Abstract
Although [18F] 2-fluoro-2-deoxy-D-glucose (FDG) is the most widely used radiopharmaceutical the world over, it is not the ideal tracer for brain imaging, owing to its high physiological cortical uptake and lack of specificity. This has paved the way for the introduction of several novel radiotracers, each with their own inherent strengths and limitations. We present the insights gained from the use of these radiotracers at our institution.
Keywords: Brain tumor, Novel Positron emission tomography radiotracers
Introduction
Positron emission tomography/computerized tomography (PET/CT) has gained widespread acceptance in tumor evaluation owing to its ability to detect tumors and define their extent, provide information that aids planning of radiotherapy and operative interventions, show response to treatment, and detect tumor recurrence. [18F] 2-fluoro-2-deoxy-D-glucose (FDG) has been aptly called the molecule of the millennium because of the impact it has had in the field of oncological imaging. In fact, the first oncological application of PET was in the assessment of brain tumors.[1] FDG PET/CT has been utilized primarily in brain tumors for grading and characterization, prognostication, guiding the selection of the site for biopsy, and evaluation of therapeutic response with modest accuracy. However, it has its limitations, primarily the lack of specificity[2] and the high physiological cortical uptake. This has led to the development and utilization of newer non-FDG radiopharmaceuticals. We present the insights gained with the use of these non-FDG radiopharmaceuticals in brain tumor evaluation at our institution.
Discussion
At present FDG is the most widely used radiopharmaceutical the world over. This is due its favorable half-life of 110 minutes (which does not necessitate an on-site cyclotron), high uptake in tumor tissue, and relatively low positron range. The basis for high FDG uptake in tumor tissue is the high metabolic rate and therefore increased glucose utilization in tumor cells. However, FDG may not be an ideal imaging agent for brain tumors as there is a high physiological glucose uptake in normal brain parenchyma, glucose being an obligatory energy substrate for brain. This leads to intense radiotracer uptake in normal brain tissue, and, as a result, low grade tumors, small tumors, and tumors with early recurrence may go undetected. Moreover, FDG uptake is relatively nonspecific and is seen to occur in inflammatory and granulomatous tissues also.
A variety of non-FDG PET radiotracers with oncological applications have been developed. These include radiotracers for amino acid synthesis, Deoxyribonucleic acid (DNA) synthesis, lipid synthesis, hypoxia, angiogenesis, and peptide receptors.
Radiotracers for amino acid synthesis
11C methionine
Protein synthesis is a fundamental prerequisite for tumor growth. There is an upregulation of protein metabolism and cell-mediated transport of amino acids in tumors. Radiotracers that can measure the increase in protein synthesis will have increased sensitivity and specificity as compared to FDG. Several such radiopharmaceuticals have been developed. Of these, 11C-labelled methionine has been the most widely studied. Unlike with FDG, the normal brain parenchyma exhibits overall reduced uptake of 11C methionine. The pituitary gland shows increased uptake [Figure 1]. It has thus proven superior to FDG in the evaluation of brain tumors as its reduced uptake in healthy brain tissue results in enhanced contrast between the brain tumor and the surrounding normal parenchyma.[3]
Figure 1 (A-D).
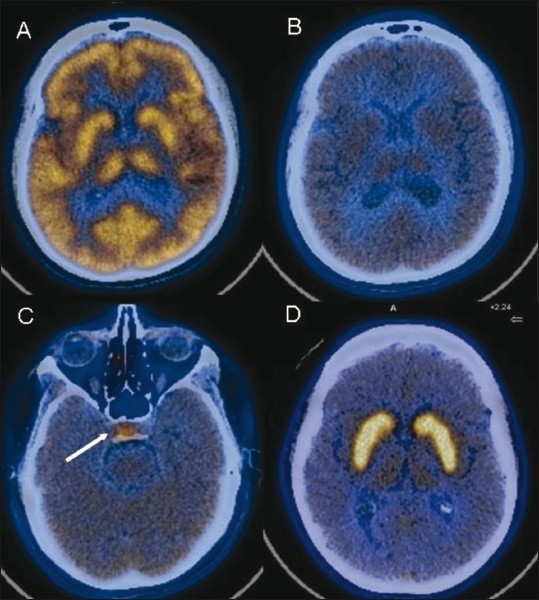
Normal radiotracer distribution pattern. Axial fused FDG PET/CT image (A) shows normal uptake in the cortex and basal ganglia. Axial fused 11C methionine PET/CT images (B,C) shows reduced parenchymal uptake with relatively increased uptake in the pituitary gland (arrow). Axial fused F-DOPA image (D) shows high tracer accumulation in both basal ganglia
Despite its obvious advantages, 11C methionine has certain limitations. The short half-life of twenty minutes necessitates an on-site cyclotron. Further, it takes some time for the radiotracer 11C to bind to various physiological substrates, further reducing the amount of radioisotope available in the final radiopharmaceutical by the time it is ready for injection. However, it has been found to be more efficacious than FDG PET/CT both for the primary detection of tumor and for delineation of its extent [Figure 2]. Additionally, it can differentiate tumorous from nontumorous lesions with a high degree of sensitivity and specificity.[4] A semiquantitative estimate of the level of radiopharmaceutical uptake in a particular tissue can be made by calculating the standardized uptake value (SUV). The ratio of radiotracer uptake in the pathological region to that in the contralateral uninvolved cortex can then be calculated. A threshold of 1.48 of relative 11C methionine uptake between tumor and normal tissue has been found to yield a high sensitivity of 83% and a specificity of 92%.[5] This is of particular relevance in the Indian setting, where the incidence of intracranial tuberculomas and other inflammatory granulomas is high. These lesions often mimic tumors clinically as well as on imaging studies. 11C methionine has demonstrated good results in distinguishing tuberculomas from neoplasia [Figures 3 and 4].
Figure 2 (A-D).
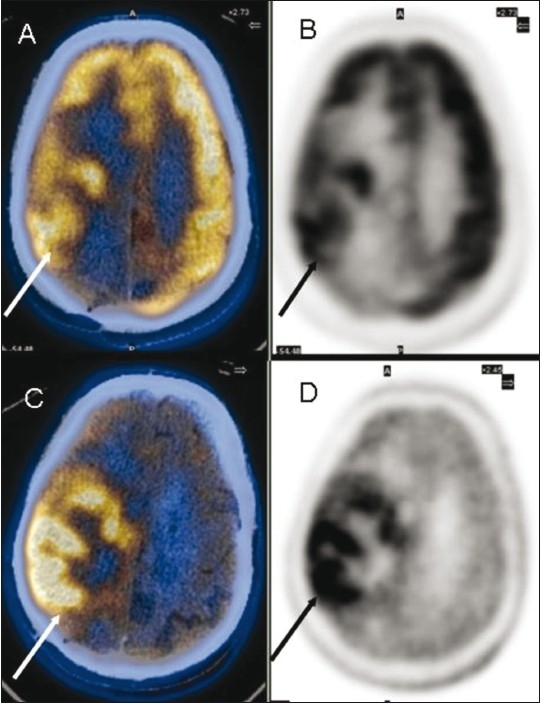
Glioblastoma multiforme in the right frontoparietal region. Axial fused FDG PET/CT (A) and PET (B) images show the extent of the lesion (arrows). Axial fused 11C methionine PET/CT (C) and PET (D) images show better visualization of the extent and margins (arrow)
Figure 3 (A-D).
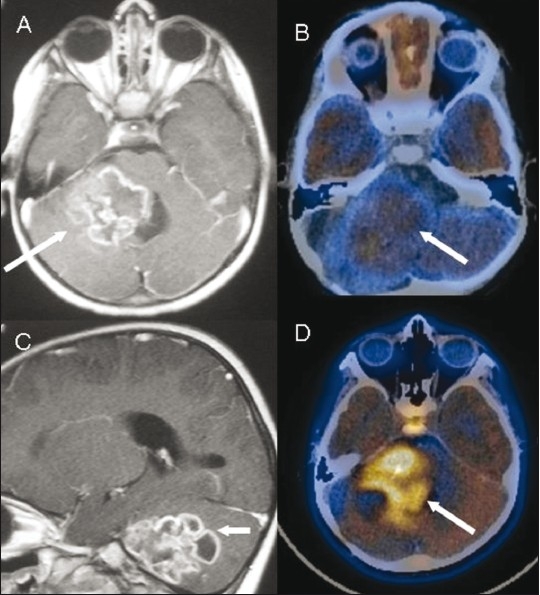
Posterior fossa glioma. Contrast-enhanced axial (A) and sagittal (C) MRI images show a conglomerate ring-enhancing lesion (arrow) that was mistaken for a tuberculoma. Axial fused FDG PET/CT image (B) shows low-grade FDG uptake (arrow). Axial fused 11C methionine PET/CT image (D) shows uptake (arrow), consistent with a high-grade malignancy. Note normal uptake in the pituitary gland
Figure 4 (A-D).
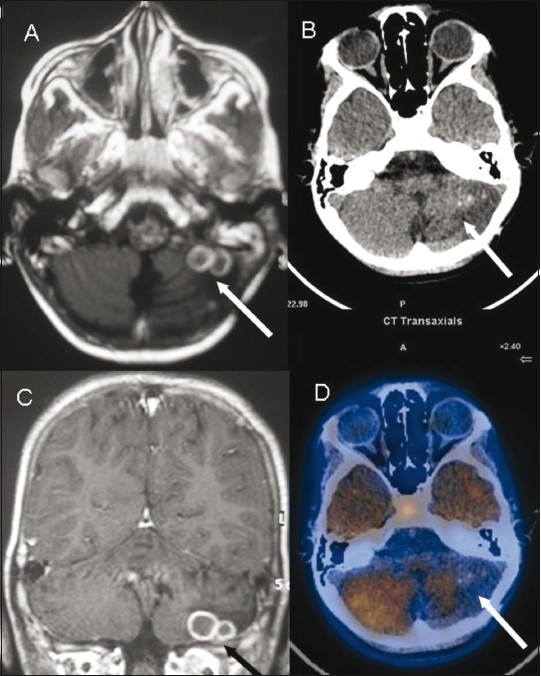
Posterior fossa tuberculoma. Axial (A) and coronal (C) contrast enhanced MRI images show conglomerate ring-enhancing lesions (arrow) in the left lesions (arrow) with surrounding edema with reduced tracer uptake cerebellar hemisphere. Axial non-contrast CT scan (B) and fused 11C methionine PET/CT (D) images show rounded Increased radiotracer uptake in the pituitary fossa region is noted again
Owing to its selective uptake in brain tumors, 11C methionine PET/CT has also been found to be a useful modality for assessing the size and margins of gliomas that may not enhance on contrast-enhanced Magnetic resonance imaging (MRI) imaging.[6] It also plays an important role as a marker for cell proliferation and angiogenesis[5] and can be used to evaluate tumor grade in gliomas, thus allowing better tumor prognosis.[6] It plays an important role when planning the radiotherapy field and for guiding selection of the site of biopsy. 11C methionine is useful for discriminating between tumor recurrence and radiation necrosis [Figures 5 and 6], a situation where CT scan, MRI, and FDG PET are often inconclusive.[7] However, there have been a few instances of 11C methionine uptake in nonmalignant conditions as well,[8] such as brain abscesses and conditions wherein the blood-brain barrier is disrupted as in intracranial hematomas. The increased radiotracer accumulation noted in some of these cases may be a reflection of overall increased cell metabolism, although the exact mechanism is unclear [Figure 7].
Figure 5 (A-D).
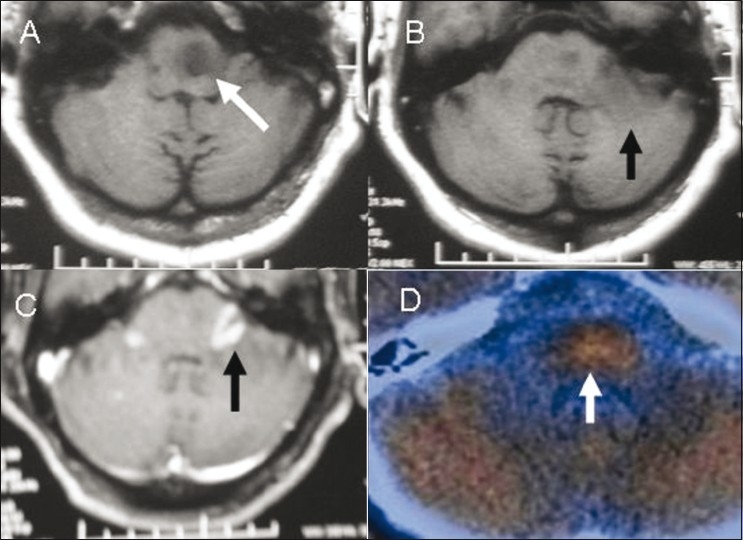
Brain stem glioma, post-radiotherapy. Axial T1W MRI images (A,B) show the glioma as a hypointense lesion (white arrow in A) that does not enhance on contrast administration with another hypointense lesion (black arrow in B) in the left middle cerebellar peduncle, which shows intense enhancement (black arrow) on a contrast-enhanced T1W axial MRI image (C). Axial fused 11C methionine PET/CT image (D) shows tracer uptake in the former, suggestive of an active lesion, with absent uptake in the latter due to post-radiotherapy changes
Figure 6 (A-D).
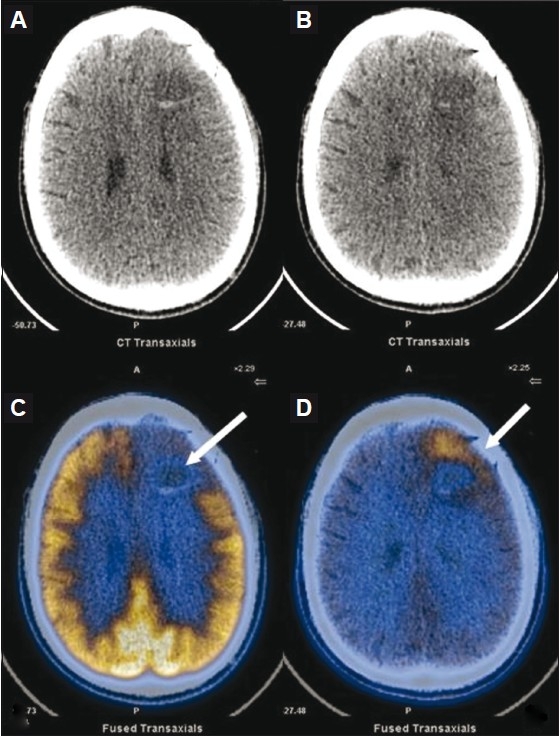
Contrast-enhanced CT scan(A,B) show a left frontal grade II fibrillary astrocytoma (post-surgery and post-radiotherapy) with a peripherally enhancing cavity (arrow) that is equivocal for tumor residue/post-radiotherapy changes, which was also the case on a contrast-enhanced MRI (not shown). Axial FDG PET/CT image (C) shows no radiotracer uptake (arrow). Axial 11C methionine PET/ CT (D) shows increased uptake anteriorly (arrow), and this was later proven to be tumor residue on biopsy
Figure 7 (A-C).
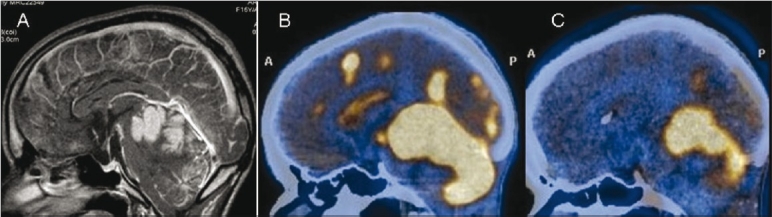
Neurocutaneous melanosis. Sagittal contrast-enhanced MRI image shows intensely enhancing areas of leptomeningeal thickening along the cerebellar folia, cerebral sulci, and ependyma (arrows). Sagittal FDG (B) and 11C methionine (C) PET/CT images show intense uptake (arrows) at the corresponding sites. However, there was no evidence of malignant transformation on biopsy
Other amino acid tracers
Malignant transformation increases the use of aminoacids for energy, protein synthesis and cell division. Owing to the short half life of 20 minutes that 11C methionine has, its use has been limited to institutions with an on-site cyclotron. Aminoacids labeled with a radiotracer having a longer half-life would be preferred in the routine clinical setting. Of these, 3,4-dihydroxy-6-18F-fluoro-L-phenylalanine (FDOPA) and O-(2-[18F]fluoroethyl)-l-tyrosine (FET) have been widely studied. Their uptake is mainly related to carrier mediated active transport, which is upregulated in tumor cells.[9] These amino acids are retained in tumor cells, which exhibit higher metabolic activities than most normal cells.
The superiority of FDOPA PET over FDG PET for imaging low-grade tumors and evaluating recurrent brain tumors has been demonstrated in past studies[10] [Figures 8 and 9]. Low-grade tumors are frequently FDG negative. However, as both high- and low-grade tumors show increased avidity for this radiotracer, it may not be useful for assessing tumor grade. One important advantage however is that tracer uptake is independent of blood–brain barrier breakdown, thus facilitating radiotracer uptake independent of tumor enhancement on contrast MRI studies.[11]
Figure 8 (A-F).
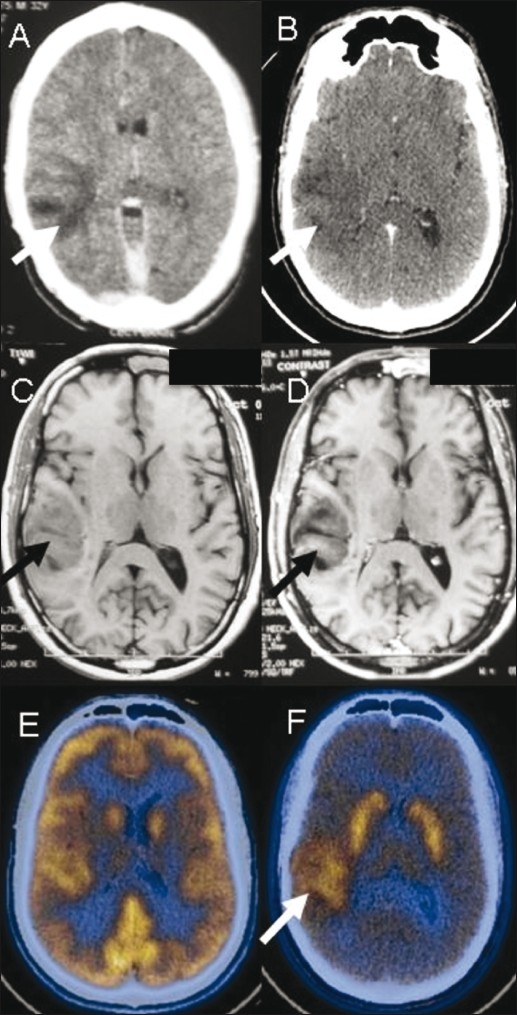
A 32-year-male presented with seizures. Contrast-enhanced CT scan (A) done in 2008 shows a poorly enhancing hypodense lesion (arrow) in the right temporal lobe. A repeat contrast-enhanced CT scan (B) in 2010 shows no change (arrow). T1W axial MRI images in 2010 (C,D) show a poorly enhancing lesion (arrow) in the cortical and subcortical white matter. FDG PET/CT image (E) is unremarkable. F-DOPA PET/CT image (F) shows increased uptake (arrow) suggestive of a low-grade malignant neoplasm eventually proven to be oligodendroglioma
Figure 9 (A-C).
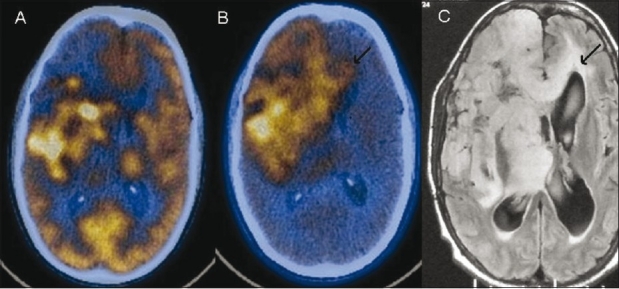
Post-operative case of low-grade right frontal astrocytoma. Axial FDG PET/CT image (A) shows residual tumor (arrows) in the right fronto-parietal region. However, the F-DOPA PET/ CT image (B) delineates the actual extent of the lesion (arrows), which is seen to cross the midline as is also seen (arrows) on the corresponding FLAIR axial MRI image (C)
FET PET when combined with MRI significantly improves the identification of cellular glioma tissue.[12] In addition, high- and low-grade brain tumors can be differentiated on the basis of the different uptake kinetics of FET. It has also proven useful in differentiating malignant from benign lesions of the brain.[13]
Radiotracers for Deoxyribonucleic acid synthesis
DNA synthesis is an important prerequisite for cellular proliferation. The most widely used radiotracer for assessing DNA synthesis is 3-deoxy-3-[18F] flurothymidine (FLT). This compound gets phosphorylated by thymidine kinase, which shows markedly increased activity in proliferating tumors, and thereafter gets trapped within the cell. The imaging of cellular proliferation has a potential advantage over glucose imaging because FLT is specific to tumors, while high levels of energy metabolism are also seen with other processes including inflammation.[14] Thus, unlike FDG it does not show uptake into inflammatory cells and has been widely used to distinguish benign from malignant pulmonary lesions. Although this differentiation may not always be feasible, the possibility of using FLT for monitoring treatment with cytostatic anticancer drugs needs to be explored.[15] Like 11C methionine, the background uptake in normal brain parenchyma is low, thus enhancing tumor detection [Figure 10].
Figure 10 (A, B).
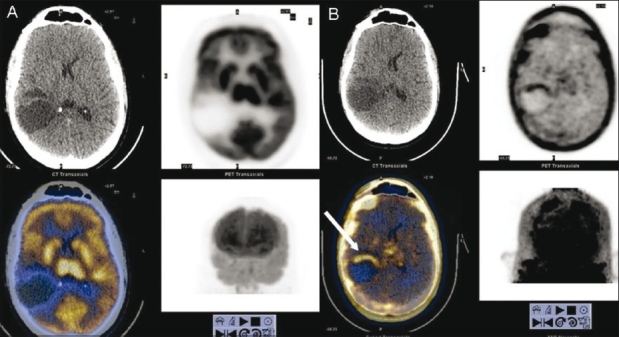
Postoperative case of grade III glioma. FDG PET/ CT image (A) shows no abnormal tracer uptake at the operative site. FLT PET/CT image (B) shows evidence of tumor recurrence anteriorly at the periphery of the lesion
Radiotracers for lipid synthesis
11C- and 18F-labeled choline have been used in brain tumors. The basis for this is the increased transport and phophorylation of choline in tumor cells, where it gets incorporated into the phospholipids of the cell membrane. It has been found useful for the differentiation of low-grade from high-grade gliomas, but not for distinguishing low-grade gliomas from nonmalignant lesions.[16] In a comparative study of FDG, 11C methionine, and 11C choline in 95 gliomas, 11C methionine was found to be the most superior among the three.[17]
Radiotracers for hypoxia
Adequate tumor vascularization is an important precondition to tumor growth. Inadequate vascularization would culminate in tumor hypoxia and eventual necrosis. Hypoxic tissue is inherently more resistant to chemotherapy or radiotherapy and this is often responsible for failure of chemo-radiotherapy and an overall poor response. Several in vivo PET tracers have been developed to assess tumor hypoxia, e.g.,[18] F fluoromisonidazole (FMISO) and 64/60Cu(II)-diacetylbis (N-4-methylthiosemicarbazone) (64/60Cu-ATSM), which have a propensity to accumulate in hypoxic rather than normoxic cells.[18] The most extensively used radiotracer for hypoxia is FMISO [Figures 11 and 12].[19] Inclusion of FMISO imaging data provides information that is complementary to FDG PET data by correlating metabolic activity to tumor hypoxia. In a comparative study on glioblastomas, the biological aggressiveness assessed by serial MRI was seen to be linked with the hypoxic tumor burden assessed on FMISO_PET.[20] This aids the selection of alternative treatments and monitoring of their therapeutic efficacy.
Figure 11 (A-C).
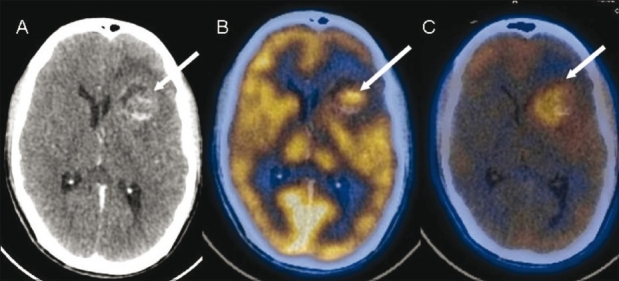
Left frontal anaplastic astrocytoma. Contrast-enhanced CT scan (A) shows a solid-cystic lesion (arrow) with heterogenous enhancement in the solid component. FDG PET/CT image (B) shows tracer uptake (arrow) in the solid portion of the lesion, while FMISO PET/CT image (C) shows diffusely increased uptake (arrow) suggestive of extensive tumor hypoxia
Figure 12 (A-D).
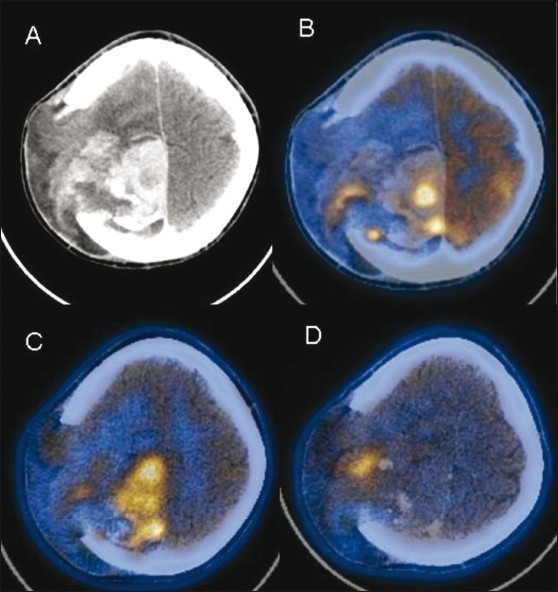
Postoperative case of gliosarcoma. Contrast-enhanced CT scan (A) shows an intensely enhancing residual lesion (arrow) in the right high frontoparietal region, herniating through a calvarial defect. FDG PET/CT image (B) shows heterogeneous uptake (arrow) in the lesion with more extensive involvement (arrow) seen on the 11C methionine PET/CT image (C). FMISO PET/CT image (D) shows a focal area of tumor hypoxia (arrow) at the site of herniation
Other radiotracers
The next generation of PET tracers includes those that will bind to specific cancer-related receptors or antigens.[2] These agents would offer tremendous opportunities for selective imaging, thus increasing the sensitivity and specificity for a particular tumor. There would also be enormous potential for directing molecular therapies targeted at these neoplasms. The development of targeted radiolabeled drugs to explore the efficacy of anticancer regimens holds promise for the future.
Conclusion
A multitude of PET radiotracers has emerged for the assessment of brain tumors, each with its inherent strengths and weakness. There is a pressing need to undertake comparative trials of the various tracers so that we can select the best tracer for any given clinical situation.
Acknowledgments
The authors gratefully acknowledge the contributions of Dr. RP Tripathi, Dr. Raunak and Dr. Anil Mishra, Institute of Nuclear Medicine and Allied Sciences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Patronas NJ, Chiro G, Brooks RA, DeLaPaz RL, Kornblith PL, Smith BH, et al. Work in progress: [18F] fluorodeoxyglucose and positron emission tomography in the evaluation of radiation necrosis of the brain. Radiology. 1982;144:885–9. doi: 10.1148/radiology.144.4.6981123. [DOI] [PubMed] [Google Scholar]
- 2.Hicks RJ. Beyond FDG: Novel PET tracers for cancer imaging. Cancer Imaging. 2004;4:22–4. doi: 10.1102/1470-7330.2003.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, et al. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: Local comparison with stereotactic histopathology. Clin Cancer Res. 2004;10:7163–71. doi: 10.1158/1078-0432.CCR-04-0262. [DOI] [PubMed] [Google Scholar]
- 4.Jager PL, Vaalburg W, Pruim J, Vries EG, Langen KJ, Piers DA. Radiolabeled amino acids: Basic aspects and clinical applications in oncology. J Nucl Med. 2001;42:432–45. [PubMed] [Google Scholar]
- 5.Galldiks N, Kracht LW, Berthold F, Miletic H, Klein JC, Herholz K, et al. [11C]-L-Methionine positron emission tomography in the management of children and young adults with brain tumors. J Neurooncol. 2010;96:231–9. doi: 10.1007/s11060-009-9953-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato T, Shinoda J, Nakayama N, Miwa K, Okumura A, Yano H, et al. Metabolic assessment of gliomas using 11C-methionine, [18F] fluorodeoxyglucose, and 11C-choline positron-emission tomography. Am J Neuroradiol. 2008;29:1176–82. doi: 10.3174/ajnr.A1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tai YF, Piccini P. Applications of positron emission tomography (PET) in neurology. J Neurol Neurosurg Psychiatry. 2004;75:669–76. doi: 10.1136/jnnp.2003.028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuyuguchi N, Sunada I, Ohata K, Iwai Y, Yamanaka K, Tanaka K, et al. Evaluation of treatment effects in brain abscess with positron emission tomography: Comparison of fluorine-18-fluorodeoxyglucose and carbon-11-methionine. Ann Nucl Med. 2003;17:47–51. doi: 10.1007/BF02988258. [DOI] [PubMed] [Google Scholar]
- 9.Shiue CY, Welch MJ. Update on PET radiopharmaceuticals: Life beyond fluorodeoxyglucose. Radiol Clin North Am. 2004;42:1033–53. doi: 10.1016/j.rcl.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Silverman DH, Delaloye S, Czernin J, Kamdar N, Pope W, et al. 18F-FDOPA PET imaging of brain tumors: Comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47:904–11. [PubMed] [Google Scholar]
- 11.Yee RE, Cheng DW, Huang SC, Namavari M, Satyamurthy N, Barrio JR. Blood brain barrier and neuronal membrane transport of 6-[F-18]flouro-L-DOPA. Biochem Pharmacol. 2001;62:1409–15. doi: 10.1016/s0006-2952(01)00787-0. [DOI] [PubMed] [Google Scholar]
- 12.Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128:678–87. doi: 10.1093/brain/awh399. [DOI] [PubMed] [Google Scholar]
- 13.Nalaf V, Kerrou K, Balogova S, Pene F, Huchet V, Gutman F, et al. Fluoroethyltyrosine 18F PET in the detection of brain tumors. Bull Cancer. 2010;97:495–506. doi: 10.1684/bdc.2010.1078. [DOI] [PubMed] [Google Scholar]
- 14.Mankoff DA, Shields AF, Krohn KA. PET imaging of cellular proliferation. Radiol Clin North Am. 2005;43:153–67. doi: 10.1016/j.rcl.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Yap CS, Czernin J, Fishbein MC, Cameron RB, Schiepers C, Phelps ME, et al. Evaluation of thoracic tumors with 18F-fluorothymidine and 18F-fluorodeoxyglucose-positron emission tomography. Chest. 2006;129:393–401. doi: 10.1378/chest.129.2.393. [DOI] [PubMed] [Google Scholar]
- 16.Ohtani T, Kurihara H, Ishiuchi S, Saito N, Oriuchi N, Inoue T, et al. Brain tumor imaging with carbon-11 choline: Comparison with FDG PET and gadolinium-enhanced MR imaging. Eur J Nucl Med. 2001;28:1664–70. doi: 10.1007/s002590100620. [DOI] [PubMed] [Google Scholar]
- 17.Kato T, Shinoda J, Nakayama N, Miwa K, Okumura A, Yano H, et al. Metabolic assessment of gliomas using 11C-methionine, [18F] fluorodeoxyglucose, and 11C-choline positron-emission tomography. Am J Neuroradiol. 2008;29:1176–82. doi: 10.3174/ajnr.A1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunn A, Linder K, Strauss HW. Nitroimidazoles and imaging hypoxia. Eur J Nucl Med. 1995;22:265–80. doi: 10.1007/BF01081524. [DOI] [PubMed] [Google Scholar]
- 19.Imam SK. Review of positron emission tomography tracers for imaging of tumor hypoxia. Cancer Biother Radiopharm. 2010;25:365–74. doi: 10.1089/cbr.2009.0740. [DOI] [PubMed] [Google Scholar]
- 20.Szeto MD, Chakraborty G, Hadley J, Rockne R, Muzi M, Alvord EC, et al. Quantitative metrics of net proliferation and invasion link biological aggressiveness assessed by MRI with hypoxia assessed by FMISO-PET in newly diagnosed glioblastomas. Cancer Res. 2009;69:4502–9. doi: 10.1158/0008-5472.CAN-08-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]


