Abstract
Background:
Spinal anaesthesia is the most common approach which is used for lower limb surgery. Dexmedetomidine is the recent drug which acts on α2-adrenergic receptors in the dorsal horn of the spinal cord to produce analgesic effects.
Aim:
Efficacy and safety of intrathecal dexmedetomidine added to ropivacaine.
Setting and Design:
Randomised double blind trial.
Methods:
Sixty patients were randomly allocated to receive intrathecally either 3 ml of 0.75% isobaric ropivacaine + 0.5 ml normal saline (Group R) or 3 ml of 0.75% isobaric ropivacaine + 5 μg dexmedetomidine in 0.5 ml of normal saline (Group D).
Results:
The mean time of sensory regression to S2 was 468.3±36.78 minutes in group D and 239.33±16.8 minutes in group R. Duration of analgesia (time to requirement of first rescue analgesic) was significantly prolonged in group D (478.4±20.9 minutes) as compared to group R (241.67±21.67 minutes). The maximum visual analogue scale score for pain was less in group D (4.4±1.4) as compared to group R (6.8±2.2).
Conclusion:
The addition of dexmedetomidine to ropivacaine intrathecally produces a prolongation in the duration of the motor and sensory block.
Keywords: Dexmedetomidine, postoperative analgesia, ropivacaine, spinal block
INTRODUCTION
Ropivacaine is a first single enantiomer-specific compound, which has a reduced risk of cardiotoxicity, neurotoxicity, and rapid recovery of motor function.[1,2] Postoperative pain relief is an important issue with ropivacaine. It has been used with many adjuvants for lower limb surgery, which has other side effects. So, our concern is of using a drug as an adjuvant with ropivacaine which provides better intraoperative haemodynamic condition as well as prolonged postoperative analgesia with minimal side effects. Dexmedetomidine is a highly selective α2-adrenergic agonist which has been used for premedication and as an adjunct to general anaesthesia. It reduces opioid and inhalational anaesthetics requirements.[3] Intrathecal α2-receptor agonists are found to have antinociceptive action for both somatic and visceral pain.[4]
Our study describes the use of intrathecal dexmedetomidine (5 μg) with ropivacaine in lower limb surgeries. The purpose of this study was to compare intrathecal isobaric ropivacaine with the combination of isobaric ropivacaine and dexmedetomidine. The primary outcomes studied were time to regression of spinal blockade below level S2 and duration of pain relief, defined as the time from intrathecal administration of dexmedetomidine to first request for supplemental analgesia by patients. Postoperative cumulative analgesic consumption and maximum visual analogue scale (VAS) pain score have been evaluated as secondary outcome.
METHODS
The study was conducted with the approval of ethical committee of the institution. A written and informed consent was obtained from all patients. Patients included for the study were all ASA physical status I or II, of either sex (18-50 years) presenting for lower limb surgeries. Patients who had contraindications to spinal anaesthesia, allergy to drug, patients of heart block and hypertension were excluded from the study groups. All patients received a tablet of diazepam 0.2 mg/kg orally the night before surgery. on arrival in the operating room, patients were preloaded with lactated ringer's solution at 15ml/kg. All patients were monitored with automated non-invasive blood pressure, pulse oximetry and electrocardiogram. Spinal needles used were either 23 or 25 gauge pencil point needles and were introduced at L3-4 or L4-5 interspace in sitting position with all aseptic precautions. Patients were randomised on the basis of a sealed envelope technique to receive one of the following into the subarachnoid block: Group R-3 ml volume of 0.75% isobaric ropivacaine and 0.5 ml normal saline and Group D-3 ml volume of 0.75% isobaric ropivacaine with 5 μg dexmedetomidine in 0.5 ml of normal saline. Injections were given over approximately 10 to 15 seconds. Immediately after completion of the block, patients were made to the supine position. Oxygen was administrated through a mask if the pulse oximetry reading decreased below 90%. Hypotension defined as a decrease in systolic blood pressure by more than 30% from baseline or less than 80 mm Hg was treated with incremental intravenous (IV) doses of ephedrine 5 mg and further boluses of IV fluid as required. Bradycardia defined as heart rate (HR) less than 50 bpm was treated with IV atropine 0.6 mg. The incidence of adverse effects such as nausea, vomiting, shivering, itching, pruritus, respiratory depression, sedation and hypotension was recorded. Sensory testing was assessed by loss of pinprick sensation to 23 G hypodermic needle and dermatomal levels were tested every 2 minutes until the highest level had stabilised for four consecutive tests. Testing was then conducted every 10 minutes until the point of two segment regression of the block. To this point, dermatomal testing was performed by an anaesthetist who was blinded to the patient group. Further testing was performed at 20 minutes intervals until the recovery of S2 dermatome. The surgeon and the observing anaesthetist were blinded to the patient groups. Data regarding the highest dermatomal level of sensory blockade, the time to reach this level from the time of injection, time to S2 sensory regression and incidence of side effects were collected. Sedation was assessed with a four-point verbal rating scale (1 = no sedation, 2 = light sedation, 3 = somnolence, 4 = deep sedation).
Postoperatively, pain scores were recorded by using VAS between 0 and 10 (0 = no pain, 10 = the most severe pain), initially every 1 hour for 2 hours, then every 2 hours for next 8 hours and then after every 4 hours till 24 hours. Injection diclofenac 75 mg intramuscular was given as rescue analgesia when VAS ≥4. Follow-up was carried out 1 week postoperatively by the blinded anaesthetist who asked about postoperative headache as well as postoperative pain and dysesthesias in the buttock, thighs, or lower limbs.
Statistical analysis was done by SPSS version 15.0 for analysing the collected data. As there was no prior historic evidence available, the sample size was kept to be large enough (n>30) for statistical purposes as per the Central Limit Theorem. Parametric data were reported as arithmetic mean±standard deviation and analysed by using student t-test. The comparison was studied using chi-squared test or the Fisher's exact test as appropriate, with the P value reported at the 95% confidence interval. P<0.05 was considered statistically significant. Post-hoc power analysis was done using Power and Sample size calculator developed by Vanderbilt University. The cut-off level for power of test was 80% (β=0.8).
Power analysis
The effect size/power of study was calculated for time of rescue analgesia (β=1) and for highest pain score on VAS scale (β=0.992). For both, the power was well above the generally accepted level of 80%. Thus, the post-hoc assessment of effect size justified the sample size.
RESULTS
The groups were comparable with respect to age, height, weight and ASA physical status. There was no significant difference in the type and duration of surgery [Table 1]. The results regarding the characteristics of sensory block are summarised in Table 2. There was no difference between group D and R in the highest level of block (T5 and T6, respectively) or in the time to reach peak level (11.65±1.73 and 12.05±1.64 minutes, respectively). Block regression was significantly slower with the addition of intrathecal dexmedetomidine as compared to ropivacaine alone, as both time to two segment regressions and time to S2 regression were significantly more with intrathecal dexmedetomidine. On statistical analysis, the maximum VAS score in the group D was lower as compared to group R up to 24 hours postoperatively [Table 2]. The duration of analgesia was significantly prolonged with the addition of dexmedetomidine as compared to ropivacaine alone (478.4±20.9 min and 241.67±21.67 min, respectively). There was no serious complication in the 60 study patients, like nausea, vomiting, shivering, itching, pruritus, sedation, respiratory depression and hypotension [Table 3].
Table 1.
Demographic characteristics
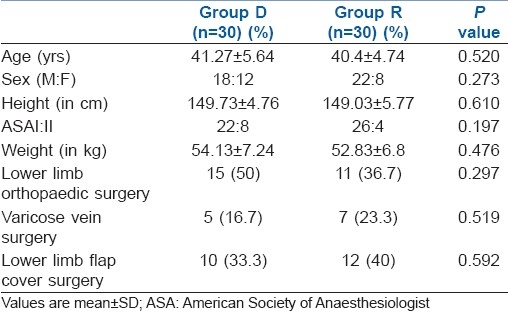
Table 2.
Summary of results
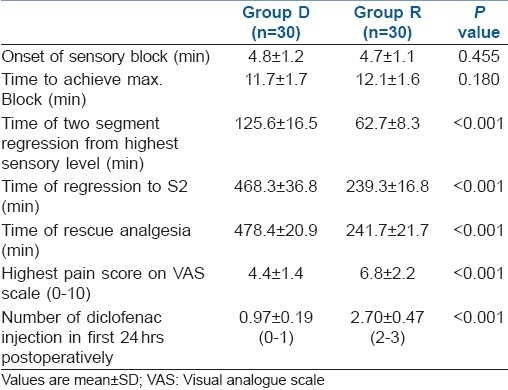
Table 3.
Side effects in two groups
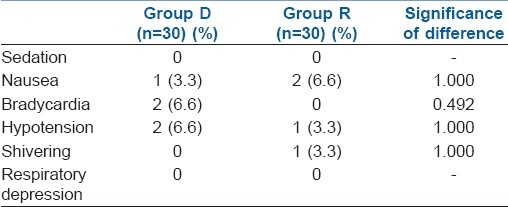
Intraoperative ephedrine requirement was more in group D (8±4 mg) as compared to group R (6±3 mg). Two patients in group D had bradycardia (HR <50/ min) that was successfully managed with atropine 0.6 mg IV. No patient had residual neurological deficit, post-dural puncture headache or transient neurological symptoms at the postoperative follow-up.
DISCUSSION
Various animal studies have been conducted in rats, rabbits, dogs and sheep using intrathecal dexmedetomidine at a dose range of 2.5 to 100 μg without any neurological deficits.[5–12] In human beings, studies using epidural dexmedetomidine have been conducted without any report of neurological deficit.[13,14] Intrathecal dexmedetomidine in combination with bupivacaine have been studied in human beings without any postoperative neurological deficit.[15–17]
Intrathecal small dose of dexmedetomidine (3 μg) used in combination with bupivacaine in human beings for spinal anaesthesia have been shown to produce a shorter onset of motor block and a prolongation in the duration of motor and sensory block with haemodynamic stability and lack of sedation.[15] Al - Ghanem et al.'s[16] study concluded that 5mg dexmedetomide seems to be alternative as adjuvant to spinal bupivacaine in surgical procedures, especially in those who need quite long time with minimal side effects and excellent quality of analgesia. In our study, we had compared the intrathecal isobaric ropivacaine with isobaric ropivacaine and dexmedetomidine for lower limb surgery. In this study, we had used dexmedetomidine as an adjuvant to ropivacaine intrathecally.
Kalso et al.[4] reported that dexmedetomidine affinity to α2-adrenoceptor agonists is 10 times as compared to clonidine. De Kock et al.[18] who used clonidine with ropivacaine intrathecally in three different doses – 15, 45 and 75 μg—for ambulatory knee arthroscopy, observed that small dose clonidine 15 μg significantly improves the quality of anaesthesia without delaying sensory and motor recovery, 45 μg prolongs the sensory blockade without any influence on motor blockade but a dose of 75 μg is associated with delayed sensory and motor recovery as well as detectable side effects as hypotension and sedation. From these studies, we had concluded that 5 μg dexmedetomidine would be safe and appropriate for our study.
The toxicity levels in terms of side effects were found to be insignificant and incidental only. No major issue related with safety of use was observed. Al-Ghanem et al.[16] have reported the use of dexmedetomidine to be associated with a decrease in heart rate and blood pressure. In present study, only two cases of bradycardia and hypotension were noticed. The reason could be combination of dexmedetomidine with ropivacaine. Ropivacaine has been shown to be a better drug in terms of cardiovascular and haemodynamic control[1,2].
In our study, we found that the analgesic effect of intrathecal ropivacaine was potentiated by intrathecal dexmedetomidine. The addition of 5 μg of intrathecal dexmedetomidine prolonged the postoperative analgesic effect of ropivacaine by approximately 8 hours. In addition, dexmedetomidine-treated group required less postoperative analgesic in the first 24 hours after surgery.
The mechanism of action by which intrathecal α2-adrenoceptor agonists prolong the motor and sensory block of local anaesthetics is not well known. The local anaesthetics act by blocking sodium channels, whereas the α2-adrenoceptor agonist acts by binding to pre-synaptic C-fibres and post-synaptic dorsal horn neurons. The analgesic action of intrathecal α2-adrenoceptor agonists is by depressing the release of C-fibre transmitters and by hyperpolarisation of post-synaptic dorsal horn neurons.[19] It may be an additive or synergistic effect secondary to the different mechanisms of action of the local anaesthetics and the α2-adrenoceptor agonist as studied by Salgado et al.[20] This antinociceptive effect may explain the prolongation of the sensory block when added to spinal anaesthetics. The prolongation of the motor block of spinal anaesthetics may result from the binding of α2-adrenoceptor agonists to motor neurons in the dorsal horn.[21,22]
The α-2 adrenergic agents also have antishivering property as observed by Talke et al.,[23] but we did not observe any incidence of shivering in both the groups. We also did not observe any side effect other than two cases of bradycardia (HR <50/ min) in dexmedetomidine group which were successfully managed with atropine 0.6 mg IV. The reason may be, we had used small doses of intrathecal dexmedetomidine (5 μg) in our study which was supported by Al-Ghanem et al.[16]
CONCLUSION
In conclusion, 5 μg dexmedetomidine seems to be an attractive alternative as an adjuvant to spinal ropivacaine in surgical procedures, especially those requiring long time. It has excellent quality of postoperative analgesia with minimal side effects. However, clinical studies to prove its efficacy and safety and varying dosages for supplementation of spinal local anaesthetics are recommended.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Yamashita A, Matsumoto M, Matsumoto S, Itoh M, Kawai K, Sakabe T. A comparison of the neurotoxic effects on the spinal cord of tetracaine, lidocaine, bupivacaine, and ropivacaine administered intrathecally in rabbits. AnesthAnalg. 2003;97:512–9. doi: 10.1213/01.ANE.0000068885.78816.5B. [DOI] [PubMed] [Google Scholar]
- 2.McNamee DA, Convery PN, Milligan KR. Total knee replacement: A comparison of ropivacaine and bupivacaine in combined femoral and sciatic block. ActaAnaesthesiolScand. 2001;45:477–81. doi: 10.1034/j.1399-6576.2001.045004477.x. [DOI] [PubMed] [Google Scholar]
- 3.Martin E, Ramsay G, Mantz J, Sum-Ping ST. The role of the alpha2-adrenreceptor agonist dexmedetomidine in post-surgical sedation inthe intensive care unit. J Intensive Care Med. 2003;18:29–34. doi: 10.1177/0885066602239122. [DOI] [PubMed] [Google Scholar]
- 4.Kalso EA, Poyhia R, Rosenberg PH. Spinal antinociception by dexmedetomidine, a highly selective a2-adrenergic agonist. PharmacolToxicol. 1991;68:140–3. doi: 10.1111/j.1600-0773.1991.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 5.Eisenach JC, Shafer SL, Bucklin BA, Jackson C, Kallio A. Pharmacokinetics and pharmacodynamics of intraspinaldexmedetomidine in sheep. Anesthesiology. 1994;80:1349–59. doi: 10.1097/00000542-199406000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Lo WC, Harris J, Clarke RW. Endogenous opioids support the spinal inhibitory action of an alpha 2-adrenoceptor agonist in the decerebratedspinalised rabbit. Neurosci Lett. 2003;340:95–8. doi: 10.1016/s0304-3940(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 7.Talke P, Xu M, Paloheimo M, Kalso E. Effects of intrathecally administered dexmedetomidine, MPV-2426 and tizanidine on EMG in rats. ActaAnaesthesiolScand. 2003;47:347–54. doi: 10.1034/j.1399-6576.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 8.Xu M, Kontinen VK, Kalso E. Effects of radolmidine, a novel alpha2-adrenergic agonist compared with dexmedetomidine in different pain models in the rat. Anesthesiology. 2000;93:473–81. doi: 10.1097/00000542-200008000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Horvath G, Joo G, Dobos I, Klimscha W, Toth G, Benedek G. The synergistic antinociceptive interactions of endomorphin-1 with dexmedetomidine and/or S (+)-ketamine in rats. AnesthAnalg. 2001;93:1018–24. doi: 10.1097/00000539-200110000-00044. [DOI] [PubMed] [Google Scholar]
- 10.Shimode N, Fukuoka T, Tanimoto M, Tashiro C, Tokunaga A, Noguchi K. The effects of dexmedetomidine and halothane on the Fos expression in the spinal dorsal horn using a rat postoperative pain model. NeurosciLett. 2003;343:45–8. doi: 10.1016/s0304-3940(03)00309-4. [DOI] [PubMed] [Google Scholar]
- 11.Onttonen T, Pertovaara A. The mechanical antihyperalgesic effect of intrathecally administered MPV-2426, a novel alpha2-adrenoceptor agonist, in a rat model of postoperative pain. Anesthesiology. 2000;92:1740–5. doi: 10.1097/00000542-200006000-00034. [DOI] [PubMed] [Google Scholar]
- 12.Takano Y, Yaksh TL. Characterization of the pharmacology of intrathecally administered alpha 2-agonists and antagonists in rats. J PharmacolExpTher. 1992;261:764–72. [PubMed] [Google Scholar]
- 13.Fukushima K, Nishimi Y, Mori K, Takeda J. Effect of epidurally administered dexmedetomidine on sympathetic activity and postoperative pain in man. AnesthAnalg. 1996;82:S121. [Google Scholar]
- 14.Maroof M, Khan SA, Jain D, Khan RM, Maroof SM. Evaluation of effect of dexmedetomidine in reducing shivering following epidural anesthesia. Anesthesiology. 2004;101:A495. [Google Scholar]
- 15.Kanazi GE, Aouad MT, Jabbour-Khoury SI, AI Jazzar, Alameddine MM, AI-Yaman R, et al. Effect of low dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. ActaAnesthesiolScand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 16.Al-Ghanem SM, Massad IM, Al-Mustafa MM, Al-Zaben KR, Qudaisat IY, Qatawneh AM, et al. Effect of adding dexmedetomidine versus fentanyl to intrathecal bupivacaine on spinal block characteristics in gynecological procedures. Am JApplSci. 2009;6:882–7. [Google Scholar]
- 17.Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, Murshidi MM, Ammari BA, Awwad ZM, et al. Effect of Dexmedetomidineadded to spinal bupivacaine for urological procedure. Saudi Med J. 2009;30:365–70. [PubMed] [Google Scholar]
- 18.De Kock M, Gautier P, Fanard L, Hody JL, Lavand’homme P. Intrathecal Ropivacaine and clonidine for ambulatory Knee arthroscopy.A dose response study. Anesthesiology. 2001;94:574–8. doi: 10.1097/00000542-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Eisanach JC, De Kock M, Klimscha W. α2 adrenergic agonists for regional anesthesia. Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Salgado PF, Sabbag AT, Silva PC, Brienze SL, Dalto HP, Módolo NS, et al. Synergistic effect between dexmedetomidine and 0.75% ropivacaine in epidural anesthesia. Rev Assoc Med Bras. 2008;54:110–5. doi: 10.1590/s0104-42302008000200011. [DOI] [PubMed] [Google Scholar]
- 21.Harada Y, Nishioka K, Kitahata LM, Kishikawa K, Collins JG. Visceral antinociceptive effects of spinal clonidine combined with morphine, enkephalin, or U50, 488H. Anesthesiology. 1995;83:344–52. doi: 10.1097/00000542-199508000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Yaksh TL, Reddy SV. Studies in primate on the analgesic effects associated with intrathecal actions of opiates, adrenergic agonists, and baclofen. Anesthesiology. 1981;54:451–67. doi: 10.1097/00000542-198106000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Talke P, Tayefeh F, Sessler DI, Jeffrey R, Noursalehi M, Richardson C. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1997;87:835–41. doi: 10.1097/00000542-199710000-00017. [DOI] [PubMed] [Google Scholar]


