Abstract
Symptoms commonly associated with sleep loss and chronic inflammation include sleepiness, fatigue, poor cognition, enhanced sensitivity to pain and kindling stimuli, excess sleep and increases in circulating levels of tumor necrosis factor α (TNF) in humans and brain levels of interleukin-1 β (IL1) and TNF in animals. Cytokines including IL1 and TNF partake in non-rapid eye movement sleep (NREMS) regulation under physiological and inflammatory conditions. Administration of exogenous IL1 or TNF mimics the accumulation of these cytokines occurring during sleep loss to the extent that it induces the aforementioned symptoms. Extracellular ATP associated with neuro- and glio-transmission, acting via purine type 2 receptors, e.g., the P2X7 receptor, has a role in glia release of IL1 and TNF. These substances in turn act on neurons to change their intrinsic membrane properties and sensitivities to neurotransmitters and neuromodulators such as adenosine, glutamate and GABA. These actions change the network input-output properties, i.e., a state shift for the network. State oscillations occur locally within cortical columns and are defined using evoked response potentials. One such state, so defined, shares properties with whole animal sleep in that it is dependent on prior cellular activity—it shows homeostasis. The cortical column sleep-like state is induced by TNF and is associated with experimental performance detriments. ATP released extracellularly as a consequence of cellular activity is posited to initiate a mechanism by which the brain tracks its prior sleep-state history to induce/prohibit sleep. Thus, sleep is an emergent property of populations of local neural networks undergoing state transitions. Specific neuronal groups participating in sleep depend upon prior network use driving local network state changes via the ATP-cytokine-adenosine mechanism. Such considerations add complexity to finding biochemical markers for sleepiness.
Citation:
Clinton JM; Davis CJ; Zielinski MR; Jewett KA; Krueger JM. Biochemical regulation of sleep and sleep biomarkers. J Clin Sleep Med 2011;7(5):Supplement S38-S42.
Sleep is associated with changes in the expression of many molecules,1,2 some of which are directly involved in sleep regulation.3,4 It is thus an attractive idea that one or more of these molecules, or the downstream changes in molecular events induced by these molecules, could serve as an index to sleepiness and prior time awake, predict how long someone may sleep if allowed, correlate with an individual’s performance status, or be useful in the diagnosis of primary sleep disorders. Herein, we conclude that the simultaneous determination of several of such molecules may indeed be useful for these purposes. However, another conclusion will be that the biological actions of every identified sleep regulatory molecule are numerous and thus every sleep regulatory molecule lacks specificity for sleep.
The modern era of investigations aimed at determining the humoral, or chemical, causes of sleep began with Ishimori’s demonstration in 1909 that cerebral homogenates from sleep deprived dogs could induce sleep when injected into non-sleep deprived dogs. Similar studies were reported by Legendre and Piéron5 a few years later. Over the next few decades there were sporadic reports of unidentified sleep promoting substances, for example Schnedorf and Ivy.6 However, it was not until the 1960s and 1970s that attempts to isolate and characterize such substances were successful. One of the first sleep promoting substances, called “Factor S” was isolated and purified at Harvard Medical School.7 This substance progressively increased in the cerebral spinal fluid during sleep deprivation. In the 1980s, Factor S was chemically characterized as a muramyl peptide, a class of compounds previously known as immune adjuvants and as components of microbial cell walls.8 That work, and the work of others, led to our current knowledge of the biochemical regulation of sleep. Some of that knowledge is illustrated in Figure 1. This review will focus on the role that cytokines such as interleukin-1 β (IL1) and tumor necrosis factor α (TNF) play in sleep regulation because they are currently the best characterized sleep regulatory substances (SRS) and many of their downstream biochemical mechanisms are also implicated in sleep regulation, e.g., adenosine, nitric oxide, prostaglandins, and others.
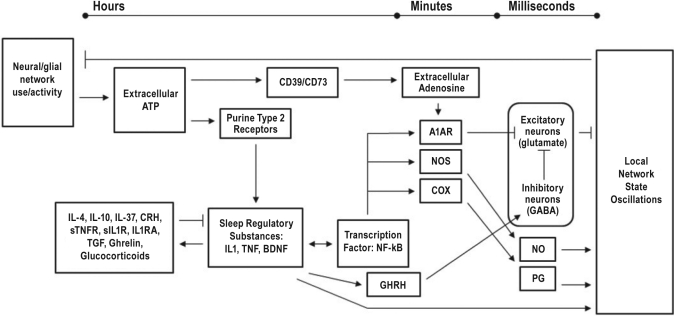
Figure 1.
The Sleep Homeostat
Sleep regulatory substances (SRS) form complex biochemical pathways involving positive and negative feedback loops, changes in gene transcription, as well as the processing of intermediary products. These SRSs act in concert with many other molecules to facilitate local sleep state oscillations. SRSs act over multiple time scales. Multiple components of the homeostat will likely have to be simultaneously assessed for use as a biomarker for sleepiness. Abbreviations: ATP, adenosine triphosphate; CD39, ecto-nucleotidase CD39; CD73, 5[prime]-ecto-nucleotidase CD73; IL-4, interleukin-4; IL-10, interleukin-10; IL-37, interleukin-37; CRH, corticotrophin releasing hormone; sTNFR, soluble tumor necrosis factor α receptor; sIL1R, soluble interleukin-1 receptor; TGF, transforming growth factor β; IL1, interleukin-1 β; TNF, tumor necrosis factor α; BDNF, brain derived neurotrophic factor; NF-kB, nuclear factor κ β; A1AR, adenosine A1 receptor; NOS, nitric oxide synthase; COX, cyclo-oxygenase; GHRH, growth hormone releasing hormone; NO, nitric oxide; PG, prostaglandin.
Sleep Regulatory Substance Criteria
For a substance to be classified as a putative SRS several criteria need to be met.4,9,10 These include: 1) the substance and/or its receptor oscillates with sleep propensity; 2) sleep is increased or decreased with administration of the substance; 3) blocking the action or inhibiting the production of the substance changes sleep; 4) disease states, e.g., infection, associated with altered sleep also change levels of the putative SRS; and finally 5) the substance acts on known sleep regulatory circuits. While many substances meet some of these criteria including microRNAs, metabolites, hormones, growth factors, transcription factors, and various proteins and their receptors, only a few meet all the required characteristics to be considered an SRS. These include IL1, TNF and growth hormone releasing hormone (GHRH) for non-rapid eye movement sleep (NREMS) and prolactin and nitric oxide (NO) for rapid eye movement sleep (REMS). These SRSs act within complex biochemical cascades, e.g., Figure 1, to form the sleep homeostat, a network of molecules that regulate sleep over different time scales.4,11
Interleukin-1 Beta and Tumor Necrosis Factor Alpha
IL1 and TNF are cytokines. They are released in response to many stimuli including sleep loss, tissue injury, and infection. Cytokines act via juxtacrine, autocrine, and paracrine signaling but can also serve as endocrines. IL1 and TNF are pleiotropic, serving both physiological and pathological functions including modulation of memory, mood, inflammation and sleep. Sleep loss and altered cytokine levels are associated with enhanced sensitivity to pain12,13 and kindling stimuli,14 fatigue,15–17 sleepiness and rebound sleep, metabolic syndrome18,19 including type-2 diabetes,20 and impaired cognition21,22 and memory.23–25 These sleep loss-associated symptoms can be elicited by injections of TNF or IL1.
TNF or IL1 induces sleep in humans and animals when administered centrally or systemically, even when given in low non-pyrogenic doses.4 At lower doses the increases in sleep are restricted to NREMS. As the dose is raised, NREMS increases further at the expense of decreased REMS and fever may occur. At high doses both NREMS and REMS are inhibited. For example, a single intraperitoneal injection of TNF in mice induces an additional 90 min of sleep during the first 9 h post-injection. Microinjection of TNF or IL1 into or near hypothalamic and other sleep regulatory circuits enhances NREMS.26–29
In addition to increasing time spent sleeping, these cytokines after central injection can enhance electroencephalographic (EEG) δ power (a measure of sleep intensity) during NREMS. This effect is similar to that seen after sleep deprivation30 suggesting that IL1 and TNF injection mimics the effect of sleep loss.
Blocking the action or inhibiting the production via pharmacological agents or genetic manipulation of IL1 or TNF decreases spontaneous sleep.4 For instance, injection of antibodies to these cytokines, their soluble receptors, or small interfering RNA (siRNA) targeting IL1 or TNF reduces NREMS and/or EEG δ power, e.g. Taishi et al.31 Mice lacking the TNF55kD receptor,32 or the type 1 IL1 receptor,33 or both TNF receptors34 sleep less than control mice. Some endogenous molecules inhibit IL1 and TNF and also attenuate spontaneous NREMS including IL-4, IL-10, IL-13,3 corticotrophin releasing hormone,35 IL1-receptor antagonist,4 and others (see Figure 1). We have recently found that transgenic mice with the ability to express human IL-37b, a suppressor of multiple pro-somnogenic cytokines36 sleep less after sleep deprivation than under baseline conditions.37
Finally, IL1 and TNF vary with sleep propensity. For example, TNF mRNA38 and protein39 vary approximately twofold and 10-fold respectively, across the day in the cortex and hypothalamus and increase with sleep propensity. In humans, plasma TNF levels are correlated with EEG δ power.40 Sleep deprivation increases IL1 mRNA in the brain,41 and blocking TNF blunts the typical sleep rebound following sleep deprivation.3 TNF also has other roles in the brain including its involvement in synaptic scaling42 and glutamatergic gliotransmission.43
Brain and circulating levels of TNF and IL1 are altered in many pathologies that exhibit sleep disturbances including sleep apnea, insomnia, HIV, AIDS, post-dialysis fatigue, chronic inflammation, excessive daytime sleepiness, myocardial infarction, preeclampsia, alcoholism, chronic fatigue syndrome, post-viral fatigue syndrome, influenza infection, and rheumatoid arthritis.44,45 A polymorphic variant of TNF, G-308A, has been linked to metabolic syndrome46,47 and sleep apnea.48 Clinically available inhibitors of IL1 or TNF reduce the sleepiness and fatigue associated with rheumatoid arthritis49 and sleep apnea.50 Furthermore, surgical treatment of obstructive sleep apnea reduces plasma levels of TNF as well as sleepiness.51 The link between pathology, cytokine levels, and sleep suggest that some primary sleep disorders such as sleep apnea or insomnia are chronic inflammatory disorders.
Only now are we beginning to understand how wakefulness leads to increases in SRS production and release and how SRSs subsequently promote and alter sleep. Regardless, IL1 and TNF, as well as other SRSs not discussed herein, are clearly involved in sleep/wake regulation. Their measurement may provide an index for sleepiness, although as mentioned, because they are pleiotropic, they may index other processes as well. Specificity for sleep likely emerges from the actions of multiple such substances. Thus simultaneous measurement of multiple SRSs may be necessary to develop an assay with greater specificity for sleep.
ATP-Cytokine-Adenosine Hypothesis
Although we know that sleep loss up-regulates SRSs, the exact property of wakefulness responsible for the enhanced expression of SRSs has only recently been explored. We posited that the brain tracks prior wakefulness via extracellular adenosine triphosphate (ATP) released during neuro- and glio-transmission.52 The transient dynamic changes in ATP released into the extracellular space stimulate the production and release of longer-lived SRSs. ATP binds to purine type 2X7 receptors (P2X7R) on nearby cells to trigger the processing and release of IL1 and TNF from glia.53–56 Intracerebroventricular administration of an ATP agonist or ATP antagonists increases or decrease sleep, respectively.57 P2X7R knockout animals have reduced time spent in NREMS and EEG slow wave activity after sleep deprivation. There are diurnal variations in cortical P2X7R mRNA levels.57 ATP is also catabolized by ecto- enzymes into adenosine, a well characterized SRS (Figures 1 and 2).58 However, knockout mice lacking ecto-enzyme CD73, required for the conversion of extracellular adenosine monophosphate (AMP) to adenosine, have increased spontaneous NREMS suggesting that our understanding of adenosine sleep mechanisms is incomplete.59 IL1 and TNF likely alter neuronal/glial network sensitivity by causing dynamic changes in neuro- or glio-transmitter receptor expression and thereby alter network state.52 For example, TNF and IL1 activate nuclear factor κ β, a transcription factor for numerous genes linked to sleep regulation including IL1, TNF, the adenonsine A1 receptor (A1AR), the gluR1 subunit of AMPA receptors (gluR1), cyclo-oxygenase (COX), and nitric oxide synthase (NOS) (Figure 1).52 The release and actions of extracellular ATP are fundamentally a local event, altering network state in response to cell activity. A logical implication of this mechanism is that sleep is initiated at the local network level and is a consequence of cell activity. ATP and associated molecules (Figure 2) also provide additional potential targets to measure for characterization of state.
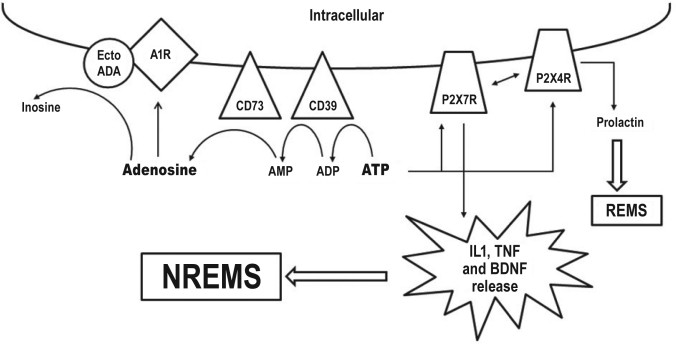
Figure 2.
The ATP-cytokine-adenosine hypothesis
Extracellular adenosine triphosphate (ATP) is catabolized to adenosine via conversion to adenosine diphosphate (ADP) and adenosine monophosphate (AMP) by the ecto-enzymes CD39 and CD73, respectively. Adenosine can promote non rapid eye movement sleep (NREMS) by signaling through purine type 1 receptors (A1R). Adenosine is converted into inosine by membrane bound ecto-adenosine deaminase (ectoADA) which co-localizes with A1R. ATP also acts on purine type 2X7 receptors (P2X7R) to induce the processing and release of interleukin 1 β (IL1), tumor necrosis factor α (TNF) and brain derived neurotrophic factor (BDNF). IL1, TNF and BDNF in turn promote non-rapid eye movement sleep (NREMS). ATP can also bind to purine type 2X4 receptors (P2X4R) to stimulate the release of prolactin to promote rapid eye movement sleep (REMS).
State Oscillations in Local Neuronal Networks
The logic, evidence, and need for a local use-dependent sleep process are presented in detail elsewhere.52 To provide an experimental example, we briefly discuss work from Rector’s laboratory demonstrating a sleep-like state in cortical columns. We broach the local use-dependent sleep hypothesis here because it provides a different view of the mechanisms responsible for sleepiness and it has bearing on whether it is worthwhile to pursue biochemical markers of sleep.
Rector and colleagues have characterized cortical column states using evoked response potentials initiated by sensory input and measured using electrode arrays placed over the cortical areas receiving the sensory input. He showed, for example, that rat somatosensory cortical columns (well-characterized neuronal/glial networks receiving input from mystacial whiskers) have two distinct states, one of which shares properties of whole animal sleep. For instance, the probability of a particular column being in the sleep-like state depends on the prior duration of its wake-like state.60 If the whole animal is asleep, most cortical columns are in the sleep-like state, and conversely, if the animal is awake, most columns are in wake-like state. Further, enhanced afferent activity (e.g., by repeatedly twitching a single whisker), as well as local micro-injection of TNF, induce columns to switch to the sleep-like state.11 Such data suggest that whole animal sleep may be a consequence of the fraction of individual local neuronal/glial networks in the sleep-like state. Thus, if sleep is a fundamental property of local networks semi-autonomously oscillating between states, sleepiness may be an emergent property dependent upon the number of local networks in a sleep-like state but not necessarily a consequence of the same networks being in a sleep-like state every time sleepiness manifests. This view stands in contrast to the prevailing paradigm in sleep research that sleep regulatory circuits impose sleep and sleepiness on the brain. The inconsistencies and incompleteness of the dominant paradigm, and its fundamental dualist nature, are reviewed elsewhere52; sleep regulatory circuits likely help orchestrate and synchronize local states. Regardless, the local use-dependent sleep hypothesis suggests that perhaps global changes in SRSs (derived from many local networks) may possibly index sleepiness but following SRS expression in any one network would be futile for this purpose.
Conclusions
Sleep is a use-dependent phenomenon distributed throughout the brain and biochemically regulated in part by SRSs, including IL1 and TNF. Extracellular ATP, released in response to neuro- and glio- transmission, binds to purine type 2 receptors to mediate SRS production and release within local networks. These and other substances which participate in sleep regulation may serve as useful indicators of sleep history, necessity, or dysfunction. Due to their pleiotropic nature, multiple SRSs will need to be monitored simultaneously to provide a useful biomarker for sleepiness.
DISCLOSURE STATEMENT
The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (USA) grant numbers NS 25,378, NS 31,453 and HD 36,520. Editing of the conference proceedings supported by HL104874.
REFERENCES
- 1.Kilduff TS, Lein ES, de la Iglesia H, Sakurai T, Fu YH, Shaw P. New developments in sleep research: molecular genetics, gene expression, and systems neurobiology. J Neurosci. 2008;28:11814–8. doi: 10.1523/JNEUROSCI.3768-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–21. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 3.Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:d520–50. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- 4.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14:3408–16. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legendre R, Piéron H. Recherches sur le besoin de sommeil consécutif á une veille prlongée. Z Allg Physiol. 1913;14:235–62. [Google Scholar]
- 6.Schnedorf J, Ivy A. An examination of the hypnotoxin theory of sleep. Am J Physiol. 1939:13. [Google Scholar]
- 7.Pappenheimer JR, Miller TB, Goodrich CA. Sleep-promoting effects of cerebrospinal fluid from sleep-deprived goats. Proc Natl Acad Sci U S A. 1967;58:513–7. doi: 10.1073/pnas.58.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krueger JM, Pappenheimer JR, Karnovsky ML. The composition of sleep-promoting factor isolated from human urine. J Biol Chem. 1982;257:1664–9. [PubMed] [Google Scholar]
- 9.Jouvet M. Neuromediators and hypnogenic factors. Rev Neurol (Paris) 1984;140:389–400. [PubMed] [Google Scholar]
- 10.Borbely AA, Tobler I. Endogenous sleep-promoting substances and sleep regulation. Physiol Rev. 1989;69:605–70. doi: 10.1152/physrev.1989.69.2.605. [DOI] [PubMed] [Google Scholar]
- 11.Churchill L, Rector DM, Yasuda K, et al. Tumor necrosis factor alpha: activity dependent expression and promotion of cortical column sleep in rats. Neuroscience. 2008;156:71–80. doi: 10.1016/j.neuroscience.2008.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kundermann B, Hemmeter-Spernal J, Huber MT, Krieg JC, Lautenbacher S. Effects of total sleep deprivation in major depression: overnight improvement of mood is accompanied by increased pain sensitivity and augmented pain complaints. Psychosom Med. 2008;70:92–101. doi: 10.1097/PSY.0b013e31815c1b5d. [DOI] [PubMed] [Google Scholar]
- 13.Honore P, Donnelly-Roberts D, Namovic MT, et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319:1376–85. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- 14.Yi PL, Tsai CH, Lin JG, Lee CC, Chang FC. Kindling stimuli delivered at different times in the sleep-wake cycle. Sleep. 2004;27:203–12. doi: 10.1093/sleep/27.2.203. [DOI] [PubMed] [Google Scholar]
- 15.Anisman H, Merali Z. Cytokines, stress and depressive illness: brain-immune interactions. Ann Med. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- 16.Carmichael MD, Davis JM, Murphy EA, et al. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1344–8. doi: 10.1152/ajpregu.00141.2006. [DOI] [PubMed] [Google Scholar]
- 17.Omdal R, Gunnarsson R. The effect of interleukin-1 blockade on fatigue in rheumatoid arthritis-a pilot study. Rheumatol Int. 2005;25:481–4. doi: 10.1007/s00296-004-0463-z. [DOI] [PubMed] [Google Scholar]
- 18.Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148:241–51. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hristova M, Aloe L. Metabolic syndrome-neurotrophic hypothesis. Med Hypotheses. 2006;66:545–9. doi: 10.1016/j.mehy.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 20.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 21.Baune BT, Ponath G, Rothermundt M, Riess O, Funke H, Berger K. Association between genetic variants of IL-1beta, IL-6 and TNF-alpha cytokines and cognitive performance in the elderly general population of the MEMO-study. Psychoneuroendocrinology. 2008;33:68–76. doi: 10.1016/j.psyneuen.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Trompet S, de Craen AJ, Slagboom P, et al. Genetic variation in the interleukin-1 beta-converting enzyme associates with cognitive function. The PROSPER study. Brain. 2008;131:1069–77. doi: 10.1093/brain/awn023. [DOI] [PubMed] [Google Scholar]
- 23.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–28. [PMC free article] [PubMed] [Google Scholar]
- 24.Pickering M, O’Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res. 2007;163:339–54. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- 25.Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 26.Kubota T, Li N, Guan Z, Brown RA, Krueger JM. Intrapreoptic microinjection of TNF-alpha enhances non-REM sleep in rats. Brain Res. 2002;932:37–44. doi: 10.1016/s0006-8993(02)02262-x. [DOI] [PubMed] [Google Scholar]
- 27.Terao A, Matsumura H, Yoneda H, Saito M. Enhancement of slow-wave sleep by tumor necrosis factor-alpha is mediated by cyclooxygenase-2 in rats. Neuroreport. 1998;9:3791–6. doi: 10.1097/00001756-199812010-00005. [DOI] [PubMed] [Google Scholar]
- 28.De Sarro G, Gareri P, Sinopoli VA, David E, Rotiroti D. Comparative, behavioural and electrocortical effects of tumor necrosis factor-alpha and interleukin-1 microinjected into the locus coeruleus of rat. Life Sci. 1997;60:555–64. doi: 10.1016/s0024-3205(96)00692-3. [DOI] [PubMed] [Google Scholar]
- 29.Manfridi A, Brambilla D, Bianchi S, Mariotti M, Opp MR, Imeri L. Interleukin-1beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur J Neurosci. 2003;18:1041–9. doi: 10.1046/j.1460-9568.2003.02836.x. [DOI] [PubMed] [Google Scholar]
- 30.Pappenheimer JR, Koski G, Fencl V, Karnovsky ML, Krueger J. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol. 1975;38:1299–311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- 31.Taishi P, Churchill L, Wang M, et al. TNFalpha siRNA reduces brain TNF and EEG delta wave activity in rats. Brain Res. 2007;1156:125–32. doi: 10.1016/j.brainres.2007.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFalpha treatment. J Neurosci. 1997;17:5949–55. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang J, Wang Y, Krueger JM. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am J Physiol. 1998;274:R655–60. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- 34.Kapas L, Bohnet SG, Traynor TR, et al. Spontaneous and influenza virus-induced sleep are altered in TNF-alpha double-receptor deficient mice. J Appl Physiol. 2008;105:1187–98. doi: 10.1152/japplphysiol.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opp MR. Corticotropin-releasing hormone involvement in stressor-induced alterations in sleep and in the regulation of waking. Adv Neuroimmunol. 1995;5:127–43. doi: 10.1016/0960-5428(95)00004-l. [DOI] [PubMed] [Google Scholar]
- 36.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–22. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boucher A, Zielinski M, Dinarello C, Krueger J. Mice with the human interleukin-37 transgene sleep less after sleep deprivation. In press. [Google Scholar]
- 38.Bredow S, Guha-Thakurta N, Taishi P, Obal F, Jr, Krueger JM. Diurnal variations of tumor necrosis factor alpha mRNA and alpha-tubulin mRNA in rat brain. Neuroimmunomodulation. 1997;4:84–90. doi: 10.1159/000097325. [DOI] [PubMed] [Google Scholar]
- 39.Floyd RA, Krueger JM. Diurnal variation of TNF alpha in the rat brain. Neuroreport. 1997;8:915–8. doi: 10.1097/00001756-199703030-00020. [DOI] [PubMed] [Google Scholar]
- 40.Darko DF, Miller JC, Gallen C, et al. Sleep electroencephalogram delta-frequency amplitude, night plasma levels of tumor necrosis factor alpha, and human immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1995;92:12080–4. doi: 10.1073/pnas.92.26.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taishi P, Chen Z, Obal F, Jr, et al. Sleep-associated changes in interleukin-1beta mRNA in the brain. J Interferon Cytokine Res. 1998;18:793–8. doi: 10.1089/jir.1998.18.793. [DOI] [PubMed] [Google Scholar]
- 42.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–9. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 43.Santello M, Bezzi P, Volterra A. TNFalpha Controls Glutamatergic Gliotransmission in the Hippocampal Dentate Gyrus. Neuron. 2011;69:988–1001. doi: 10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116:1188–98. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Krueger JM, Rector DM, Churchill L. Sleep and Cytokines. Sleep Med Clin. 2007;2:161–9. doi: 10.1016/j.jsmc.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sookoian SC, Gonzalez C, Pirola CJ. Meta-analysis on the G-308A tumor necrosis factor alpha gene variant and phenotypes associated with the metabolic syndrome. Obes Res. 2005;13:2122–31. doi: 10.1038/oby.2005.263. [DOI] [PubMed] [Google Scholar]
- 47.Sookoian S, Garcia SI, Gianotti TF, Dieuzeide G, Gonzalez CD, Pirola CJ. The G-308A promoter variant of the tumor necrosis factor-alpha gene is associated with hypertension in adolescents harboring the metabolic syndrome. Am J Hypertens. 2005;18:1271–5. doi: 10.1016/j.amjhyper.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Riha RL, Brander P, Vennelle M, et al. Tumour necrosis factor-alpha (-308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur Respir J. 2005;26:673–8. doi: 10.1183/09031936.05.00130804. [DOI] [PubMed] [Google Scholar]
- 49.Franklin CM. Clinical experience with soluble TNF p75 receptor in rheumatoid arthritis. Semin Arthritis Rheum. 1999;29:172–81. doi: 10.1016/s0049-0172(99)80028-6. [DOI] [PubMed] [Google Scholar]
- 50.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–13. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 51.Kataoka T, Enomoto F, Kim R, et al. The effect of surgical treatment of obstructive sleep apnea syndrome on the plasma TNF-alpha levels. Tohoku J Exp Med. 2004;204:267–72. doi: 10.1620/tjem.204.267. [DOI] [PubMed] [Google Scholar]
- 52.Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–9. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bianco F, Pravettoni E, Colombo A, et al. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–77. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 54.Gabel CA. P2 purinergic receptor modulation of cytokine production. Purinergic Signal. 2007;3:27–38. doi: 10.1007/s11302-006-9034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hide I, Tanaka M, Inoue A, et al. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem. 2000;75:965–72. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krueger JM, Taishi P, De A, et al. ATP and the purine type 2 X7 receptor affect sleep. J Appl Physiol. 2010;109:1318–27. doi: 10.1152/japplphysiol.00586.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bjorness TE, Greene RW. Adenosine and sleep. Curr Neuropharmacol. 2009;7:238–45. doi: 10.2174/157015909789152182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zielinski MR, Taishi P, Krueger JM. CD73 in sleep regulation. Sleep Abstract Supplement. 2011;34:A14. [Google Scholar]
- 60.Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]