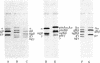Abstract
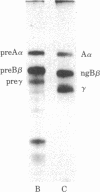
Vertebrate fibrinogen consists of two sets of three nonidentical polypeptides that are synthesized in the liver. The subunits of fibrinogen have been synthesized in a cell-free, membrane-free translation system and compared with (alpha), polypeptides of fibrinogen purified from rat plasma and (b) subunits synthesized and secreted by hepatoma cells grown in culture. Rat hepatoma monolayers were grown with or without tunicamycin to prevent or allow glycosylation of the B beta and gamma subunits, respectively. Sodium dodecyl sulfate polyacrylamide gel analysis indicated that each of the polypeptides translated in vitro from mRNA is larger than its corresponding nonglycosylated fibrinogen chain. The primary translation A alpha, B beta, and gamma chains are larger than their authentic nonglycosylated counterparts by 600, 1100, and 3000 daltons, respectively. Furthermore, the preA alpha and preB beta translation products are thrombin sensitive. These results strongly imply that signal peptides exist on each of the primary translation products of fibrinogen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman L. W., Kuehl W. M. Temporal relationship of translation and glycosylation of immunoglobulin heavy and light chains. Biochemistry. 1978 Nov 28;17(24):5174–5180. doi: 10.1021/bi00617a017. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bouma H., 3rd, Fuller F. M. Partial chemical characterization of rat fibrinogen. J Biol Chem. 1975 Jun 25;250(12):4678–4683. [PubMed] [Google Scholar]
- Bouma H., 3rd, Kwan S. W., Fuller G. M. Radioimmunological identification of polysomes synthesizing fibrinogen polypeptide chains. Biochemistry. 1975 Nov 4;14(22):4787–4792. doi: 10.1021/bi00693a002. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Structural aspects of the fibrinogen to fibrin conversion. Adv Protein Chem. 1973;27:1–109. doi: 10.1016/s0065-3233(08)60446-5. [DOI] [PubMed] [Google Scholar]
- Gaffney P. J. Localisation of carbohydrate in the subunits of human fibinogen and its plasmin induced fragments. Biochim Biophys Acta. 1972 Apr 15;263(2):453–458. doi: 10.1016/0005-2795(72)90099-2. [DOI] [PubMed] [Google Scholar]
- Goldberg I. H., Stewart M. L., Ayuso M., Kappen L. S. On the mechanisms of inhibition of polypeptide synthesis by the antibiotics sparsomycin and pactamycin. Fed Proc. 1973 Jun;32(6):1688–1697. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kwan S. W., Fuller G. M. Immunochemical characterization of fibrinogen induction in rat liver. Biochim Biophys Acta. 1977 Apr 19;475(4):659–668. doi: 10.1016/0005-2787(77)90326-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Blout E. R. Heterogeneity of bovine fibrinogen and fibrin. J Biol Chem. 1973 Oct 10;248(19):6896–6903. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Van Ruijven-Vermeer I. A., Nieuwenhuizen W. Purification of rat fibrinogen and its constituent chains. Biochem J. 1978 Mar 1;169(3):653–658. doi: 10.1042/bj1690653. [DOI] [PMC free article] [PubMed] [Google Scholar]