Abstract
Posterior reversible encephalopathy syndrome (PRES) is a cliniconeuroradiological syndrome associated with various clinical conditions, presenting with headache, encephalopathy, seizures, cortical visual disturbances or blindness. Imaging predominantly shows parieto-occipital white matter changes, with vasogenic oedema being the most accepted pathophysiology. We report a 25-year-old primigravida who presented in term pregnancy with seizures and blindness, scheduled for emergency caesarean section. She was managed peroperatively under general anaesthesia and shifted to intensive care unit. Postoperative computed tomography brain revealed an intra-axial hypodensity involving predominantly white matter regions of bilateral parieto-occipital lobes, right caudate nucleus and right cerebellum, suggestive of PRES. Clinical improvement with complete resolution of visual disturbances was observed with supportive treatment. The importance of prompt suspicion and management in preventing short- and long-term neurological deficits in reversible condition like PRES is highlighted.
Keywords: Leukoencephalopathy, posterior leukoencephalopathy syndrome, posterior reversible encephalopathy syndrome, pregnancy, reversible posterior cerebral oedema syndrome, reversible posterior leukoencephalopathy syndrome
INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) or reversible posterior leukoencephalopathy syndrome (RPLS) is a rare cliniconeuroradiological entity introduced as late as 1996 by Hinchey et al.[1] Others have referred to the syndrome as reversible posterior cerebral oedema syndrome or posterior leukoencephalopathy syndrome. Multiple clinical associations for PRES have been described. Common clinical conditions include hypertensive encephalopathy, renal failure, autoimmune disorders and treatment with immunosuppressant or cytotoxic medications. Uncommon clinical conditions include acute intermittent porphyria and cryoglobulinemia.[2] Clinical features include headache, encephalopathy, seizures, cortical visual disturbances or blindness and parieto-occipital white matter changes on neuroimaging.[3,4] A high level of suspicion is necessary to recognise and prevent the long-term sequelae of reversible conditions like PRES.
CASE REPORT
A 25-year-old primigravida at 36 weeks gestation, weighing 60 kg, presented with complaints of acute onset headache, loss of vision, altered sensorium and one episode of seizure. There was no past history of hypertension, cardiac diseases, vision abnormalities or seizures. She was managed conservatively in a local hospital with IV magnesium sulphate (MgSO4) and referred to our centre on the same day. On arrival, the patient was disoriented, restless, afebrile with bilateral pedal oedema and unable to perceive hand movements. Blood pressure was 150/90 mmHg with a heart rate of 110 beats per minute. Her respiratory rate was 18 breaths per minute and chest was clear on auscultation. Room air saturation was 98%. Pupils were equal and reactive to light, but fundoscopy was not possible. Bilateral plantar reflexes were flexor. Other system examinations were normal. Complete blood picture (Hb of 10.8 g %), renal and liver function tests, clotting parameters, and electrocardiogram were normal. Urine analysis revealed proteinuria 2+. A provisional diagnosis of eclampsia was made and she was immediately shifted for an emergency caesarean section. In the operation room, she developed an episode of generalised tonic–clonic seizure, following which her trachea was intubated with rapid sequence induction using thiopentone and succinylcholine. Anaesthesia was maintained with oxygen, air and isoflurane. Intraoperative hypertension was managed with IV labetalol with invasive blood pressure monitoring. A 1.7-kg, low-birth weight baby was extracted and transferred to a neonatal intensive care unit. Analgesia was achieved with 150 μg of IV fentanyl. Intraoperative desaturation with thin frothy secretions in the endotracheal tube and fine basal crepitations on auscultation of chest were noted after extraction of the baby and managed with a positive end expiratory pressure of 10 mmHg and 0.5 FiO2 on intermittent positive pressure ventilation. A total of 700 ml of Ringer's lactate was infused during the entire procedure which lasted for 1 hour. Urine output was approximately 100 ml in 1 hour. Postoperatively, she was shifted to an intensive care unit (ICU) for planned mechanical ventilation and invasive monitoring. Her treatment included MgSO4 infusion, amlodipine, clonidine, frusemide and mannitol. Neurology consultation was obtained in the immediate postoperative period and computed tomography (CT) brain [Figure 1a, b] revealed an intra-axial hypodensity involving predominantly white matter regions of bilateral parieto-occipital lobes, right caudate nucleus and right cerebellum. Chest X-ray revealed features of pulmonary oedema. Her trachea was extubated as her sensorium, vision and ventilatory parameters improved on the second postoperative day. On the third postoperative day, there was complete recovery of vision (6/6) and she was shifted to a step-down ICU. Rest of her hospital stay was uneventful till she was discharged.
Figure 1.
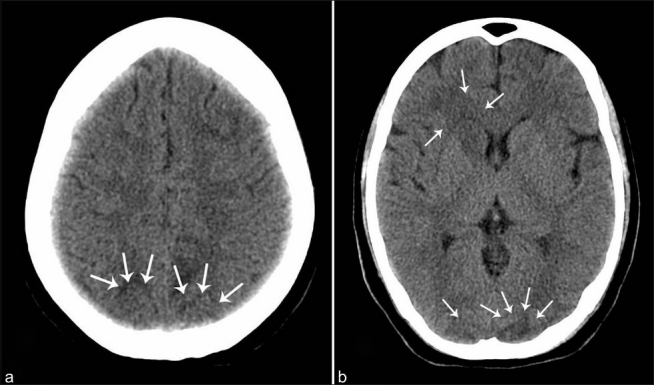
(a) Plain CT study of brain showing intra-axial hypodensities involving white matter regions of bilateral occipital lobes. (b) Plain CT brain showing intra-axial hypodensities involving white matters of bilateral parieto-occipital lobes and right caudate nucleus
DISCUSSION
A primigravida presenting at term with seizures and blindness puts every clinician in dilemma of varied possibilities. The possibilities could be cerebrovascular accidents complicating pregnancy, eclampsia and clinical syndromes like PRES.[5] PRES is a recently described syndrome in literature, which constitutes a recognisable syndrome characterised by headache, altered mental status, seizures, and visual loss.[1] Altered mental status could range from lethargy, somnolence, restlessness, agitation, confusion to stupor and coma. Multiple seizures are more common than a single event.[2] The three most common visual complications of preeclampsia and eclampsia are hypertensive retinopathy, exudative retinal detachment and cortical blindness.[6] Current opinion suggests that blindness in severe preeclampsia is mostly associated with cortical aetiology. Cortical blindness is a clinical syndrome characterised by intact pupillary reflexes and normal fundoscopic findings. The lost vision is usually regained within 4 hours to 8 days.[6]
Preeclampsia and eclampsia may be the most common causes of PRES and most cases are managed without neuroimaging, and the incidence remains unknown. However, it is uncertain whether a cause and effect relationship truly exists between the two or if these represent independent processes with some element of clinical overlap.[2]
Multiple theories have been proposed on the pathophysiology of PRES, the most accepted being the vasogenic oedema.[1] Cerebral autoregulation maintains a constant blood flow to the brain despite alterations in the systemic pressures. Once this mechanism gets disrupted, increased perfusion pressure is sufficient to overcome the blood–brain barrier, allowing extravasation of fluid, macromolecules and even red blood cells. So, PRES represents vasogenic rather than cytotoxic oedema in the majority of cases.
Clinical improvement always follows the treatment of elevated blood pressure and withdrawal of offending agents. Magnesium therapy should be initiated as soon as eclampsia or PRES in pregnancy is suspected, as it treats both seizures and hypertension. Finsterer and associates[7] found that treating pre-eclamptic patients with nitroglycerin infusion needs caution, as it may worsen PRES. Even mild fluctuations in blood pressure during or after anaesthesia, or changes in serum electrolytes, notably magnesium, may be sufficient to precipitate PRES in susceptible patients.[8] Early treatment usually results in complete reversal of the deficits over few days to several weeks.
Neuroimaging CT shows oedema as bilateral symmetrical hypodensities involving the white matter typically in the parieto-occipital regions. This is explained by better autoregulation of the anterior circulation due to better sympathetic innervations as compared to the posterior circulation.[9] Magnetic resonance imaging shows high signal intensity on T2-weighted and fluid attenuated inversion recovery (FLAIR) sequences.[4] FLAIR provides T2-weighted images while suppressing the signal from CSF by use of an inversion pulse. FLAIR frequently allows detection of what otherwise would be subtle findings on conventional spin-echo T2-weighted images. “PRES” remains the term preferred over earlier descriptions involving the word “leukoencephalopathy”. The latter suggests that only white matter is involved, so is quite misleading since grey matter lesions are present in as many as 94% of cases. Nonetheless, “posterior reversible encephalopathy syndrome” is also a misnomer because the image changes and clinical features may not be limited to the posterior cerebral hemispheres. Also, reversibility of PRES may be clinically or radiologically incomplete, the condition may be complicated by ischaemic or haemorrhagic stroke, and may lead to a chronic seizure disorder or death.[10]
The case described here presented to the emergency room with high blood pressure, seizures and blindness. Initial diagnosis of eclampsia was made and managed accordingly. However, postoperative CT helped us to diagnose PRES. Follow-up neuroimaging was not considered in view of rapid clinical recovery of the patient. Thus, this case report emphasises the need for early diagnosis and prompt treatment of PRES to avert short- and long-term neurological sequelae. Though the association of PRES and pregnancy-induced hypertension is well documented, the cause and effect relationship is unfounded. A pregnant patient presenting with seizures and blindness is not necessarily eclamptic; possible presence of PRES with proper preoperative neurological evaluation and perioperative management is worth a consideration.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posteriorleukoencephalopathy syndrome. Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 2.Long TR, Hein BD, Brown MJ, Rydberg CH, Wass CT. Posterior reversible encephalopathy syndrome during pregnancy: Seizures in a previously healthy parturient. J Clin Anesth. 2007;19:145–8. doi: 10.1016/j.jclinane.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007;28:1320–7. doi: 10.3174/ajnr.A0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: Utility of fluid-attenuated inversion recoveryMR imaging in the detection of cortical and subcortical lesions. Am J Neuroradiol. 2000;21:1199–206. [PMC free article] [PubMed] [Google Scholar]
- 5.Thackeray EM, Tielborg MC. Posterior reversible encephalopathy syndrome in a patient with severe preeclampsia. Anesth analg. 2007;105:184–6. doi: 10.1213/01.ane.0000265553.36391.96. [DOI] [PubMed] [Google Scholar]
- 6.Swende TZ, Abwa T. Reversible blindness in fulminating preeclampsia. Ann Afr Med. 2009;8:189–91. doi: 10.4103/1596-3519.57247. [DOI] [PubMed] [Google Scholar]
- 7.Finsterer J, Schlager T, Kopsa W, Wild E. Nitroglycerin-aggravated pre-eclamptic posterior reversible encephalopathy syndrome (PRES) Neurology. 2003;1:715–6. doi: 10.1212/01.wnl.0000080369.87484.06. [DOI] [PubMed] [Google Scholar]
- 8.Rangi PS, Partridge WJ, Newlands ES, Waldman AD. Posterior reversible encephalopathysyndrome: A possible late interaction between cytotoxic agents and general anaesthesia. Neuroradiology. 2005;47:586–90. doi: 10.1007/s00234-005-1376-6. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz RB, Mulkern RV, Gudbjartsson H, Jolesz F. Diffusion-weighted MR imaging in hypertensive encephalopathy: Clues topathogenesis. AJNR Am J Neuroradiol. 1998;19:859–62. [PMC free article] [PubMed] [Google Scholar]
- 10.Pratap JN, Down JF. Posterior reversible encephalopathy syndrome: A report of a case with atypical features. Anaesthesia. 2008;63:1245–8. doi: 10.1111/j.1365-2044.2008.05587.x. [DOI] [PubMed] [Google Scholar]


