doi: 10.1002/ijc.11277. [DOI] [PubMed] [Google Scholar]
- 41.Tannenbaum A. The initiation and growth of tumors. Introduction. I. Effects of underfeeding. Am J Cancer. 1940;38:335–50. [Google Scholar]
- 42.Carlson AJ, Hoelzel F. Apparent prolongation of the life span of rats by intermittent fasting. J Nutr. 1946;31:363–75. doi: 10.1093/jn/31.3.363. [DOI] [PubMed] [Google Scholar]
- 43.Shao RP, Dao ML, Day NK, Good RA. Dietary manipulation of mammary tumor development in adult C3H/Bi mice. Proc Soc Exp Biol Med. 1990;193:313–7. doi: 10.3181/00379727-193-43041. [DOI] [PubMed] [Google Scholar]
- 44.Chen RF, Good RA, Engelman RW, Hamada N, Tanaka A, Nonoyama M, et al. Suppression of mouse mammary tumor proviral DNA and protooncogene expression: association with nutritional regulation of mammary tumor development. Proc Natl Acad Sci U S A. 1990;87:2385–9. doi: 10.1073/pnas.87.7.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shankaraiah K, Halberg F, Yunis E, Watson LM. Alternate-day feeding alters the circadian system, reduces breast cancer incidence and prolongs life. In: Halberg F, Reale L, Tarquini B, editors. Proceedings of II International Symposium on Chronobiologic Approach to Social Medicine. Rome: Instituto Itaniano di Medicina Sociale; 1984. pp. 633–48. [Google Scholar]
- 46.Engelman RW, Day NK, Chen RF, Tomita Y, Bauer-Sardiña I, Dao ML, et al. Calorie consumption level influences development of C3H/Ou breast adenocarcinoma with indifference to calorie source. Proc Soc Exp Biol Med. 1990;193:23–30. doi: 10.3181/00379727-193-42984. [DOI] [PubMed] [Google Scholar]
- 47.Harris SR, Brix AE, Broderson JR, Bunce OR. Chronic energy restriction versus energy cycling and mammary tumor promotion. Proc Soc Exp Biol Med. 1995;209:231–6. doi: 10.3181/00379727-209-43897. [DOI] [PubMed] [Google Scholar]
- 48.Tagliaferro AR, Ronan AM, Meeker LD, Thompson HJ, Scott AL, Sinha D. Cyclic food restriction alters substrate utilization and abolishes protection from mammary carcinogenesis female rats. J Nutr. 1996;126:1398–405. doi: 10.1093/jn/126.5.1398. [DOI] [PubMed] [Google Scholar]
- 49.Buison AM, Pellizzon MA, Brogan KE, Barnes MJ, Jen KL. Weight cycling did not increase tumor incidence in high fat-fed rats treated with a low-dose 7,12-dimethylbenzyl(1)anthracene. Nutrition Research. 2005;25:1097–108. [Google Scholar]
- 50.Sesca E, Premoselli F, Binasco V, Bollito E, Tessitore L. Fasting-refeeding stimulates the development of mammary tumors induced by 7,12-dimethylbenz[a]anthracene. Nutr Cancer. 1998;30:25–30. doi: 10.1080/01635589809514636. [DOI] [PubMed] [Google Scholar]
- 51.Tessitore L, Chiara M, Sesca E, Premoselli F, Binasco V, Dianzani MU. Fasting during promotion, but not during initiation, enhances the growth of methylnitrosourea-induced mammary tumours. Carcinogenesis. 1997;18:1679–81. doi: 10.1093/carcin/18.8.1679. [DOI] [PubMed] [Google Scholar]
- 52.Halter SA, Dempsey P, Matsui Y, Stokes MK, Graves-Deal R, Hogan BL, et al. Distinctive patterns of hyperplasia in transgenic mice with mouse mammary tumor virus transforming growth factor-alpha.Characterization of mammary gland and skin proliferations. Am J Pathol. 1992;140:1131–46. [PMC free article] [PubMed] [Google Scholar]
- 53.Cleary MP, Jacobson MK, Phillips FC, Getzin SC, Grande JP, Maihle NJ. Weight-cycling decreases incidence and increases latency of mammary tumors to a greater extent than does chronic caloric restriction in mouse mammary tumor virus-transforming growth factor-alpha female mice. Cancer Epidemiol Biomarkers Prev. 2002;11:836–43. [PubMed] [Google Scholar]
- 54.Cleary MP, Hu X, Grossmann ME, Juneja SC, Dogan S, Grande JP, et al. Prevention of mammary tumorigenesis by intermittent caloric restriction: does caloric intake during refeeding modulate the response? Exp Biol Med (Maywood) 2007;232:70–80. [PubMed] [Google Scholar]
- 55.Rogozina OP, Bonorden MJ, Grande JP, Cleary MP. Serum insulin-like growth factor-I and mammary tumor development in ad libitum-fed, chronic calorie-restricted, and intermittent calorie-restricted MMTV-TGF-alpha mice. Cancer Prev Res (Phila) 2009;2:712–9. doi: 10.1158/1940-6207.CAPR-09-0028. [DOI] [PubMed] [Google Scholar]
- 56.Dogan S, Rogozina OP, Lokshin A, Grande JP, Cleary MP. Effects of chronic vs.intermittent calorie restriction on mammary tumor incidence and serum adiponectin and leptin levels in MMTV-TGF-α mice at different ages. Oncol Lett. 2010;1:167–76. doi: 10.3892/ol_00000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pape-Ansorge KA, Grande JP, Christensen TA, Maihle NJ, Cleary MP. Effect of moderate caloric restriction and/or weight cycling on mammary tumor incidence and latency in MMTV-Neu female mice. Nutr Cancer. 2002;44:162–8. doi: 10.1207/S15327914NC4402_07. [DOI] [PubMed] [Google Scholar]
- 58.Bonorden MJ, Rogozina OP, Kluczny CM, Grossmann ME, Grambsch PL, Grande JP, et al. Intermittent calorie restriction delays prostate tumor detection and increases survival time in TRAMP mice. Nutr Cancer. 2009;61:265–75. doi: 10.1080/01635580802419798. [DOI] [PubMed] [Google Scholar]
- 59.Bonorden MJ, Rogozina OP, Kluczny CM, Grossmann ME, Grande JP, Lokshin A, et al. Cross-sectional analysis of intermittent versus chronic caloric restriction in the TRAMP mouse. Prostate. 2009;69:317–26. doi: 10.1002/pros.20878. [DOI] [PubMed] [Google Scholar]
- 60.Buschemeyer WC, 3rd, Klink JC, Mavropoulos JC, Poulton SH, Demark-Wahnefried W, Hursting SD, et al. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate. 2010;70:1037–43. doi: 10.1002/pros.21136. [DOI] [PubMed] [Google Scholar]
- 61.Descamps O, Riondel J, Ducros V, Roussel AM. Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting. Mech Ageing Dev. 2005;126:1185–91. doi: 10.1016/j.mad.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23:817–22. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- 63.Pedersen CR, Hagemann I, Bock T, Buschard K. Intermittent feeding and fasting reduces diabetes incidence in BB rats. Autoimmunity. 1999;30:243–50. doi: 10.3109/08916939908993805. [DOI] [PubMed] [Google Scholar]
- 64.Tikoo K, Tripathi DN, Kabra DG, Sharma V, Gaikwad AB. Intermittent fasting prevents the progression of type I diabetic nephropathy in rats and changes the expression of Sir2 and p53. FEBS Lett. 2007;581:1071–8. doi: 10.1016/j.febslet.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Katare RG, Kakinuma Y, Arikawa M, Yamasaki F, Sato T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3K signaling pathway. J Mol Cell Cardiol. 2009;46:405–12. doi: 10.1016/j.yjmcc.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 66.Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, et al. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem. 2010;21:413–7. doi: 10.1016/j.jnutbio.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varady KA, Hudak CS, Hellerstein MK. Modified alternate-day fasting and cardioprotection: relation to adipose tissue dynamics and dietary fat intake. Metabolism. 2009;58:803–11. doi: 10.1016/j.metabol.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 68.Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90:1138–43. doi: 10.3945/ajcn.2009.28380. [DOI] [PubMed] [Google Scholar]
- 69.Varady KA, Allister CA, Roohk DJ, Hellerstein MK. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J Nutr Biochem. 2010;21:188–95. doi: 10.1016/j.jnutbio.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–74. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer. 2008;98:370–9. doi: 10.1038/sj.bjc.6604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arditi JD, Venihaki M, Karalis KP, Chrousos GP. Antiproliferative effect of adiponectin on MCF7 breast cancer cells: a potential hormonal link between obesity and cancer. Horm Metab Res. 2007;39:9–13. doi: 10.1055/s-2007-956518. [DOI] [PubMed] [Google Scholar]
- 73.Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2006;345:271–9. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 74.Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–7. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 75.Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–704. [PubMed] [Google Scholar]
- 76.Grossmann ME, Ray A, Nkhata KJ, Malakhov DA, Rogozina OP, Dogan S, et al. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–53. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 77.Rogozina OP, Bonorden MJ, Seppanen CN, Grande JP, Cleary MP. Effect of chronic and intermittent calorie restriction on serum adiponectin and leptin and mammary tumorigenesis. Cancer Prev Res (Phila) 2011;4:568–81. doi: 10.1158/1940-6207.CAPR-10-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Varady KA, Roohk DJ, Loe YC, McEvoy-Hein BK, Hellerstein MK. Effects of modified alternate-day fasting regimens on adipocyte size, triglyceride metabolism, and plasma adiponectin levels in mice. J Lipid Res. 2007;48:2212–9. doi: 10.1194/jlr.M700223-JLR200. [DOI] [PubMed] [Google Scholar]
- 79.Nkhata KJ, Ray A, Schuster TF, Grossmann ME, Cleary MP. Effects of adiponectin and leptin co-treatment on human breast cancer cell growth. Oncology Rep. 2009;21:1611–9. doi: 10.3892/or_00000395. [DOI] [PubMed] [Google Scholar]
- 80.Chen GC, Huang CY, Chang MY, Chen CH, Chen SW, Huang CJ, et al. Two unhealthy dietary habits featuring a high fat content and a sucrose-containing beverage intake, alone or in combination, on inducing metabolic syndrome in Wistar rats and C57BL/6J mice. Metabolism. 2011;60:155–64. doi: 10.1016/j.metabol.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 81.Bondanelli M, Margutti A, Ambrosio MR, Plaino L, Cobellis L, Petraglia F, et al. Blood growth hormone-binding protein levels in premenopausal and postmenopausal women: roles of body weight and estrogen levels. J Clin Endocrinol Metab. 2001;86:1973–80. doi: 10.1210/jcem.86.5.7485. [DOI] [PubMed] [Google Scholar]
- 82.Laud K, Gourdou I, Pessemesse L, Peyrat JP, Djiane J. Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Mol Cell Endocrinol. 2002;188:219–26. doi: 10.1016/s0303-7207(01)00678-5. [DOI] [PubMed] [Google Scholar]
- 83.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94:1704–11. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 84.Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2002;293:622–8. doi: 10.1016/S0006-291X(02)00205-X. [DOI] [PubMed] [Google Scholar]
- 85.Cleary MP, Phillips FC, Getzin SC, Jacobson TL, Jacobson MK, Christensen TA, et al. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003;77:205–15. doi: 10.1023/a:1021891825399. [DOI] [PubMed] [Google Scholar]
- 86.Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med (Maywood) 2004;229:182–93. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 87.Woo HY, Park H, Ki CS, Park YL, Bae WG. Relationships among serum leptin, leptin receptor gene polymorphisms, and breast cancer in Korea. Cancer Lett. 2006;237:137–42. doi: 10.1016/j.canlet.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 88.Coskun U, Günel N, Toruner FB, Sancak B, Onuk E, Bayram O, et al. Serum leptin, prolactin and vascular endothelial growth factor (VEGF) levels in patients with breast cancer. Neoplasma. 2003;50:41–6. [PubMed] [Google Scholar]
- 89.Ryan AS, Berman DM, Nicklas BJ, Sinha M, Gingerich RL, Meneilly GS, et al. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26:2383–8. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 90.Grossmann ME, Ray A, Dogan S, Mizuno NK, Cleary MP. Balance of adiponectin and leptin modulates breast cancer cell growth. Cell Res. 2008;18:1154–6. doi: 10.1038/cr.2008.293. [DOI] [PubMed] [Google Scholar]
- 91.Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–14. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 92.Zhu Z, Jiang W, Thompson HJ. An experimental paradigm for studying the cellular and molecular mechanisms of cancer inhibition by energy restriction. Mol Carcinog. 2002;35:51–6. doi: 10.1002/mc.10073. [DOI] [PubMed] [Google Scholar]
- 93.Rajabi A, Parinejad N, Ahmadi K, Khorramizadeh MR, Raza M. Anti-inflammatory effects of serum isolated from animals on intermittent feeding in C6 glioma cell line. Neurosci Lett. 2011;487:32–5. doi: 10.1016/j.neulet.2010.09.068. [DOI] [PubMed] [Google Scholar]
- 94.Mellemkjaer L, Emborg C, Gridley G, Munk-Jørgensen P, Johansen C, Tjønneland A, et al. Anorexia nervosa and cancer risk. Cancer Causes Control. 2001;12:173–7. doi: 10.1023/a:1008974414116. [DOI] [PubMed] [Google Scholar]
- 95.Michels KB, Ekbom A. Caloric restriction and incidence of breast cancer. JAMA. 2004;291:1226–30. doi: 10.1001/jama.291.10.1226. [DOI] [PubMed] [Google Scholar]
- 96.Coleman KJ, Toussi R, Fujioka K. Do gastric bypass patient characteristics, behavior, and health differ depending upon how successful weight loss is defined? Obes Surg. 2010;20:1385–92. doi: 10.1007/s11695-010-0223-y. [DOI] [PubMed] [Google Scholar]
- 97.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35:714–27. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]


