Abstract
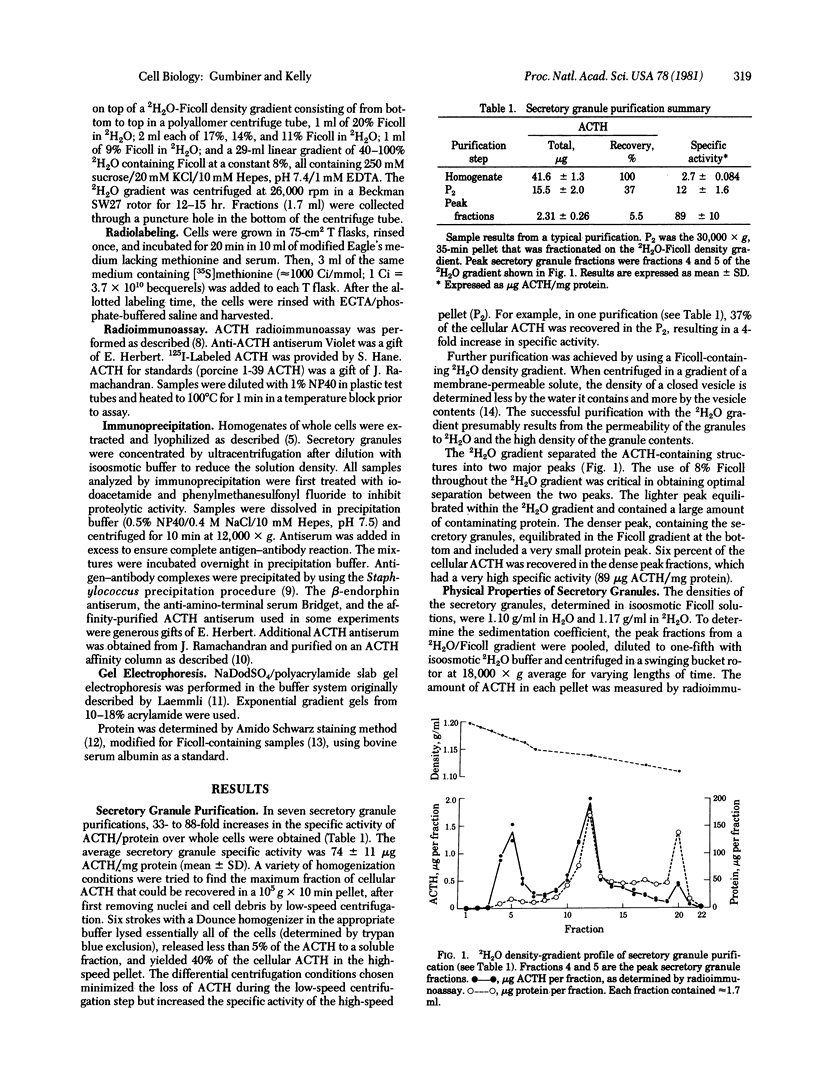
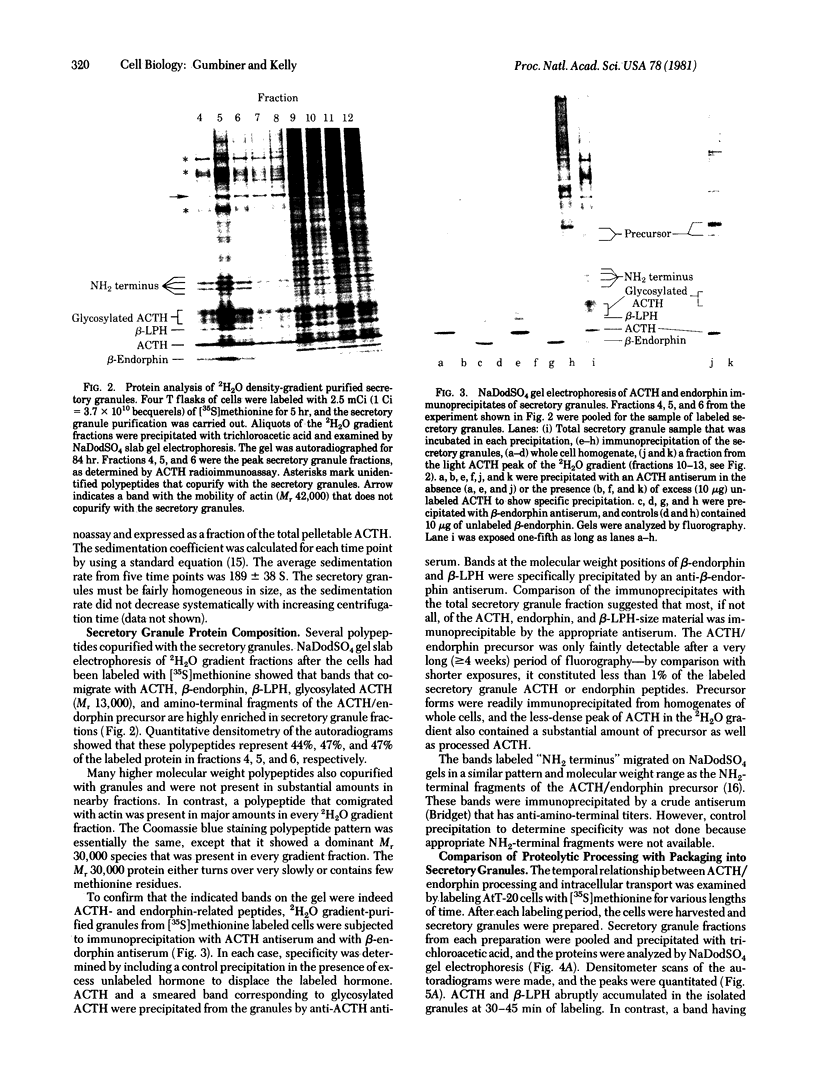
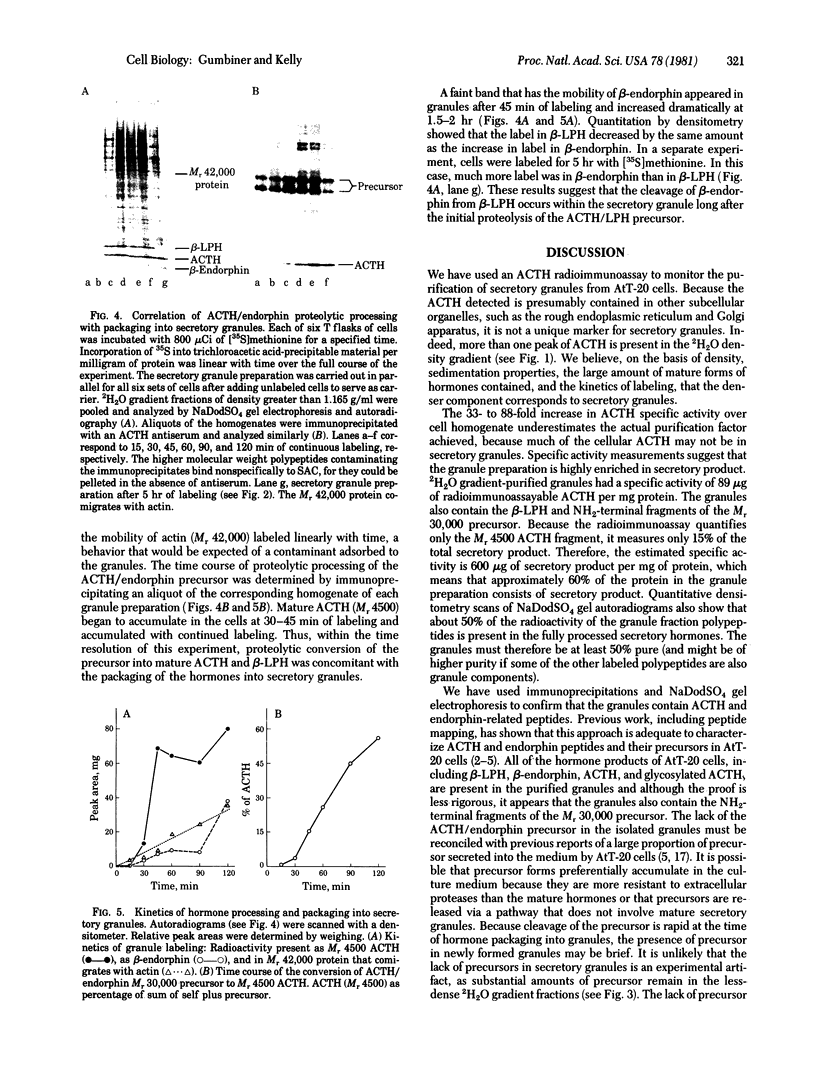
The pituitary cell line, AtT-20, synthesizes the precursor to corticotropin (adrenocorticotropic hormone; ACTH) and beta-endorphin and correctly glycosylates and cleaves it to make the mature forms of the hormones before they are secreted. This cell line was used to study the intracellular transport, packaging, and secretion of these hormones. Secretory granules from the cells were isolated by homogenization and differential centrifugation and isopycnic sedimentation on a 2H2O-Ficoll gradient to give a preparation having a specific activity of 90micrograms ACTH per mg of protein, which is 30- to 90-fold greater than that of whole cells. The granules have density characteristics and a sedimentation coefficient that are appropriate for spheres of 1000 A radius. They contain all of the fragments of the initial ACTH/endorphin precursor but almost undetectable amounts of the intact precursor. The fragments constitute about 50% of the protein in the secretory granule fraction and, from density measurements, we estimate that they are present in approximately 60,000 copies per vesicle. The cell line secretory granules appear, therefore, to be similar to mature secretory granules in normal differentiated tissues. ACTH first appears in the secretory granule at 30-45 min after synthesis. Cleavage of the precursor to mature ACTH occurs at about the same time in the whole cell. Therefore, proteolysis of the prohormone to ACTH and to beta-lipotropin is a metabolic event that can be correlated with the packaging of the hormone into a mature secretory granule. Cleavage of beta-lipotropin to beta-endorphin occurs later, probably in the secretory granule.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownstein M. J., Russell J. T., Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980 Jan 25;207(4429):373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Carlson S. S., Wagner J. A., Kelly R. B. Purification of synaptic vesicles from elasmobranch electric organ and the use of biophysical criteria to demonstrate purity. Biochemistry. 1978 Apr 4;17(7):1188–1199. doi: 10.1021/bi00600a009. [DOI] [PubMed] [Google Scholar]
- Cotman C., Brown D. H., Harrell B. W., Anderson N. G. Analytical differential centrifugation: an analysis of the sedimentation properties of synaptosomes, mitochondria and lysosomes from rat brain homogenates. Arch Biochem Biophys. 1970 Feb;136(2):436–447. doi: 10.1016/0003-9861(70)90215-8. [DOI] [PubMed] [Google Scholar]
- Cross B. A., Dyball R. E., Dyer R. G., Jones C. W., Lincoln D. W., Morris J. F., Pickering B. T. Endocrine neurons. Recent Prog Horm Res. 1975;31:243–294. doi: 10.1016/b978-0-12-571131-9.50011-6. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Keutmann H. T., Eipper B. A., Mains R. E. Partial characterization of a glycoprotein comprising the NH2-terminal region of mouse tumor cell pro-adrenocorticotropic hormone/endorphin. J Biol Chem. 1979 Sep 25;254(18):9204–9208. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem. 1976 Jul 10;251(13):4115–4120. [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Coordinate synthesis of corticotropins and endorphins by mouse pituitary tumor cells. J Biol Chem. 1978 Feb 10;253(3):651–655. [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Rees L. H., Cook D. M., Kendall J. W., Allen C. F., Kramer R. M., Ratcliffe J. G., Knight R. A. A radioimmunoassay for rat plasma ACTH. Endocrinology. 1971 Jul;89(1):254–261. doi: 10.1210/endo-89-1-254. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: cell-free synthesis of the precursor and identification of corticotropin peptides in the molecule. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4826–4830. doi: 10.1073/pnas.74.11.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: identification of beta-lipotropin peptides and their arrangement relative to corticotropin in the precursor synthesized in a cell-free system. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5300–5304. doi: 10.1073/pnas.74.12.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. L., Phillips M., Rosa P. A., Herbert E. Steps involved in the processing of common precursor forms of adrenocorticotropin and endorphin in cultures of mouse pituitary cells. Biochemistry. 1978 Aug 22;17(17):3609–3618. doi: 10.1021/bi00610a030. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Siperstein E. R., Miller K. J. Further cytophysiologic evidence for the dentity of the cells that produce adrenocorticotrophic hormone. Endocrinology. 1970 Mar;86(3):451–486. doi: 10.1210/endo-86-3-451. [DOI] [PubMed] [Google Scholar]
- Wagner J. A., Carlson S. S., Kelly R. B. Chemical and physical characterization of cholinergic synaptic vesicles. Biochemistry. 1978 Apr 4;17(7):1199–1206. doi: 10.1021/bi00600a010. [DOI] [PubMed] [Google Scholar]