Abstract
Due to its characterized progression from recognized premalignant oral epithelial changes (i.e., oral epithelial dysplasia) to invasive cancer, oral squamous cell carcinoma represents an optimal disease for chemopreventive intervention prior to malignant transformation. The primary goal of oral cancer chemoprevention is to reverse, suppress, or inhibit the progression of premalignant lesions to cancer. Over the last several decades, numerous oral cancer chemoprevention clinical trials have assessed the therapeutic efficacy of diverse chemopreventive agents. The standard of care for more advanced oral dysplastic lesions entails surgical excision and close clinical follow-up due to the potential (~33%) for local recurrence at a similar or more advanced histological stage. The purpose of this review was to identify prominent oral cancer chemoprevention clinical trials, assess their overall therapeutic efficacy, and delineate effects of local versus systemic drug administration. In addition, these compiled clinical trial data present concepts for consideration in the design and conduction of future clinical trials.
Keywords: Chemoprevention, local drug delivery, oral cancer, pharmacologic advantage
INTRODUCTION
Cancer treatment delivery strategies – Past and present
Optimal cancer therapies preferentially target cancerous cells while sparing normal, non-tumorigenic cells. These treatment outcomes, however, are rarely realized with current systemic chemotherapy regimens. Often severe dose-limiting toxicities impede the progression of treatment and/or mandate decreased therapeutic dosing.[1] These treatment alterations result in an increased risk of selecting for chemotherapy-resistant cell populations due to the low-dose induction of detoxification enzymes (i.e., phase I/II enzymes and ATP binding cassette-efflux transporters).[2] In an effort to minimize toxicity-induced treatment reductions, innovative strategies for concentrating therapeutics in tumors have been developed for systemic delivery. Notably, these novel delivery methods often exploit tumor microenvironment and protein expression profiles to preferentially target delivery to tumors (e.g., concentration of macrotherapeutics via the enhanced permeability and retention effect, antibody–therapeutic conjugates against tumor specific receptors, and hypoxia-activated prodrugs).[3–6] Another strategy introduced by Folkman et al. directed treatment toward tumor-associated angiogenesis rather than tumor cells; however, systemic delivery of angiostatic agents has not been highly successful in human applications.[7] Although tumor-targeted strategies are becoming more commonplace in clinical practice, the standard of treatment for numerous cancers remains the systemic administration of highly toxic compounds with severe side effects.[1,8,9]
Many cancers necessitate systemic delivery due to their anatomical locations, high metastatic potential, and/or advanced stage upon diagnosis. Classic examples of such malignancies include leukemia, advanced stage lymphoma, lung and hepatocellular cancers. These cancers are ideal candidates for systemic administration of chemotherapeutic compounds due to their systemic distribution and/or origin from richly vascularized tissues.[9,10] In contrast, several cancers (e.g., oral, cutaneous, and cervical carcinomas) arise at visibly accessible locations and progress through well-characterized, recognizable premalignant lesions. These cancers are, therefore, more amenable to the use of local delivery-based preventive therapies for either primary chemoprevention (suppressing progression of premalignant lesions to cancer) or secondary chemoprevention (inhibition of cancer recurrence). Despite their suitable location for local delivery strategies, numerous chemoprevention clinical trials have failed to deviate from the standard systemic delivery paradigm. Notably, in order for systemically administered agents to affect epithelial lesions, they require transport through systemic vasculature, perfusion from blood vessels in the underlying connective tissue, diffusion through interstitial spaces/epithelial basement membrane, and subsequent absorption and retention within the epithelium. This method of delivery likely requires supra-therapeutic serum concentrations (i.e., potentially toxic concentrations) to obtain therapeutically relevant drug levels in the target tissues. In contrast to these extensive delivery considerations, local delivery formulations provide targeted delivery methods that impart a pharmacologic advantage over systemic delivery schemes (i.e., optimal drug concentrations in target epithelium, reduced drug dosing and less exposure of normal tissues resulting in minimal side effects, and increased stability and apparent solubility of drugs in physiological fluids thus facilitating drug permeability across biological membranes).[11,12] Furthermore, compounds delivered locally are not subjected to first-pass hepatic metabolism, thereby limiting premature metabolic inactivation and excretion prior to the exertion of therapeutic effects in the target tissues.[12] Collectively, local formulations represent favorable treatment modalities for drug delivery to site-specific premalignant epithelial lesions.
Chemopreventive therapies to suppress the development of oral squamous cell carcinoma (OSCC), the focus of our laboratory's research, entail the use of natural and/or synthetic compounds to suppress, inhibit, or reverse the malignant transformation of oral epithelial dysplasia to OSCC.[13,14] While many promising chemopreventive agents have been evaluated in both local and systemic delivery clinical trials, only the local delivery studies have shown both chemopreventive efficacy and negligible dose-limiting side effects. Notably, the success of intraoral drug delivery strategies is governed by the effectiveness of the delivery vehicles (i.e., ability to provide effective drug localization and maximize patient compliance). Hence, numerous polymeric carriers, such as mucoadhesive gels, patches, tablets, rinses, sprays, and lozenges, have been studied for intraoral local delivery of oral medications.[13–22] Although many of these delivery strategies have been employed in oral cancer chemoprevention trials, most of these studies have failed to optimize drug delivery through the assessment of local pharmacokinetic parameters (e.g., determining oral intraepithelial drug concentrations, metabolite formation, and drug stability/release kinetics).[23–31] In addition, inconsistent enrollment criteria (e.g., the inclusion of patients with benign hyperkeratotic lesions and lack of tobacco cessation) complicate the comparative analysis of inter-trial chemopreventive efficacy. Therefore, the purpose of this review was to identify prominent oral cancer chemoprevention clinical trials, assess their overall therapeutic outcomes in those patients with histologically confirmed premalignant lesions (i.e., regression of oral epithelial dysplastic lesions), and evaluate the chemopreventive efficacy depending on the method of drug delivery (i.e., local versus systemic administration).
ORAL CANCER CHEMOPREVENTION CLINICAL TRIALS: SYSTEMIC DELIVERY
Previous oral cancer chemoprevention clinical trials that utilized systemic delivery systems have provided mixed results. The most prominent of these studies, which have evaluated the delivery of several classes of compounds, i) vitamin A derivatives, ii) cyclooxygenase 2 (COX-2) inhibitors, and iii) natural products, are reviewed [Table 1].
Table 1.
Systemic delivery oral cancer chemoprevention trials
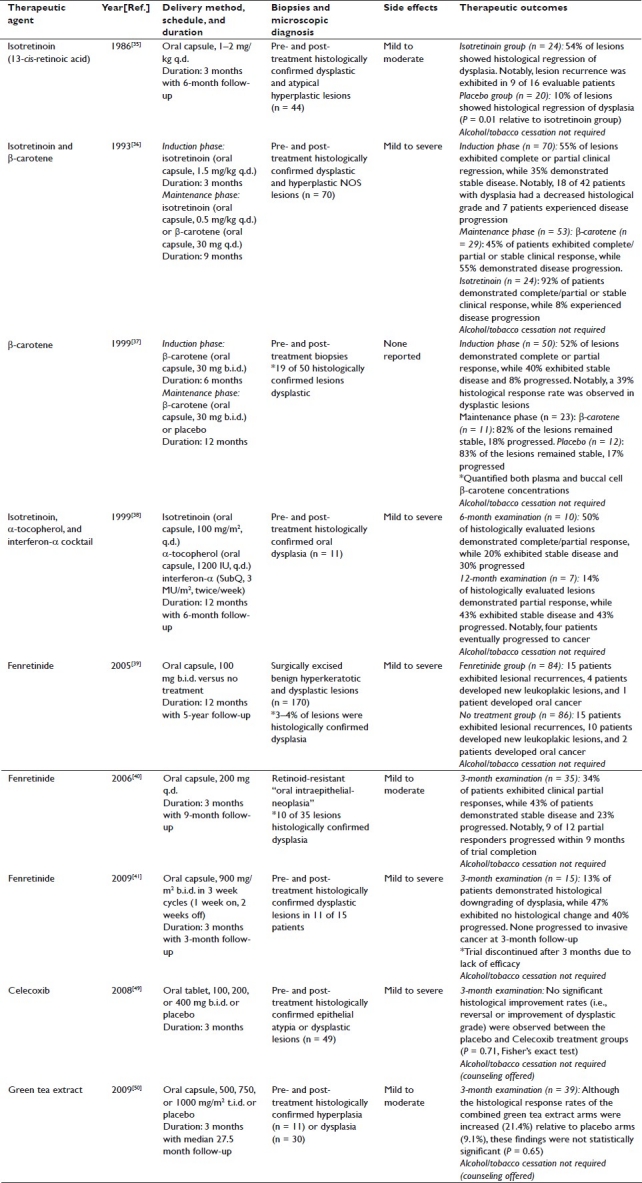
Vitamin A derivatives
Natural and synthetic vitamin A derivatives elicit the induction of terminal differentiation and apoptosis in epithelial cells, both promising therapeutic endpoints for oral cancer chemoprevention.[32–34] Therapeutic outcome with these derivatives is often dependent on the duration of treatment, concentration within the target site, and specific vitamin A compound under evaluation.[35–41] Notably, the vitamin A derivatives investigated in systemic delivery oral cancer chemoprevention trials include: 13-cis-retinoic acid (isotretinoin), β-carotene, an isotretinoin/α-tocopherol/interferon-α cocktail, and 4-hydroxyphenylretinamide (fenretinide).[35–41]
In 1986, Hong et al. evaluated the effects of isotretinoin administration (oral capsule, 1–2 mg/kg q.d.) on histologically confirmed oral dysplasia.[35] Although the trial results were promising (i.e., 54% of the treated lesions showed histological reversal of dysplasia relative to 10% in the placebo group), the favorable response rate of these lesions was offset by the wide range of adverse side effects encountered throughout the study [i.e., mild (cheilitis, skin dryness, facial erythema) to moderate (conjunctivitis and hypertriglyceridemia)].[35] In an effort to expand upon the promising therapeutic outcome of Hong et al.'s studies, Lippman et al. evaluated an introductory high-dose isotretinoin treatment (oral capsule, 1.5 mg/kg q.d. for 3 months) followed by maintenance doses of either isotretinoin (oral capsule, 0.5 mg/kg q.d.) or β-carotene (oral capsule, 30 mg q.d.) for 9 months.[36] The goal of this study was to preserve responsiveness while concurrently reducing side effects. Variable side effects, however, were encountered and the inclusion of both histologically confirmed dysplasia and hyperplastic not otherwise specified (NOS) lesions complicated the interpretation of results.[36] Unlike dysplastic lesions, reactive hyperplastic lesions do not have an established malignant transformation potential, and therefore, inclusion of these lesions introduced bias toward increased therapeutic efficacy.[42,43]
Similar data evaluation challenges were encountered in a later β-carotene study (oral capsule, 30 mg b.i.d. for 6 months, followed by randomization to placebo or 30 mg b.i.d. β-carotene for 12 months), which was conducted in 50 patients (only 19 presenting with histologically confirmed dysplastic lesions) who were permitted to continue tobacco and/or alcohol use.[37] Following 6 months of treatment, 4% of the participants’ lesions demonstrated complete clinical response while 48% exhibited partial clinical response (39% histological response rate in dysplastic lesions).[37] Although the indiscriminate enrollment of patients with non-dysplastic lesions and the lack of risk factor cessation weaken the study, the quantification of β-carotene levels in both plasma and buccal cells provides a basis for target tissue concentrations in future chemoprevention studies.[37]
Furthermore, in 1999, Papadimitrakopoulou et al. published the results of a 12-month study evaluating a chemoprevention cocktail designed to minimize the side effects commonly associated with vitamin A treatments [i.e., isotretinoin (oral capsule, 100 mg/m2 q.d.), α-tocopherol (oral capsule, 1200 IU q.d.), and interferon-α (subcutaneous injection, 3 MU/m2 twice weekly)].[38,44,45] Despite the inclusion of α-tocopherol, side effects ranging up to Grade 3 toxicities were observed.[38] In addition, both histologically confirmed dysplastic laryngeal and oral lesions were included in the trial. Whereas laryngeal lesions responded favorably, oral lesions were recalcitrant to the cocktail chemopreventive approach (i.e., only 14% of histologically evaluated oral lesions demonstrated a partial response at 12 months).[38] Importantly, oral cancer chemoprevention will likely require prolonged treatment regimens. These treatment considerations combined with the adverse side effects encountered in these studies warranted the evaluation of other prospective chemopreventive compounds.
Likewise, several oral cancer chemoprevention trials have evaluated the clinical efficacy of the synthetic retinoid fenretinide based on its reduced toxicity profile and retinoid receptor-dependent and -independent effects.[39–41] In 2005, the results were published from a long-term study that evaluated the effects of low-dose treatment (oral capsules, 100 mg b.i.d.) versus no treatment for 1 year on surgically resected oral lesions, which included both histologically confirmed benign hyperkeratotic and dysplastic lesions.[39] Although this study demonstrated that fenretinide treatment protected against recurrent lesions, the inclusion of benign lesions obfuscated the determination of actual chemopreventive efficacy.[39] An additional low-dose fenretinide study (oral capsules, 200 mg q.d. for 3 months) in non-excised retinoid-resistant histologically confirmed oral dysplastic lesions demonstrated the reduction of clinical size in 34% of the patients.[40] Subsequently, a study investigating the effects of high-dose fenretinide (oral capsules, 900 mg/m2 b.i.d. in 3 week cycles, with 1 week on, 2 weeks off) on histologically confirmed oral dysplastic lesions was discontinued after 12 weeks due to low lesional response rates.[41] Despite moderate, yet transient, treatment responses in the low-dose trials, all of these fenretinide studies encountered a range of mild to severe systemic side effects, some of which required trial discontinuation.[39–41] Notably, these studies failed to obtain the in vitro-established therapeutically relevant fenretinide levels of 1–10 μM in serum (i.e., high-dose treatment: 0.122 ± 0.093 μM, low-dose continuous treatment: 0.23 μM), and were undetermined by Chiesa et al.[39–41,46]
Collectively, the systemic delivery of vitamin A derivatives provided modest therapeutic efficacy and were accompanied by systemic toxicities.[35–41] With the exception of the β-carotene trial by Garewal et al., none of these vitamin A derivative studies evaluated oral intraepithelial compound levels, making it difficult to determine whether the negative results are due to the lack of compound efficacy or insufficient therapeutic levels within the target tissues.
COX-2 Inhibitors
COX-2 is often elevated in premalignant and malignant epithelial lesions, and is associated with suppression of apoptosis and production of reactive oxygen species, both of which are pro-tumorigenic.[47,48] Based on these well-established roles of COX-2, Papadimitrakopoulou et al. conducted a clinical trial evaluating the therapeutic effect of Celecoxib, a COX-2 inhibitor, in patients with histologically confirmed epithelial atypia or dysplasia.[49] Patients were assigned to placebo or Celecoxib groups (oral tablet, 100, 200, or 400 mg b.i.d. for 3 months), and pre- and post-treatment biopsies were obtained to evaluate lesional response.[49] Notably, none of the Celecoxib recipients demonstrated clinical or microscopic differences relative to placebo recipients.[49] Again, these negative results are difficult to decipher since oral intraepithelial Celecoxib levels were not determined, thus failing to demonstrate achieved therapeutic levels in the targeted epithelial lesions.
Natural products
Based on the extended duration of treatment likely required for oral cancer chemoprevention, natural products represent a promising group of compounds due to their decreased dose-limiting toxicity profiles. A recent clinical trial assessed the chemopreventive effects of green tea extract (oral capsules, 500, 750, 1000 mg/m2 t.i.d. for 3 months) relative to placebo in patients with both histologically confirmed premalignant dysplastic lesions and benign hyperplastic lesions.[50] Although a dose–dependent trend was associated with clinical responsiveness, no significant differences were observed with regard to lesional progression to OSCC.[50] Furthermore, the inclusion of benign lesions and failure to quantify intraepithelial levels of the bioactive compounds complicate the evaluation of therapeutic efficacy.
Systemic delivery summary
These collective systemic delivery oral cancer chem-oprevention trials have yielded modest results with regard to clinical efficacy (i.e., histological and clinical lesional regression). Moreover, the failure to quantify intraepithelial chemopreventive concentrations and the frequent inclusion of benign lesions raise concerns regarding the efficiency of drug delivery and relevancy of therapeutic outcomes. In addition, the likely need for sustained treatment regimens combined with the presence of mild to severe systemic toxicities negate the clinical utility of these chemopreventive compounds via systemic delivery methods, and accentuate the need for an alternative dosing scheme (e.g., local intraoral delivery).
ORAL CANCER CHEMOPREVENTION CLINICAL TRIALS: LOCAL DELIVERY
Previous oral cancer chemoprevention clinical trials that utilized local delivery strategies have evaluated the delivery of several classes of compounds/therapeutics [Table 2]: i) vitamin A derivatives, ii) adenoviruses, iii) cancer chemotherapy agents, iv) COX inhibitors, and v) natural products.[23–31,51,52–56]
Table 2.
Local delivery oral cancer chemoprevention trials
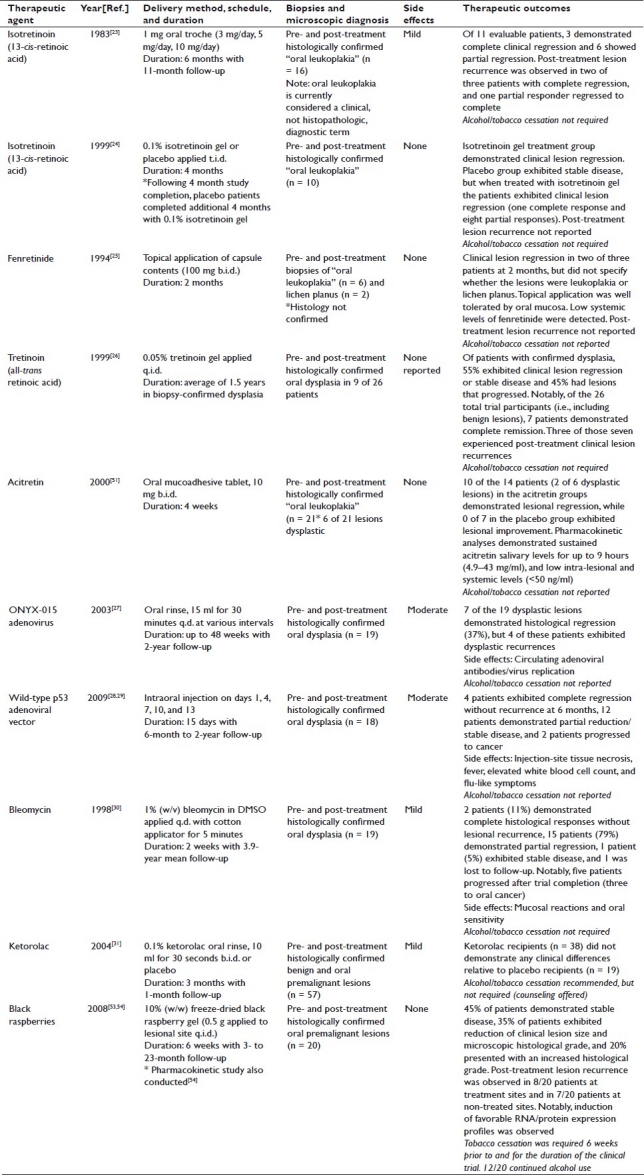
Vitamin A derivatives
Separate studies conducted in 1983 and 1999 evaluated the topical application of isotretinoin on oral leukoplakic lesions.[23,24] Notably, oral leukoplakia is currently considered a clinical, not histopathologic, diagnostic term. Therefore, the description of clinical lesions as “oral leukoplakia” does not differentiate between premalignant dysplastic lesions and nonmalignant reactive hyperplastic lesions. The first study in 16 patients evaluated three treatment groups (1 mg troche formulation; 3, 5, 10 mg/day for 6 months) and obtained both pre- and post-treatment biopsies.[23] Notably, of 11 evaluable patients, 3 exhibited complete clinical lesion regression, 6 had partial clinical regression, and none of the trial participants experienced “unacceptable” levels of toxicity.[23] The second study, by Piattelli et al., evaluated isotretinoin gel application (0.1% isotretinoin gel t.i.d. for 4 months) or placebo in 10 patients with “biopsy-proven oral leukoplakic lesions”.[24] Patients in the treatment group exhibited unspecified clinical lesional regression, while the placebo group demonstrated stable disease.[24] Following completion of the 4-month study, patients in the placebo group completed an additional 4-month study using the 0.1% isotretinoin gel and demonstrated clinical lesional regression.[24] The final results showed one complete clinical response and eight partial clinical responses with negligible side effects.[24]
In 1994, a study by Tradati et al. evaluated topical fenretinide application in patients with oral leukoplakia/lichen planus (100 mg b.i.d. for 2 months).[25] Although the method of topical application lacked a controlled drug delivery system (i.e., patients broke open and applied the contents of 100 mg capsules), this study demonstrated clinical regression of pre-malignant leukoplakic lesions and benign lichen planus, no adverse side effects, and minimal drug levels in serum.[25] Notably, due to the method of intraoral delivery and likely ingestion of the capsule contents, the presence of detectable serum levels is expected. Despite the positive outcome of this small pilot study, additional local fenretinide studies were not conducted.
In 1999, Epstein and Gorsky reported their findings from a trial assessing the effects of q.i.d. topical application of a 0.05% all-trans retinoic acid (tretinoin) gel on oral dysplastic lesions (histologically confirmed in 9 of 26 patients).[26] Of those patients with confirmed dysplasia, five exhibited clinical lesion regression or stable disease, while four had lesions that progressed, and toxic side effects were not observed.[26] Notably, the duration and frequency of gel application varied amongst the participants, some of whom continued to smoke over the duration of the trial.[26] Clearly, lack of smoking cessation adds a major confounding variable, which hampers the proper evaluation of the trial results.
A study conducted in 2000 by Gaeta et al. evaluated the chemopreventive efficacy of two acitretin mucoadhesive tablet formulations (10 mg b.i.d. for 4 weeks) relative to a placebo control in 21 patients with histologically confirmed “oral leukoplakia”.[51] While 10 of 14 patients in the treatment groups exhibited clinical lesion regression relative to 0 of 7 in the placebo group, only 2 of 6 patients with confirmed epithelial dysplasia demonstrated histological regression.[51] While this study demonstrated the sustainability of acitretin in saliva for 4–9 hours (formulation-dependent), a 1000-fold decrease of intra-lesional and serum levels was observed relative to salivary levels.[51] Although the sustainability in saliva is promising, delivery of therapeutically relevant levels of hydrophobic compounds (e.g., retinoids) to premalignant epithelial cells will likely require formulations containing solubility and permeability enhancers (e.g., surfactants) to facilitate compound stabilization and penetration into the oral epithelium.[55]
Adenovirus vectors
In contrast to compound-based chemoprevention through the induction of differentiation and/or apoptosis, two separate studies evaluated the therapeutic efficacy of adenovirus vector delivery for targeted cytotoxicity or gene therapy [i.e., adenovirus (ONYX-015)-mediated cytotoxicity toward cells with mutated p53 and an adenoviral vector used to insert wild-type p53].[27–29] The ONYX-015 studies, which used a mouthwash-mediated local delivery system (oral rinse, 30 minutes, 15 ml q.d. for various intervals up to 48 weeks), demonstrated histological regression of dysplastic lesions in 7 of 19 patients. The future clinical applications, however, may be limited due to the presence of circulating adenoviral antibody titers in one of the seven patients evaluated and actively replicating viruses in the oral mucosa in two of three patients.[27]
In contrast to the ONYX-015 mouthwash studies, Li et al. aimed to inject an adenoviral vector (injections on days 1, 4, 7, 10, and 13) to insert wild-type p53 into pre-malignant oral epithelial cells.[28,29] Results of this study demonstrated significant tissue necrosis at the injection site in 18 of 22 patients with significant side effects (e.g., circulating adenoviral antibodies, fever, elevated white blood cell counts, and flu-like symptoms).[28,29] Notably, this study did not include a vehicle control group, which hinders the ability to determine if the physical nature of the injection or the adenovirus itself was the cause of tissue necrosis.[28,29] Positive clinical outcomes, however, were noted (i.e., 22% complete clinical regression without recurrence at 6 months and 66% with partial clinical regression or stable disease).[28,29]
Cancer chemotherapy agents
Several oral cancer chemoprevention studies have evaluated the therapeutic efficacy of bleomycin, a potent DNA-damaging antibiotic.[30,57–62] The most recent of these topical studies, which evaluated bleomycin treatment [1% w/v in dimethylsulfoxide (DMSO) q.d. for 2 weeks] in patients with histologically confirmed oral dysplastic lesions, demonstrated histological responses in 89% of the patients evaluated.[30] Notably, 5 of the 19 evaluable patients demonstrated clinical lesion progression following treatment cessation (3 progressed to OSCCs).[30] In addition, this study did not assess systemic levels of bleomycin following topical application.[30] Given the known deleterious effects of bleomycin administration (i.e., pulmonary fibrosis), long-term treatment with this compound would likely be contraindicated.[63] In contrast, short-term inductive bleomycin therapy followed by long-term treatment with a less-toxic chemopreventive compound (e.g., natural products) could provide improved chemopreventive efficacy while minimizing the risk of potential deleterious systemic effects.
Cyclooxygenase inhibitors
Similar to the outcome of systemic COX-2 inhibitor studies, local application of the nonspecific COX inhibitor Ketorolac (oral rinse, 10 ml for 30 seconds b.i.d. for 3 months) exhibited comparable reductions in lesional sizes relative to placebo without eliciting any significant toxic side effects.[31] Notably, this study did not exclude benign lesions or require smoking cessation. In addition, although the authors speculated that Ketorolac was unable to penetrate the cornified epithelial layer to reach the proliferating basal cells, they did not determine the intraepithelial Ketorolac concentration.[31]
Natural products
Black raspberry anthocyanins are potent antioxidants capable of suppressing pro-tumorigenic activation pathways (e.g., quenching reactive oxygen species-mediated signal transduction, suppressing angiogenesis, and inhibiting oxidant-responsive proinflammatory enzymes).[64–72] Due to these established effects in vitro and in vivo, our laboratories recently conducted Phase I/II oral cancer chemoprevention studies to evaluate the safety and chemopreventive efficacy of a 10% (w/w) freeze-dried black raspberry (BRB) bioadhesive gel in normal volunteers (Phase I) and in histologically confirmed premalignant oral epithelial lesions (Phase II).[53,54] Phase I studies in 10 healthy volunteers (0.5 g applied to normal mucosa q.i.d. for 6 weeks) demonstrated gel tolerability through the absence of any deleterious side effects.[53,54] Phase II studies were conducted in 20 patients (0.5 g applied to lesional site q.i.d. for 6 weeks) with oral premalignant lesions and demonstrated a significant decrease in loss of heterozygosity indices at key tumor suppressor gene loci, decreased expression of genes associated with recycling of growth factors, apoptosis inhibition, and RNA processing, and significantly reduced levels of COX-2 protein in lesional epithelium.[53,54] Notably, 45% of trial participants exhibited stable disease and 35% demonstrated a reduction of both gross lesional size and microscopic histological grade, decreased microvascular density in underlying connective tissues, and increased expression of genes associated with epithelial terminal differentiation.[53] Due to the pre- and post-treatment biopsies of confirmed premalignant lesions and required tobacco cessation, this trial facilitates critical analysis of the findings.
Interestingly, these data revealed a cohort of high-level responders, which did not correlate with a lower grade oral dysplastic lesion at the onset of treatment (i.e., lesional regression was seen in persons with moderate to higher epithelial dysplasia).[53] The basis for this variation in lesional responsiveness was speculated to reflect inter-patient variations in local pharmacokinetics including penetration, bioactivation, and sustainability of the BRB chemopreventives. Subsequent pharmacokinetic analyses in healthy volunteers (0.5 g gel applied sublingually or to retromolar pad) assessed the distribution of four main black raspberry constituents (i.e., anthocyanin metabolites) in saliva, oral mucosal tissue, and plasma.[56] Although considerable inter-patient variations of absorption and retention in saliva and oral tissue were observed, oral chemopreventive levels were significantly greater than their corresponding plasma concentrations in all participants.[56] These findings demonstrate the pharmacologic advantage provided by local delivery formulations (i.e., successful achievement of therapeutically relevant levels within the target tissue while minimizing systemic exposure).[11,12,56]
Furthermore, ongoing studies in our lab have demonstrated highly variable anthocyanin-relevant metabolic enzyme profiles (i.e., hydrolytic and Phase I/II enzymes and ATP binding cassette transporters) in 15 normal human oral mucosal samples.[73] In addition, these studies also showed participant-specific contribution of oral microflora and salivary proteins to the local recycling of anthocyanins, which may effectively increase oral epithelial exposure to the chemopreventive compounds.[73] Ultimately, these pharmacokinetic and metabolic profiling data can be used to guide future chemoprevention studies through the use of permeability enhancers and/or bioactive metabolites that are not dependent on individual metabolic profiles for bioactivation.[73] This approach would, therefore, de-emphasize metabolism-related pharmacogenomic contributions to therapeutic efficacy.
Collectively, these data formed the foundation for an ongoing National Cancer Institute supported multicenter oral cancer chemoprevention trial (NCT01192204) evaluating the effects of the 10% BRB gel in histopathologically confirmed premalignant oral lesions. This trial, conducted under the auspices of a Food and Drug Administration (FDA)-approved Investigational New Drug, is partnered with investigators at the University of Louisville and the University of North Carolina at Chapel Hill and will extend the treatment time from 6 weeks (Phase I/II trial) to 12 weeks. Notably, Phase I/II evaluative parameters (i.e., microscopic histological grading of oral premalignant lesions pre- and post-treatment, loss of heterozygosity analysis, alteration of gene expression, COX-2 and inducible nitric oxide synthase levels, and angiogenesis) and additional parameters have been included to guide post-trial assessment of therapeutic efficacy (e.g., pre-treatment oral metabolic profiling of anthocyanin-relevant bioactivation enzymes, determination of promoter DNA methylation level, and the inclusion of an investigator- and patient-blinded placebo gel cohort).
Local delivery summary
Taken together, these local delivery oral cancer chem-oprevention trials have demonstrated a pharmacologic advantage over systemic delivery trials. Although adenovirus trials resulted in significant side effects, local delivery of chemopreventive compounds has been well tolerated.[23–26,30,31,53–56] In addition, to facilitate the reliable evaluation of future oral cancer chemoprevention trials, adoption of the following study parameters is recommended: exclusive enrollment of patients with histopathologically confirmed premalignant oral epithelial lesions, alcohol/tobacco cessation, pre- and post-treatment microscopic histological grading, minimum of 12-week study duration (allows repopulation of surface epithelium since turnover occurs approximately every 28 days, and thus negates the “biopsy effect”, i.e., clearing of a dysplastic lesion through frequent biopsies), determination of intraepithelial chemopreventive compound levels, and correlation of local metabolism to therapeutic efficacy.
FUTURE DIRECTIONS AND DISCUSSION
The success of intraoral local drug delivery strategies is mainly dependent on the ability of polymeric carriers to provide: i) increased apparent solubility and stability in physiological fluids, e.g., saliva, ii) appropriate rate of drug release for an optimized effect, iii) facilitated penetration and local distribution of chemopreventive compounds, and iv) flexibility to allow controlled drug delivery to various oral mucosal sites. Furthermore, due to the risk factors associated with oral cancer (i.e., tobacco and/or alcohol use), the entire oral mucosa is hypothesized to have undergone field cancerization.[74] Therefore, optimal therapeutic efficacy for oral cancer chemoprevention would likely entail both lesion-specific (topical agent) and field coverage (rinse) components. Hence, our laboratories have developed optimal intraoral drug delivery strategies for numerous chemopreventive agents by manipulating the properties of drugs (e.g., improving the apparent solubility, stability, and tissue distribution of drugs), utilizing polymeric carriers (e.g., developing mucoadhesive gels and patches, millicylindrical implants for long-term delivery, and nanoparticles), and using various delivery approaches (e.g., short- and sustained-duration drug release formulations).[13,55,75–78]
Lesion-specific, targeted therapies are currently under evaluation in our labs, and include strategies for the delivery of black raspberry extract, fenretinide, and the matrix metalloproteinase inhibitor, N-acetylcysteine.[75–77] In addition to the 10% BRB gel currently under Phase III evaluation, sustained release poly(dl-lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA) implant formulations have been developed for the sustained delivery of black raspberry anthocyanins (PLA), fenretinide (PLGA), and N-acetylcysteine (PLGA).[13,75–77] Notably, these injectable implants demonstrated sustained release for 4–5 weeks in vitro (black raspberry extract, N-acetylcysteine Ca2+ and Mg2+ salts, and fenretinide) and 4 weeks in vivo (black raspberry extract), thus providing a potential local chemoprevention delivery method independent of daily patient compliance.[75–77] Furthermore, preliminary in vitro and in vivo studies on the development and evaluation of a novel mucoadhesive fenretinide patch demonstrate both burst and sustained release patterns imparting therapeutically relevant levels in rabbit oral mucosa.[55] In addition, our lab has demonstrated the feasibility of patch-mediated nanoparticle delivery to the basal epithelial cells and underlying connective tissue of human oral mucosal explants.[78] This nanoparticle study provides yet another mechanism for drug stabilization and subsequent local delivery to the oral epithelium.
Recent studies in our labs have also demonstrated long-term sustainability of a black raspberry oral rinse formulation designed to provide a field coverage effect.[73] Notably, rinse administration exhibited greater sustained salivary levels of anthocyanins relative to corresponding levels in pharmacokinetic studies of the 10% BRB gel.[56,73]
Based on the collective results of the local delivery chemoprevention trials, which demonstrated a pharmacologic advantage over systemic strategies by minimizing systemic toxicities while obtaining therapeutically relevant local levels, these local intraoral delivery strategies developed in our laboratories warrant further evaluation for clinical efficacy in oral cancer chemoprevention. In addition, future oral cancer chemoprevention trials should focus on similar local delivery strategies and utilize the recommended study design parameters outlined within this review.
DECLARATION OF COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the writing and revision of this review paper.
AUTHOR’S PROFILE
Mr. Andrew S. Holpuch is a D.D.S./Ph.D. graduate fellow at The Ohio State University College of Dentistry
Dr. Kashappa-Goud H. Desai, Ph.D. is an Assistant Research Scientist in the Department of Pharmaceutical Sciences at the University of Michigan
Dr. Steven P. Schwendeman, Ph.D. is the Ara G. Paul Professor and Chair of Pharmaceutical Sciences at the University of Michigan
Dr. Susan R. Mallery, D.D.S., Ph.D. is a Professor in the Division of Oral and Maxillofacial Surgery, Pathology, and Anesthesiology at The Ohio State University College of Dentistry
ACKNOWLEDGMENTS
The ongoing studies in our laboratories are funded by the Fanconi Anemia Research Fund and NIH grants: (R01 CA129609, RC2 CA148099, R21 CA132138 to Susan R. Mallery) (R01 HL68345 to Steven P. Schwendeman) (F30 DE020992 and T32 DE14320 to Andrew S. Holpuch).
REFERENCES
- 1.Deeken JF, Slack R, Marshall JL. Irinotecan and uridine diphosphate glucuronosyltransferase 1A1 pharmacogenetics: to test or not to test, that is the question. Cancer. 2008;113:1502–10. doi: 10.1002/cncr.23777. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 3.Baban DF, Seymour LW. Control of tumour vascular permeability. Adv Drug Deliv Rev. 1998;34:109–19. doi: 10.1016/s0169-409x(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 4.Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release. 2001;74:47–61. doi: 10.1016/s0168-3659(01)00309-1. [DOI] [PubMed] [Google Scholar]
- 5.Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–71. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 6.Denny WA. The role of hypoxia-activated prodrugs in cancer therapy. Lancet Oncol. 2000;1:25–9. doi: 10.1016/S1470-2045(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 7.Abdollahi A, Folkman J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist Updat. 2010;13:16–28. doi: 10.1016/j.drup.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Murray N, Turrisi AT., 3rd A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270–8. doi: 10.1016/s1556-0864(15)31579-3. [DOI] [PubMed] [Google Scholar]
- 9.Scagliotti G, Govindan R. Targeting angiogenesis with multitargeted tyrosine kinase inhibitors in the treatment of non-small cell lung cancer. Oncologist. 2010;15:436–46. doi: 10.1634/theoncologist.2009-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen YC, Hsu C, Cheng AL. Molecular targeted therapy for advanced hepatocellular carcinoma: current status and future perspectives. J Gastroenterol. 2010;45:794–807. doi: 10.1007/s00535-010-0270-0. [DOI] [PubMed] [Google Scholar]
- 11.Shen Z, Shen T, Wientjes MG, O’Donnell MA, Au JL. Intravesical treatments of bladder cancer: review. Pharm Res. 2008;25:1500–10. doi: 10.1007/s11095-008-9566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sood S, Shiff SJ, Yang CS, Chen X. Selection of topically applied non-steroidal anti-inflammatory drugs for oral cancer chemoprevention. Oral Oncol. 2005;41:562–7. doi: 10.1016/j.oraloncology.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Mallery SR, Stoner GD, Larsen PE, Fields HW, Rodrigo KA, Schwartz SJ, et al. Formulation and in-vitro and in-vivo evaluation of a mucoadhesive gel containing freeze-dried black raspberries: implications for oral cancer chemoprevention. Pharm Res. 2007;24:728–37. doi: 10.1007/s11095-006-9192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35:1322–28. [PubMed] [Google Scholar]
- 15.Senel S, Ikinci G, Kaş S, Yousefi-Rad A, Sargon MF, Hincal AA. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int J Pharm. 2000;193:197–203. doi: 10.1016/s0378-5173(99)00334-8. [DOI] [PubMed] [Google Scholar]
- 16.Hersh EV, DeRossi SS, Ciarrocca KN, Secreto SA, Ghassemi A. Efficacy and tolerability of an intraoral benzocaine patch in the relief of spontaneous toothache pain. J Clin Dent. 2003;14:1–6. [PubMed] [Google Scholar]
- 17.Perugini P, Genta I, Conti B, Modena T, Pavanetto F. Periodontal delivery of ipriflavone: new chitosan/PLGA film delivery system for a lipophilic drug. Int J Pharm. 2003;252:1–9. doi: 10.1016/s0378-5173(02)00602-6. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki S, Nakayama A, Oda M, Takada M, Attwood D. Chitosan and sodium alginate based bioadhesive tablets for intraoral drug delivery. Biol Pharm Bull. 1994;17:745–7. doi: 10.1248/bpb.17.745. [DOI] [PubMed] [Google Scholar]
- 19.Gunsolley JC. Clinical efficacy of antimicrobial mouthrinses. J Dent. 2010;38(Suppl 1):S6–10. doi: 10.1016/S0300-5712(10)70004-X. [DOI] [PubMed] [Google Scholar]
- 20.Paschos E, Huth KC, Benz C, Reeka-Bardschmidt A, Hickel R. Efficacy of intraoral topical anesthetics in children. J Dent. 2006;34:398–404. doi: 10.1016/j.jdent.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Gravenmade EJ, Vissink A. Mucin-containing lozenges in the treatment of intraoral problems associated with Sjogren's syndrome.A double-blind crossover study in 42 patients. Oral Surg Oral Med Oral Pathol. 1993;75:466–71. doi: 10.1016/0030-4220(93)90172-z. [DOI] [PubMed] [Google Scholar]
- 22.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery – a promising option for orally less efficient drugs. J Control Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Shah JP, Strong EW, DeCosse JJ, Itri L, Sellers P. Effect of retinoids on oral leukoplakia. Am J Surg. 1983;146:466–70. doi: 10.1016/0002-9610(83)90232-5. [DOI] [PubMed] [Google Scholar]
- 24.Piattelli A, Fioroni M, Santinelli A, Rubini C. bcl-2 expression and apoptotic bodies in 13-cis-retinoic acid (isotretinoin)-topically treated oral leukoplakia: a pilot study. Oral Oncol. 1999;35:314–20. doi: 10.1016/s1368-8375(98)00095-5. [DOI] [PubMed] [Google Scholar]
- 25.Tradati N, Chiesa F, Rossi N, Grigolato R, Formelli F, Costa A, et al. Successful topical treatment of oral lichen planus and leukoplakias with fenretinide (4-HPR) Cancer Lett. 1994;76:109–11. doi: 10.1016/0304-3835(94)90385-9. [DOI] [PubMed] [Google Scholar]
- 26.Epstein JB, Gorsky M. Topical application of vitamin A to oral leukoplakia: A clinical case series. Cancer. 1999;86:921–7. doi: 10.1002/(sici)1097-0142(19990915)86:6<921::aid-cncr5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Rudin CM, Cohen EE, Papadimitrakopoulou VA, Silverman S, Jr, Recant W, El-Naggar AK, et al. An attenuated adenovirus, ONYX-015, as mouthwash therapy for premalignant oral dysplasia. J Clin Oncol. 2003;21:4546–52. doi: 10.1200/JCO.2003.03.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Li LJ, Zhang ST, Wang LJ, Zhang Z, Gao N, et al. In vitro and clinical studies of gene therapy with recombinant adenovirus-p53 injection for oral leukoplakia. Clin Cancer Res. 2009;15:6724–31. doi: 10.1158/1078-0432.CCR-09-1296. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, Li Y, Li L, Zhang Y, Gao N, Zhang Z, et al. Phase I study of repeated intraepithelial delivery of adenoviral p53 in patients with dysplastic oral leukoplakia. J Oral Maxillofac Surg. 2009;67:1074–82. doi: 10.1016/j.joms.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 30.Epstein JB, Gorsky M, Wong FL, Millner A. Topical bleomycin for the treatment of dysplastic oral leukoplakia. Cancer. 1998;83:629–34. doi: 10.1002/(sici)1097-0142(19980815)83:4<629::aid-cncr1>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 31.Mulshine JL, Atkinson JC, Greer RO, Papadimitrakopoulou VA, Van Waes C, Rudy S, et al. Randomized, double-blind, placebo-controlled phase IIb trial of the cyclooxygenase inhibitor ketorolac as an oral rinse in oropharyngeal leukoplakia. Clin Cancer Res. 2004;10:1565–73. doi: 10.1158/1078-0432.ccr-1020-3. [DOI] [PubMed] [Google Scholar]
- 32.Freemantle SJ, Spinella MJ, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003;22:7305–15. doi: 10.1038/sj.onc.1206936. [DOI] [PubMed] [Google Scholar]
- 33.Smith W, Saba N. Retinoids as chemoprevention for head and neck cancer: where do we go from here? Crit Rev Oncol Hematol. 2005;55:143–52. doi: 10.1016/j.critrevonc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Hail N, Jr, Kim HJ, Lotan R. Mechanisms of fenretinide-induced apoptosis. Apoptosis. 2006;11:1677–94. doi: 10.1007/s10495-006-9289-3. [DOI] [PubMed] [Google Scholar]
- 35.Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–5. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 36.Lippman SM, Batsakis JG, Toth BB, Weber RS, Lee JJ, Martin JW, et al. Comparison of low-dose isotretinoin with beta-carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 37.Garewal HS, Katz RV, Meyskens F, Pitcock J, Morse D, Friedman S, et al. Beta-carotene produces sustained remissions in patients with oral leukoplakia: results of a multicenter prospective trial. Arch Otolaryngol Head Neck Surg. 1999;125:1305–10. doi: 10.1001/archotol.125.12.1305. [DOI] [PubMed] [Google Scholar]
- 38.Papadimitrakopoulou VA, Clayman GL, Shin DM, Myers JN, Gillenwater AM, Goepfert H, et al. Biochemoprevention for dysplastic lesions of the upper aerodigestive tract. Arch Otolaryngol Head Neck Surg. 1999;125:1083–9. doi: 10.1001/archotol.125.10.1083. [DOI] [PubMed] [Google Scholar]
- 39.Chiesa F, Tradati N, Grigolato R, Boracchi P, Biganzoli E, Crose N, et al. Randomized trial of fenretinide (4-HPR) to prevent recurrences, new localizations and carcinomas in patients operated on for oral leukoplakia: long-term results. Int J Cancer. 2005;115:625–9. doi: 10.1002/ijc.20923. [DOI] [PubMed] [Google Scholar]
- 40.Lippman SM, Lee JJ, Martin JW, El-Naggar AK, Xu X, Shin DM, et al. Fenretinide activity in retinoid-resistant oral leukoplakia. Clin Cancer Res. 2006;12:3109–14. doi: 10.1158/1078-0432.CCR-05-2636. [DOI] [PubMed] [Google Scholar]
- 41.William WN, Jr, Lee JJ, Lippman SM, Martin JW, Chakravarti N, Tran HT, et al. High-dose fenretinide in oral leukoplakia. Cancer Prev Res (Phila) 2009;2:22–6. doi: 10.1158/1940-6207.CAPR-08-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silverman S, Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation.A follow-up study of 257 patients. Cancer. 1984;53:563–8. doi: 10.1002/1097-0142(19840201)53:3<563::aid-cncr2820530332>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 43.Scuibba JJ. Oral leukoplakia. Crit Rev Oral Biol Med. 1995;6:147–60. doi: 10.1177/10454411950060020401. [DOI] [PubMed] [Google Scholar]
- 44.Dimery IW, Hong WK, Lee JJ, Guillory-Perez C, Pham F, Fritsche HA, Jr, et al. Phase I trial of alpha-tocopherol effects on 13-cis-retinoic acid toxicity. Ann Oncol. 1997;8:85–9. doi: 10.1023/a:1008209525671. [DOI] [PubMed] [Google Scholar]
- 45.Besa EC, Abrahm JL, Bartholomew MJ, Hyzinski M, Nowell PC. Treatment with 13-cis-retinoic acid in transfusion-dependent patients with myelodysplastic syndrome and decreased toxicity with addition of alpha-tocopherol. Am J Med. 1990;89:739–47. doi: 10.1016/0002-9343(90)90215-y. [DOI] [PubMed] [Google Scholar]
- 46.Clifford JL, Menter DG, Wang M, Lotan R, Lippman SM. Retinoid receptor-dependent and -independent effects of N-(4-hydroxyphenyl)retinamide in F9 embryonal carcinoma cells. Cancer Res. 1999;59:14–8. [PubMed] [Google Scholar]
- 47.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365–72. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 48.Prescott SM, Fitzpatrick FA. Cyclooxygenase-2 and carcinogenesis. Biochim Biophys Acta. 2000;1470:M69–78. doi: 10.1016/s0304-419x(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 49.Papadimitrakopoulou VA, William WN, Jr, Dannenberg AJ, Lippman SM, Lee JJ, Ondrey FG, et al. Pilot randomized phase II study of celecoxib in oral premalignant lesions. Clin Cancer Res. 2008;14:2095–101. doi: 10.1158/1078-0432.CCR-07-4024. [DOI] [PubMed] [Google Scholar]
- 50.Tsao AS, Liu D, Martin J, Tang XM, Lee JJ, El-Naggar AK, et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev Res (Phila) 2009;2:931–41. doi: 10.1158/1940-6207.CAPR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaeta GM, Gombos F, Femiano F, Battista C, Minghetti P, Montanari L, et al. Acitretin and treatment of oral leucoplakias.A model to have an active molecules release. J Eur Acad Dermatol Venereol. 2000;14:473–8. doi: 10.1046/j.1468-3083.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- 52.Holpuch AS, Desai KG, Phelps MP, Han B, Schwendeman SP, Mallery SR. Evaluation of a fenretinide mucoadhesive patch for local intraoral delivery [abstract] [Last accessed on 2011 Apr 5];J Dent Res. 2011 90:146204. Available from: http//www.dentalresearch.org . [Google Scholar]
- 53.Mallery SR, Zwick JC, Pei P, Tong M, Larsen PE, Shumway BS, et al. Topical application of bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Res. 2008;68:4945–57. doi: 10.1158/0008-5472.CAN-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shumway BS, Kresty LA, Larsen PE, Zwick JC, Lu B, Fields HW, et al. Effects of topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin Cancer Res. 2008;14:2421–30. doi: 10.1158/1078-0432.CCR-07-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desai KG, Mallery SR, Holpuch AS, Schwendeman SP. Development and In Vitro-In Vivo Evaluation of Fenretinide-Loaded Oral Mucoadhesive Patches for Site-Specific Chemoprevention of Oral Cancer. Pharm Res. 2011 doi: 10.1007/s11095-011-0489-3. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ugalde CM, Liu Z, Ren C, Chan KK, Rodrigo KA, Ling Y, et al. Distribution of anthocyanins delivered from a bioadhesive black raspberry gel following topical intraoral application in normal healthy volunteers. harm Res. 2009;26:977–86. doi: 10.1007/s11095-008-9806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayasaki K, Kitamura T, Kaneko T, Tachibana M, Kobayashi N, Tosaka K, et al. Application of bleomycin-iontophoresis for tumour therapy of the head and neck area. Nihon Gan Chiryo Gakkai Shi. 1977;12:522–7. [PubMed] [Google Scholar]
- 58.Hisano Y, Satoh T, Suzuki M, Kanai Y. An effective case of local injection therapy of oral leukoplakia with bleomycin. Shigaku. 1978;66:125–8. [PubMed] [Google Scholar]
- 59.Hammersley N, Ferguson MM, Rennie JS. Topical bleomycin in the treatment of oral leukoplakia: a pilot study. Br J Oral Maxillofac Surg. 1985;23:251–8. [PubMed] [Google Scholar]
- 60.Malmström M, Hietanen J, Sane J, Sysmäläinen M. Topical treatment of oral leukoplakia with bleomycin. Br J Oral Maxillofac Surg. 1988;26:491–98. doi: 10.1016/0266-4356(88)90071-x. [DOI] [PubMed] [Google Scholar]
- 61.Wong F, Epstein J, Millner A. Treatment of oral leukoplakia with topical bleomycin: a pilot study. Cancer. 1989;64:361–5. doi: 10.1002/1097-0142(19890715)64:2<361::aid-cncr2820640203>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 62.Epstein JB, Wong FL, Millner A, Le ND. Topical bleomycin treatment of oral leukoplakia: a randomized double-blind clinical trial. Head Neck. 1994;16:539–44. doi: 10.1002/hed.2880160607. [DOI] [PubMed] [Google Scholar]
- 63.De Lena M, Guzzon A, Monfardini S, Bonadonna G. Clinical, radiologic, and histopathologic studies on pulmonary toxicity induced by treatment with bleomycin (NSC-125066) Cancer Chemother Rep. 1972;56:343–56. [PubMed] [Google Scholar]
- 64.Huang C, Huang Y, Li J, Hu W, Aziz R, Tang MS, et al. Inhibition of benzo(a)pyrene diol-epoxide-induced transactivation of activated protein 1 and nuclear factor ĸB by black raspberry extracts. Cancer Res. 2002;62:6857–63. [PubMed] [Google Scholar]
- 65.Huang C, Li J, Song L, Zhang D, Tong Q, Ding M, et al. Black raspberry extracts inhibit benzo(a)pyrene diol-epoxide-induced activator protein 1 activation and VEGF transcription by targeting the phosphotidylinositol 3-kinase/Akt pathway. Cancer Res. 2006;66:581–7. doi: 10.1158/0008-5472.CAN-05-1951. [DOI] [PubMed] [Google Scholar]
- 66.Hecht SS, Huang C, Stoner GD, Li J, Kenney PM, Sturla SJ, et al. Identification of cyanidin glycosides as constituents of freeze-dried black raspberries which inhibit anti-benzo[a]pyrene-7,8-diol-9,10-epoxide induced NFkappaB and AP-1 activity. Carcinogenesis. 2006;27:1617–26. doi: 10.1093/carcin/bgi366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang LS, Hecht SS, Carmella SG, Yu N, Larue B, Henry C, et al. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res (Phila) 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodrigo KA, Rawal Y, Renner RJ, Schwartz SJ, Tian Q, Larsen PE, et al. Suppression of the tumorigenic phenotype in human oral squamous cell carcinoma cells by an ethanol extract derived from freeze-dried black raspberries. Nutr Cancer. 2006;54:58–68. doi: 10.1207/s15327914nc5401_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen T, Rose ME, Hwang H, Nines RG, Stoner GD. Black raspberries inhibit N-nitrosomethylbenzylamine (NMBA)-induced angiogenesis in rat esophagus parallel to the suppression of COX-2 and iNOS. Carcinogenesis. 2006;27:2301–7. doi: 10.1093/carcin/bgl109. [DOI] [PubMed] [Google Scholar]
- 70.Chen T, Hwang H, Rose ME, Nines RG, Stoner GD. Chemopreventive properties of black raspberries in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis: down-regulation of cyclooxygenase 2, inducible nitric oxide synthase, and c-Jun. Cancer Res. 2006;66:2853–9. doi: 10.1158/0008-5472.CAN-05-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, Gupta A, et al. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61:6112–9. [PubMed] [Google Scholar]
- 72.Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29:1665–74. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mallery SR, Budendorf DE, Larsen MP, Pei P, Tong M, Holpuch AS, et al. Effects of human oral mucosal tissue, saliva and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev Res (Phila) 2011;4:1209–21. doi: 10.1158/1940-6207.CAPR-11-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium.Clinical Implications of Multicentric Origin. Cancer. 1953;6:963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 75.Desai KG, Olsen KF, Mallery SR, Stoner GD, Schwendeman SP. Formulation and in vitro-in vivo evaluation of black raspberry extract-loaded PLGA/PLA injectable millicylindrical implants for sustained delivery of chemopreventive anthocyanins. Pharm Res. 2010;27:628–43. doi: 10.1007/s11095-009-0038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wischke C, Zhang Y, Mittal S, Schwendeman SP. Development of PLGA-based injectable delivery systems for hydrophobic fenretini. Pharm Res. 2010;27:2063–74. doi: 10.1007/s11095-010-0202-y. [DOI] [PubMed] [Google Scholar]
- 77.Desai KG, Mallery SR, Schwendeman SP. Formulation and characterization of injectable Poly(DL-lactide-co-glycolide) implants loaded with N-acetylcysteine, a MMP inhibitor. Pharm Res. 2008;25:586–97. doi: 10.1007/s11095-007-9430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holpuch AS, Hummel GJ, Tong M, Seghi GA, Pei P, Ma P, et al. Nanoparticles for local delivery to the oral mucosa: proof of principle studies. Pharm Res. 2010;27:1224–36. doi: 10.1007/s11095-010-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


