Abstract
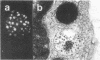
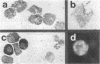
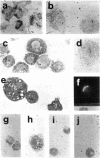
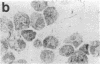
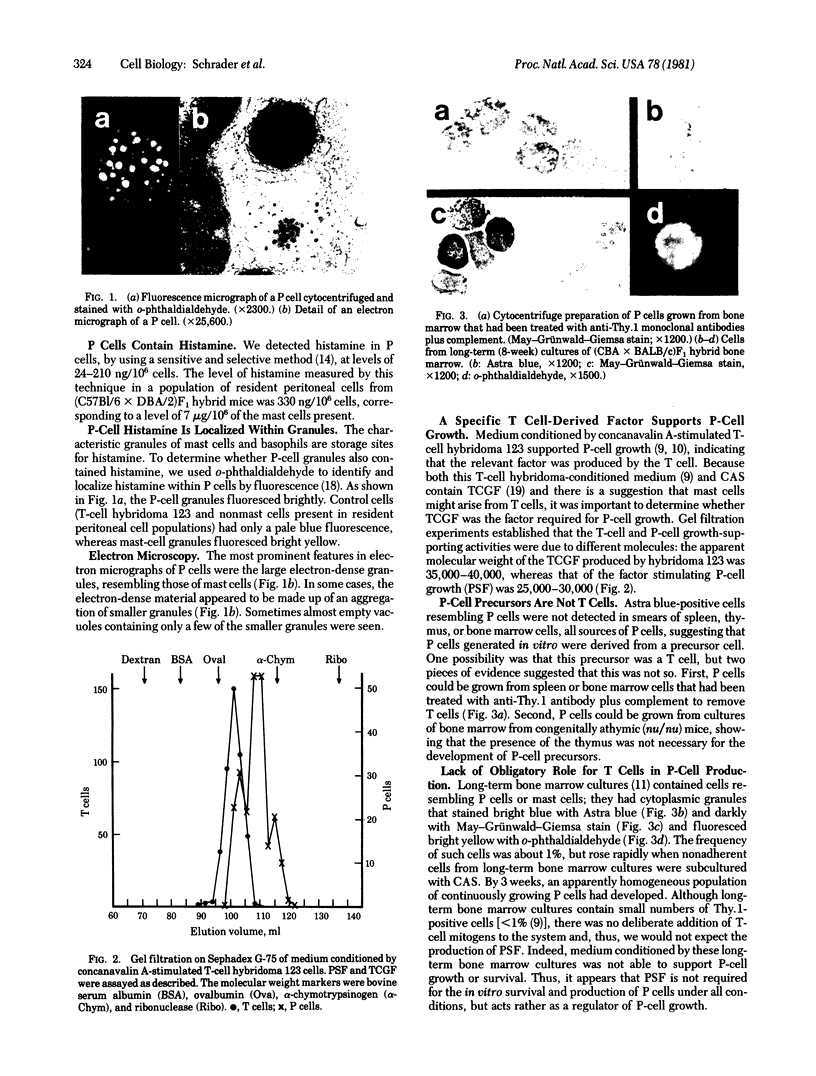
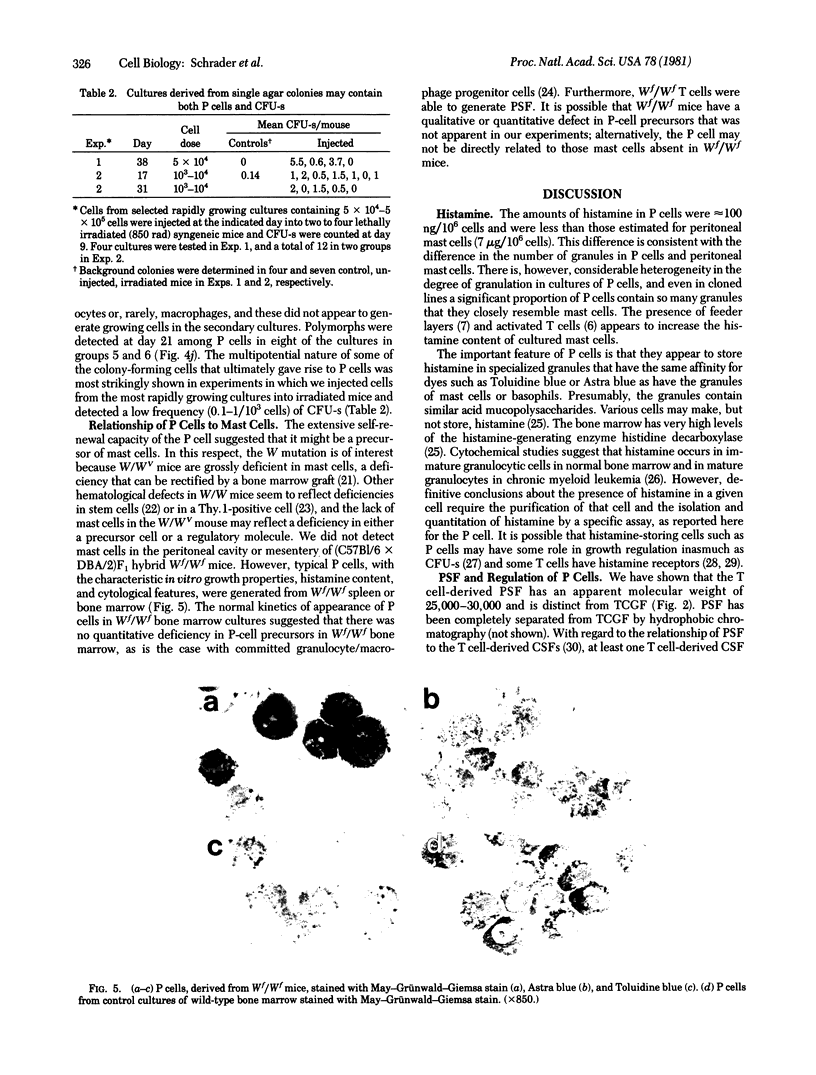
Histamine was detected at levels of 100 ng/10(6) cells in the metachromatic granules of the persisting (P) cell, which appears in cultures of murine lymphoid or bone marrow cells and is capable of long-term growth in vitro in the presence of a T cell-derived growth factor. This factor, which we termed P-cell stimulating factor, was distinct from t-cell growth factor and had an apparent molecular weight of 25,000-30,000. P cells did not originate from Thy.1-positive cells nor was the thymus necessary for the development of their precursors. Moreover, P cells grew directly from colonies generated in agar cultures of bone marrow cells, the nature of the colonies indicating that P cells shared a common precursor with hemopoietic cells. Mutant Wf/Wf mice, although deficient in certain mast cells, possessed P-cell precursors. It is hypothesized that P cells are related to a specialized subset of mast cells, derived from a bone marrow progenitor but regulated by activated T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenase P. W. Role of basophils, mast cells, and vasoamines in hypersensitivity reactions with a delayed time course. Prog Allergy. 1977;23:199–320. [PubMed] [Google Scholar]
- BLOOM G., KELLY J. W. The copper phthalocyanin dye "Astrablau" and its staining properties, especially the staining of mast cells. Z Zellforch Microsk Anat Histochem. 1960;2:48–57. doi: 10.1007/BF00736491. [DOI] [PubMed] [Google Scholar]
- BURNET F. M. MAST CELLS IN THE THYMUS OF NZB MICE. J Pathol Bacteriol. 1965 Jan;89:271–284. [PubMed] [Google Scholar]
- Burnet F. M. The probable relationship of some or all mast cells to the T-cell system. Cell Immunol. 1977 May;30(2):358–360. doi: 10.1016/0008-8749(77)90079-x. [DOI] [PubMed] [Google Scholar]
- Catini C., Gheri G., Miliani A. Cytochemical detection of histamine in the human granulopoietic series of healthy subjects and of patients affected by chronic myeloid leukaemia. A spectrophotofluorimetric test for checking OPT-induced fluorescence in isolated cells. Histochem J. 1977 Mar;9(2):141–151. doi: 10.1007/BF01003626. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Ginsburg H., Nir I., Hammel I., Eren R., Weissman B. A., Naot Y. Differentiation and activity of mast cells following immunization in cultures of lymph-node cells. Immunology. 1978 Sep;35(3):485–502. [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Adachi T., Chang T-H, Ishizaka K. Development of mast cells in vitro. II. Biologic function of cultured mast cells. J Immunol. 1977 Jan;118(1):211–217. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Biology of immunoglobulin E. Molecular basis of reaginic hypersensitivity. Prog Allergy. 1975;19:60–121. [PubMed] [Google Scholar]
- Juhlin L., Shelley W. B. Detection of histamine by a new fluorescent omicron-phthalaldehyde stain. J Histochem Cytochem. 1966 Jul;14(7):525–528. doi: 10.1177/14.7.525. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- Lewis S. J., Fennessy M. R., Laska F. J., Taylor D. A. A modified method for the isolation and determination of brain histamine using the Bio-Rex 70. Agents Actions. 1980 Jun;10(3):197–206. doi: 10.1007/BF02025936. [DOI] [PubMed] [Google Scholar]
- MCCULLOCH E. A., SIMINOVITCH L., TILL J. E. SPLEEN-COLONY FORMATION IN ANEMIC MICE OF GENOTYPE WW. Science. 1964 May 15;144(3620):844–846. doi: 10.1126/science.144.3620.844. [DOI] [PubMed] [Google Scholar]
- Mayrhofer G., Bazin H., Gowans J. L. Nature of cells binding anti-IgE in rats immunized with Nippostrongylus brasiliensis: IgE synthesis in regional nodes and concentration in mucosal mast cells. Eur J Immunol. 1976 Aug;6(8):537–545. doi: 10.1002/eji.1830060803. [DOI] [PubMed] [Google Scholar]
- McCarthy J. H., Mandel T. E., Garson O. M., Metcalf D. The presence of mast cells in agar cultures. Exp Hematol. 1980 May;8(5):562–567. [PubMed] [Google Scholar]
- Plaut M., Lichtenstein L. M., Gillespie E., Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. IV. Specificity of the histamine receptor on effector T cells. J Immunol. 1973 Aug;111(2):389–394. [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976 Nov 18;264(5583):258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Arnold B., Clark-Lewis I. A con A-stimulated T-cell hybridoma releases factors affecting haematopoietic colony-forming cells and B-cell antibody responses. Nature. 1980 Jan 10;283(5743):197–199. doi: 10.1038/283197a0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Schrader S. In vitro studies on lymphocyte differentiation. I. Long term in vitro culture of cells giving rise to functional lymphocytes in irradiated mice. J Exp Med. 1978 Sep 1;148(3):823–828. doi: 10.1084/jem.148.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M., Weinstein Y., Melmon K. L. Enhancement of immune response potential of mouse lymphoid cells fractionated over insolubilized conjugated histamine columns. J Immunol. 1974 Aug;113(2):597–607. [PubMed] [Google Scholar]
- Stanley E. R. Colony-stimulating factor (CSF) radioimmunoassay: detection of a CSF subclass stimulating macrophage production. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2969–2973. doi: 10.1073/pnas.76.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Sharkie S., Ahmed A., Sell K. W., Santos G. W. Theta-sensitive cell and erythropoiesis: identification of a defect in W/Wv anemic mice. Science. 1977 Apr 15;196(4287):313–315. doi: 10.1126/science.322288. [DOI] [PubMed] [Google Scholar]