Abstract
TAM family of receptors (Tyro3, Axl, and Mertk) plays an important role in the negative regulation of response of DCs and macrophages to pathogenic stimuli and mice lacking this receptor family develop spontaneous lupus-like systemic autoimmunity against a variety of tissues, including retina. To study the molecular mechanism underlying the TAM regulation of APC functions and subsequent effects on the induction of an autoimmune response against the eye, we examined CD4 T cell differentiation following retinal self-antigen immunization. CD4 T cells prepared from naïve or interphotoreceptor retinoid-binding protein (IRBP)1-20-immunized Axl and Mertk double knockout (dko) mice reacted to activation using anti-CD3 and anti-CD28 antibodies or to bolster by self-antigen in vitro with a predominantly Th1 effector response, as characterized by increased IFN-γ production and higher frequency of IFN-γ-positive CD4 T cells. The Th17 effector response to IRBP immunization was similar in dko mice to that in WT controls, as shown by ELISA measurement of IL-17A in the culture medium and flow cytometric analysis of IL-17A-secreting CD4 T cells. Interestingly, APCs or DCs isolated from IRBP-immunized dko mice exhibited a greater ability to drive the Th1 response. The production of two driving cytokines for Th1 differentiation, IL-12 and IL-18, was dramatically increased in dko DCs and macrophages, and LPS stimulation bolstered their production. The preferential development into the Th1 subset in dko mice suggests that the cytokine milieu produced by the mutant mice in vivo or by mutant APCs in vitro selectively creates a differentiation environment favoring the Th1 effector response.
Introduction
Professional antigen-presenting cells (APCs), including dendritic cells (DCs), macrophages, and B cells, are able to sense pathogens and endogenous antigens and play critical roles in initiating and regulating immune responses (1, 2). When they encounter pathogens or other stimuli, APCs undergo maturation leading to proinflammatory cytokine secretion and the expression of MHC and costimulatory molecules on the cell surface (2). These mature APCs are able to present antigens to T cells, leading to T cell activation (3–5). The magnitude and fate of an antigen-specific T cell response are determined by the interaction of the CD4+ T cell receptor with the antigen presented by MHC II molecules and the extent and nature of local cytokines.
On encountering cognate antigens presented by APCs, such as DCs, naïve CD4 T cells differentiate into several effector subsets, including Th1, Th2, Th17 and regulatory T cells (Treg), characterized by the production of distinct cytokines and effector functions (6–10). Th1 cells produce interferon (IFN)-γ and lymphotoxin (LT), which are responsible for immunity against intracellular pathogens, and other Th1 cytokines that are responsible for autoimmune responses. Th2 cells, producing interleukin (IL)-4, IL-5, IL-13, and IL-25, are essential for the generation of appropriate classes of antibodies and play critical roles in asthma and other allergic diseases. Th17 cells are characterized by the production of IL-17 and other cytokines primarily acting against extracellular pathogens and are associated with the pathogenesis of several organ-specific autoimmune diseases (11–13). The Treg CD4 T cell subset expresses CD25 on the cell surface and the intracellular transcription factor Foxp3 (14, 15) and acts as an inhibitory cell type by releasing inhibitory cytokines, e.g., IL-10 and tumor growth factor (TGF)-β, and plays a critical role in T-cell-dependent peripheral tolerance (16–19). Developmental or functional anomalies, or alteration in the number, of Treg cells have been linked to several chronic inflammatory and autoimmune diseases, such as multiple sclerosis (20), rheumatoid arthritis (21), and systemic lupus erythematosus (22).
The cytokine milieu plays an important role in T cell polarization, and different combinations of the surrounding cytokines induce specific transcriptional factors that control T cell differentiation. For example, during Th1 cell differentiation, IFN-γ causes induction of T-bet, a master regulator of Th1 cell differentiation that promotes Th1 polarization (23, 24). For Th2 cell differentiation, activation of Stat6 is necessary and sufficient to transduce IL-4 signaling (25).The differentiation of the Th17 cell is driven and stabilized by IL-6, TGF-β, IL-21, and IL-23, and the transcription factors STAT3 and RORγt are essential for the initial differentiation of Th17 cells (26, 27).
APCs affect T cell polarization by secreting specific cytokines, a notable example of which is IL-12, which selectively enhances Th1 cell growth by induction of IFN-γ production through activation of Stat4 (28). IL-18, originally known as IFN-γ-inducing factor, also provides an important accelerating and amplifying signal for Th1 proliferation and IFN-γ production (29). IL-12 and IL-18 act synergistically to drive Th1 activation (30–33) and are implicated in the pathogenesis of arthritis (34). Elevated levels of IL-18 and IL-12 are often correlated with the severity of autoimmune pathologies in experimental models and in clinical situations (33). Excessive production of IL-18 is seen in the blood of patients with rheumatoid arthritis (34, 35), lupus nephritis (36), and systemic lupus erythematosus (22, 37, 38).
Experimental autoimmune uveitis (EAU), an animal model for several human ocular autoimmune disorders (39, 40), can be elicited by immunization with retinal antigens in complete Freund's adjuvant (CFA), adoptive transfer of retinal autoantigen-specific CD4 T cells, or adoptive transfer of DCs pre-pulsed with specific retinal autoantigens and can develop spontaneously in some gene knockout or transgenic mice (39, 41–45). Depending on the approach used to elicit the disease, either Th1 or Th17 effector responses can be induced and lead to the development of EAU, (39, 41). Th17 cells contribute to the development of EAU induced by immunization with retinal antigens in CFA in an IL-23 dependent manner and this requires priming by an IFN-γ producing effector T cell (41, 46, 47). However, transfer of DCs pre-pulsed with IRBP1-20 or an uveitogenic CD4 Th1 cell line specific for IRBP1-20 induces an IFN-γ-dependent Th1 effector response, leading to development of this disease (41, 48).
Triple knockout of the TAM family of receptors causes autoimmune disorders due to two relevant events: (i) defective phagocytosis, around weaning, there is a rapid accumulation of apoptotic debris that is constantly exposed to the immune system and (ii) overreactivity of activated APCs (49–51). We previously showed that TAM triple knockout mice have increased numbers of DCs with higher surface levels of MHC II and B7 (49, 51). The CD4 T cell population is also increased in triple knockout mice (49). Negative regulation of activated DCs is essential to prevent overproduction of cytokines and hyperactivation of T cell responses, and the suppressor of cytokine signaling (SOCS) protein plays very important roles in the negative regulation of pathogen-induced macrophage activation (52) and the suppression of systemic autoimmune responses caused by DCs (53). DCs express both Axl and Mertk but not Tyro3 (51), and pathogen-stimulation enhances expression of Axl and Mertk, predominantly the Axl, which in turn upregulate the expression of the inducible negative regulator SOCS during the late stage of pathogen-induced DC activation (51, 54). In the absence of SOCS, DCs are hyperactivated and show enhanced antigen-presentation and cause an increased Th1 immune response (55, 56).
Autoreactive T cells can escape central selection in the thymus, but they are normally inactive due to immunologic peripheral tolerance to self-antigens. Increased amounts of self-antigens from the accumulation of apoptotic cell (AC) debris and unrestricted antigen presentation by hyper-reactive APCs can break such self-tolerance and induce pathological autoimmune responses against normal tissues that express self-antigens. Mertk has been shown to play critical role in AC-induced inhibition of DC activation and maturation (57), most likely through inhibition of NF-κB pathway (58). Without Mertk, mice develop spontaneous autoimmune disorder (59).
We have recently shown that naïve mice lacking all three members of TAM family of receptors develop autoimmunity against the retina-specific self-antigen interphotoreceptor retinoid-binding protein (IRBP) characterized by the presence of IRBP-specific CD4 T cells and retina infiltration of lymphocytes (60). In the present study, we further showed invasion of lymphocytes in the degenerating AM dko retina and the IRBP-specific CD4 T cells also existed in the dko mice. Based on the facts that DCs predominantly express Axl and Mertk receptors, and both are important for constraining DC activation and effector functions (51, 57), we further set out to investigate how DCs lacking both Axl and Mertk (AM) affect CD4 T cell polarization and how these two receptors regulate the DC response to self-antigen, we analyzed CD4 T effector responses to immunization with the peptide IRBP1-20. Our data showed that the double knockout (dko) mice exhibited hyper-polarization into the Th1 phenotype and prolonged effector responses following immunization with the IRBP peptide IRBP1-20. This dominant Th1 response in the dko mice is most likely attributable to increased production of pro-inflammatory cytokines, e.g., IL-12 and IL-18, by APCs that lack AM receptors.
Materials and Methods
Animal and reagents
The AM gene knockout mice, which were created on the C57BL/6 and 129 mixed background (50), have been backcrossed to the wild-type pure C57BL/6 background for at least 11 generations in our laboratory. All animals were housed in a pathogen-free facility and were handled according to the regulations of the Institutional Animal Care and Use Committee (IACUC), and all procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Reagents and Materials
Human IRBP1-20 peptide (amino acids 1–20 of IRBP; GPTHLFQPSLVLDMAKVLLD) was synthesized by GenScript (New Jersey, USA). Recombinant mouse IL-12, IL-6, IL-4 and TGF- β were purchased from PeproTech (New Jersey, USA). Recombinant mouse IL-23, ELISA kits for IFN-γ, IL-17A, IL-4, IL-10, and TGF-β monoclonal rat anti-mouse CD4 antibody (GK1.5 or RM4-5, FITC, APC-, or PE-conjugated), anti-mouse IFN-γ antibody (clone XMG1.2, purified, PE or FITC-conjugated), anti-mouse IL-4 antibody (clone 11B11, purified or PE-conjugated), anti-mouse CD25 antibody (PC61.5, PE, or APC-conjugated), anti-mouse Foxp3 antibody (clone FJK-16s, PE-conjugated), neutralizing anti-IL-12 antibody (C17.8), neutralizing rat IgG-κ (eBRG1) isotype antibody control and PE-conjugated rat IgG2a or IgG2b isotype antibody controls were purchased from eBiosciences (San Diego, CA). Monoclonal rat anti-mouse IL-4 antibody (clone 11B11), anti-mouse IL-12; anti-mouse CD16/32 antibody (clone 2.4G2), anti-mouse IL-17 antibody (Clone TC11-18H10), and anti-mouse CD3 antibody (clone 17A2) and monoclonal hamster anti-mouse CD28 antibody (clone 37.51) were from BD Biosciences (San Diego, CA). Rat anti-mouse IL-18 neutralizing antibody (93-10C) was purchased from MBL-International Corp (Woburn, MA). Brefeldin A was obtained from Sigma-Aldrich (St. Louis, MO). Recombinant mouse GM-CSF was purchased from R&D Systems (Minneapolis, MN).
Induction of EAU by active immunization
Two hours after intraperitoneal injection with Bordetella pertussis toxin (0.2 μg/mouse; Sigma-Aldrich), female mice aged 6–8 weeks were immunized subcutaneously over six spots at the tail base and the flank with 250 μg of human IRBP1-20 in 0.2 ml of a 1:1 v/v emulsion in CFA containing Mycobacterium tuberculosis strain H37RA (2.5 mg/ml; Difco), then, on day 0, 14, or 21 after immunization, the retina, spleen, and draining lymph nodes (inguinal, iliac) were collected and used for RNA preparation or to prepare a single cell suspension for flow cytometric analysis or in vitro cytokine production assays.
Flow cytometry
Single cell suspensions were prepared from the spleens and draining lymph nodes (inguinal and iliac) of naïve wild-type (WT) and dko mice or at day 14 post-immunization (14pi) or day 21pi after immunization with IRBP1-20 in CFA. After lysis of red blood cells using ACK Buffer (0.83% ammonium chloride, 0.1% potassium bicarbonate, 0.037% EDTA, pH 7.2), Fc receptors were blocked with anti-CD16/32 antibody before antibody staining.
To stain cell surface antigens, 106 cells in 50 μl of staining buffer [phosphate-buffered saline (PBS) containing 3% bovine serum albumin and 0.1% sodium azide] were incubated at 4°C for 30 min with fluorochrome-conjugated monoclonal antibody specific for CD4, CD25, or isotype controls.
For regulatory T cell staining, after surface staining for CD4 and CD25 and washes with staining buffer, the cells were fixed and permeabilized using Foxp3 fixation/permeabilization buffer (eBioscience) for 20 min at 4°C, then were stained for 30 min at 4°C with anti-Foxp3 antibody or rat IgG2a isotype controls.
For intracellular cytokine staining, the cells were pretreated for 4 h at 37°C with 50 ng/ml of PMA and 500 ng/ml of ionomycin, then for 2 h at 37°C with 10 μg/ml of brefeldin A (Sigma-Aldrich) prior to surface antigen staining. After surface antigen staining and washes, the cells were fixed and permeabilized for 20 min at 4°C with Cytofix/Cytoperm buffer (BD Biosciences), then stained for intracellular cytokines with antibodies against IFN-γ, IL-4, IL-17, or IL-12 and analyzed on a 4-color BD-FACSCalibur (BD Biosciences), the data being processed with CellQuestPro 5.1.1 software (BD Siosciences).
Cytokine ELISA assays
The pooled cells from the spleen and lymph nodes (inguinal, iliac, axillary and submandibular) from naïve or IRBP1-20-immunized WT and dko mice were initially separated by Nylon-wool column filtration and subsequently purified by EasySep™ mouse CD4 positive selection kit following manufacturer's instruction (StemCell Technologies). For antibody activation assays, the purified CD4+ T cells were stimulated with plate-bound anti-CD3 (5 μg ml−1) soluble anti-CD28 (1 μg ml–1) antibodies; both anti-CD3 and anti-CD28 antibodies were omitted from the controls. For naïve CD4 T cell polarization assays, the CD4+ T cells were co-cultured with γ-irradiated splenic APCs with or without IRBP1-20 for 48 h under T helper cell polarization conditions (Th1 conditions, 10 ng/ml of IL-12 and 10 μg/ml of anti-IL-4 antibody; Th2 conditions, 10 ng/ml of IL-4, 10 μg/ml of anti-IFN-γ antibody, and 10 μg/ml of anti-IL-12 antibody; Th17 conditions, 20 ng/ml of recombinant mouse IL-23, 3 ng/ml of TGF-β, 20 ng/ml of IL-6, and 1 μg/ml of anti-IL-4 antibody). Concentrations of IFN-γ, IL-4, IL-17A, IL-10, IL-12 and TGF-β were then measured in the culture medium using the relevant ELISA kits and following the manufacturer's instruction (eBiosciences, San Diego, CA).
Preparation of bone marrow-derived dendritic cells, bone marrow-derived macrophages, and peritoneal macrophages
For the preparation of bone marrow-derived cells, bone marrow was flushed from the femurs and tibias of WT and dko mice and red cells removed with ACK buffer, then 3×106 cells in complete medium [RPMI 1640 medium containing 10% fetal bovine serum (FBS), 50 mM 2-mercaptoethanol, 10 mM HEPES (pH 7.4), 2 mM glutamine, 100 U/ml of penicillin, and 100 mg/ml of streptomycin] were added to each well of a 6-well plate. To obtain DCs, 10 ng/ml of GM-CSF was added to the culture medium. On day 3, floating cells were gently removed and fresh medium added, then, on day 7, nonadherent cells and loosely adherent proliferating DCs were collected and 3×106 cells placed in each well of a new 6-well plate. On day 10 of culture, the cells were treated with 100 ng/ml of LPS for the indicated time. To obtain bone marrow-derived macrophages, 30% L929 cell (ATCC, Rockville, MD)-conditioned medium was added to the bone marrow cultures as a source of colony-stimulating factor 1, then, 24 h later, nonadherent cells were removed and the remaining adherent cells cultured for 6 more days in the same medium containing L929 cell-conditioned medium. On day 7, the cells were treated with 100 ng/ml of LPS for the indicated time.
To obtain peritoneal macrophages, 1 ml of 3% of thioglycollate was injected intraperitoneally (i.p.). On day 4 of induction, the cells in the peritoneum were flushed out with 9 ml of cold PBS containing 0.5 % FBS, then, after a wash with cold PBS+ 0.5% FBS, 3×106 cells were plated into each well of a 6-well plate. Nonadherent cells were removed 2 h later and the remaining adherent cells were cultured in complete medium for 3 days, then were treated with 100 ng/ml of LPS for 4 h before harvesting for flow cytometric analysis.
RNA isolation, cDNA synthesis, and real-time quantitative PCR
Total RNA was extracted from cultured cells or from the spleen, draining lymph nodes (inguinal, iliac), or retina of naïve mice or at days 14pi and 21pi after IRBP1-20 immunization using Trizol reagent, following the manufacturer's instruction (Invitrogen, San Diego, CA). The integrity of the RNA samples was checked by an approximately 2:1 ratio of 28S and 18S RNA on 1% formaldehyde denaturing RNA agarose gel and an A260/A280 ratio greater than 1.9. Two micrograms of total RNA from each sample was treated with DNase I to remove traces of genomic DNA, then was reverse transcribed into first strand cDNA using a qScript™ cDNA SuperMix kit (Quanta Biosciences, Gaithersbur, MD) for real-time qPCR analysis.
Real time qPCR was performed in a SYBR green-based PCR reaction mixture on a MX3005p system (Agilent Technologies, Inc., Santa Clara, CA), with a program of a 10-minute initial hot-start activation of Taq polymerase at 95°C, followed by 40 cycles of amplification (95°C for 10 seconds, 56°C for 5 seconds, and 72°C for 10 seconds). After amplification, a melting curve was generated by holding the reaction at 65°C for 15 seconds, then heating to 95°C, with a ramp rate of 0.1°C/s. To obtain the melting temperature for each sample, the fluorescence signal was plotted against temperature. The comparative CT (threshold cycle) method normalized to β-actin was used to analyze relative changes in gene expression.
The oligonucleotides used for qPCR were 5'-CAGCAACAGCAAGGCGAA-3' and 5'-CTGGACCTGTGGGTTGTTGAC-3' for IFN-γ 5'-AACATGAGTCCAGGGAGAGCTTCA-3' and 5'-AGTGTTTGGACACGCTGAGCTTTG-3' for IL-17A, 5'-GTGAACCCCAGACCAGACTG-3' and 5'-CCTGGAACACGTTTCTGAAAGA-3' for IL-18, 5'-TGATGATGACCCTGTGCCTTGGTA-3' and 5'-ATTCTGAAGTGCTG CGTTGATGGC-3' for IL-12p35, 5'-GCAACAGCACTGGAACCTTCACAA-3' and 5'-GCATTGCTTGAGGCTGCGTATGAT-3' for Foxp3, 5'-GTGCGGCAGCTGTACATTGACTTT-3' and 5'-TGTACTGTGTGTCCAGGCTCCAAA-3' for TGF-β 5'-CATGGCCCAGAAATCAAGGA-3' and 5'-GGAGAAATCGATGACAGCGC-3' for IL-10, 5'-TCTACACCAGCAAGAGCGATGTGT-3' and 5'-TGTTTCAGCCGATTTCCTTGACGC-3' for Axl, 5'-GAAACTGCATGTTGCGGGATGACA-3' and 5'-CAATGCGGCCTTGGCGGTAATAAT-3' for Mertk, and 5'-GGCTGTATTCCCCTCCATCG-3' and 5'-CCAGTTGGTAACAATGC-CATGT-3' for β-actin.
Results
Naïve CD4 T cells from AM dko mice are preferentially polarized into Th1 effector T cells
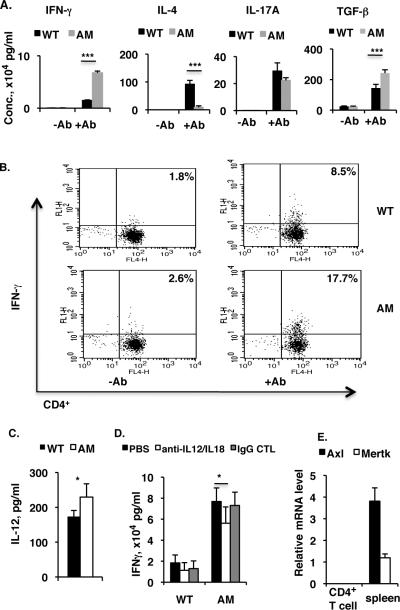
While the naïve mice lacking all three members of the TAM family of receptors produce retinal antigen-specific CD4 T cells and develop spontaneously autoimmunity against the eye (60), mice without both Axl and Mertk receptors exhibited similar lymphocytes infiltration into the degenerating retina, those included CD3-positive T cells and F4/80 positive macrophages (Fig. 1S); and these AM dko mice also generated CD4+ T cells specifically recognizing retinal specific autoantigen, IRBP (Fig. 2S). We then asked which T effector responses were involved and dominated in those dko mice. On stimulation with plate-bound anti-CD3 antibody and soluble anti-CD28 antibody, naïve CD4 T cells proliferate, differentiate into one of three major effector T cell subsets, and secrete signature cytokines. We therefore prepared CD4+ T cells from a pooled spleen and lymph node cell suspension from naive wild-type (WT) or AM dko mice and activated them using plate-bound anti-CD3 antibody and soluble anti-CD28 antibody without polarizing cytokines, then measured cytokines released into the culture medium after 48 h by ELISA. As shown in Figure 1A, in the absence of polarizing conditions, CD4+ T cells from dko mice produced dramatically higher amounts of IFN-γ (4- to 5-fold increase), slightly less IL-17A, and more TGF-β than those of WT mice. Interestingly, only a small amount of IL-4 was produced by the dko cells, compared to the 80 pg ml−1 secreted by the WT cells. In the absence of activation, levels of all of these cytokines were very low or undetectable for both WT and dko cells. To confirm the preferential Th1 differentiation in the mutant mice, we measured levels of IFN-γ-producing CD4+ T cells in the in vitro cultured T cells by flow cytometry and found that, after two days of activation by anti-CD3 and anti-CD28 antibodies, 17.7% of CD4+ T cells were IFN--producing in dko mice compared to 8.5% in the WT, although both groups showed an increase compared to the unstimulated groups (WT 1.8% and dko 2.6%) (Fig. 1B).
Figure 1. In vitro activation of CD4+ T cells by plate-bound anti-CD3 antibody and soluble anti-CD28 antibody induces more INF-γ-secreting Th1 cells in AM dko mice than in the wild-type.
A, Pooled spleen and lymph node CD4+ T cells were isolated from WT or AM dko mice by a initial Nylon-wool column filtration and followed by EasySepTM mouse CD4 positive selection kit (StemCell Technologies). 8×105 CD4+ T cells per well were plated on a 96-well plate bound with anti-CD3 (5 μg ml−1) in the presence of soluble anti-CD28 (1 μg ml−1) antibody. The control cells were plated on a 96-well plate in the absence of bothanti-CD3 and -CD28 antibodies. After two days of culture, levels of IFN-γ, IL-4, IL-17A, and TGF-β in the culture medium were measured by Ready-Set-Go ELISA kits following manufacturer's instruction (eBioscience). B, Pooled naïve spleen and lymph node CD4+ T cells from wild-type or dko mice were cultured in 96-well plates as in (A), then IFN-γ-secreting CD4+ T cells were analyzed by flow cytometry on a 4-color BD-FACSCalibur (BD Biosciences). The result is one representative of three mice in each group. C, The cell culture media prepared from (A) was subjected to IL-12 measurement by Ready-Set-Go ELISA kit (eBioscience). The concentration of IL-12 is expressed as pg/ml. D, 8×105 CD4+ T cells prepared as described in (A) were plated on a 96-well plate and stimulated for 48 h by plate-bound anti-CD3 and soluble anti-CD28 antibodies, in the presence of neutralizing antibodies against IL-12 (2 μg/ml, C17.8, eBiosciences) and IL-18 (2 μg/ml, 93–10C, MBL-International Corp); and the controls use either PBS or rat IgG1-κ isotype monoclonal antibody (2 μg/ml, eBRG1, eBiosciences) in the place of neutralizing antibodies. The culture medium after 48 h was subjected to IFN-γ measurement as in (A), and the concentration of IFN-γ is expressed as ×104 pg/ml. E. No expression of Axl and Mertk in the naïve WT CD4+ T cells. Pooled WT spleen and lymph node CD4+ T cells were first enriched by a initial Nylon-wool column filtration and then purified on a high-speed cell sorter (MoFlo, Dako Cytomatino, Fort Collins, CO), and 1×106 CD4+ T cells with purity >98% were subjected to RNA isolation and 1 μg of total RNA was used for reverse transcription and real-time qPCR analysis of Axl and Mertk expression. The total RNA from WT control spleens was used as positive control. The results in (A, C and D) are the mean ±SD for 5 wells per group in one experiment and are representative of those obtained in three independent experiments *p<5, ***p<0.001 in the one-way ANOVA test using ProStat Ver 5.5.
In vitro differentiation of naïve CD4 T cell into Th1 population is frequently conditioned by the presence of IL-12. Given the higher level of IFN-γ released from the T cells activated by plate-bound anti-CD3 and soluble anti-CD28 antibodies, we then asked whether IL-12 was present in the culture medium and found that there was indeed a small amount of IL-12 existing in both genotypes; but the mutant contained higher level of this cytokine compared to WT control (Fig. 1C). Neutralization with anti-IL-12 as well as anti-IL-18 antibodies decreased the IFN-γ release into the culture medium by approximately 25% compared to the un-neutralizing conditions, but the antibody-neutralized mutant cells still produced significantly more IFN-γ over controls (Fig. 1D).
Abnormal polarization of CD4 T cells in the dko may result from the receptor deficiency in the CD4+ T cells, however, the real-time qPCR measurement of both Axl and Mertk mRNA in the sorted CD4+ T cells (>98%) yielded undetectable signals (Fig. 1E), which was in agreement with previous publications (49, 61, 62). This suggests that abnormal CD4 T cell responses in the mutants might be caused by other cell types that normally express Axl and Mertk.
Immunization with the retinal autoantigen IRBP1-20 drives a dominant Th1 effector response
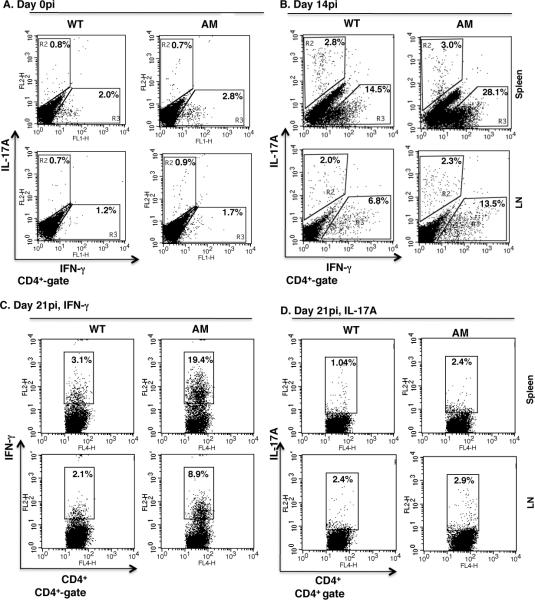
The existence of CD4 T cells with Th1 potential in the dko mice prompted us to investigate differences between the WT and dko mice in response to self-antigen immunization and to examine the T helper subsets that are preferentially induced in vivo. To assess T cell polarization in response to IRBP1-20 immunization, we analyzed effector T cell subsets by flow cytometry at days 0pi, 14pi and 21pi. As in a previous report that IRBP1-20 immunization invokes Th1 and Th17 responses in WT mice (41), we observed increases in both cell types in the dko mice, with a peak at day 14pi; however, the increase in Th1 cells was greater (Fig. 2A and B). It is generally considered that the T cell response decreases gradually after reaching a peak at 2 weeks after immunization and this was true for our WT mice, in which the spleen and lymph nodes contained, respectively, 14.5% and 6.8% IFN-γ-secreting Th1 cells at day 14pi and 3.1% and 2.1% at day 21pi (Fig. 2B and C). However, at day 21pi, the AM dko mice still had large numbers of Th1 T cells (Fig. 2C). These results suggest that the absence of AM receptors on APCs affects the duration and degree of the Th1 effector response.
Figure 2. IRBP1-20 immunization elicits a dominant Th1 effector response.
Wild-type and AM dko mice were immunized with 250 μg per animal of IRBP1-20 peptide in CFA, then Th1 and Th17 effector T cell subsets in the CD4+ T cells prepared at days 0pi, 14pi or 21pi from the spleen (top) and lymph nodes (L.N., bottom) were analyzed by fluorescence-activated cell sorting (FACS) for IL-17A (A, B and D) or IFN-γ (A, B and C) versus CD4 expression with the gates on the CD4+ T cell population. The percentages represent the percentage of cytokine-expressing CD4+ T cells in the total CD4+ T cells of the WT and AM mice (n=6). AM, Axl and Mertk double knockout.
T cells from IRBP1-20-immunized dko mice produce more IFN-γ in vitro than wild-type mice
Preferential differentiation into the Th1 lineage in the mutant mice in response to IRBP1-20-immunization was further demonstrated by the cytokine release profile of the in vitro cultured CD4 T cells. CD4+ T cells were prepared at different times post-immunization from WT and dko mice immunized with IRBP1-20 and cocultured with irradiated APCs from the same mice in the presence of IRBP1-20, then, 48 h later, cytokines released into the culture medium were measured by ELISA. As shown in Fig. 3A, IFN-γ released from cells prepared at day 14pi from immunized WT and dko mice (Fig. 3A, middle panel) was markedly increased compared to that seen with non-immunized mice (left panel). Consistent with the flow cytometry result, the dko mice produced more IFN-γ secreting Th1 T cells in response to IRBP1-20 immunization (Fig. 2B) and the CD4+ T cells prepared from dko mice at day 14pi secreted more than twice as much IFN-γ into the culture medium as WT cells (Fig. 3A, middle panel). Although IFN-γ secretion was markedly decreased in both genotypes at day 21pi, dko T cells still released approximately 5 times more IFN-γ than WT T cells (Fig. 3A, right panel). Overproduction of IFN-γ mRNA in the spleens and lymph nodes of dko mice immunized with IRBP1-20 at days 14pi and 21pi was confirmed by real-time qPCR (Fig. 3B).
Figure 3. IRBP1-20 immunization induces a greater IFN-γsecreting Th1 subset in the dko mice than in the wild-type.
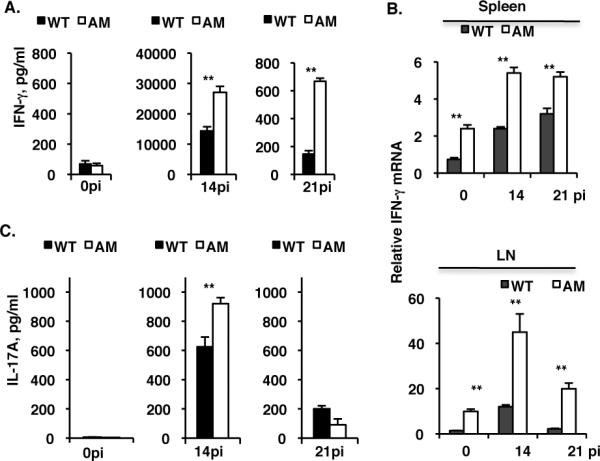
Pooled spleen and draining lymph node of CD4+ T cells and splenic APCs were isolated from naïve (0pi) mice and from mice at day 14pi or day 21pi with 250 μg of IRBP1-20 peptide in CFA per mouse, then 4×105 CD4+ T cells and syngeneic 1×105 APCs were co-cultured for 48 h in the presence of IRBP1-20 and levels of IFN-γ (A) and IL-17A (C) in the culture medium were measured by Ready-setgo ELISA kits (eBiosciences). B, qPCR analysis of IFN-γ mRNA in total RNA prepared from the spleen (top) or lymph nodes (bottom) of IRBP1-20-immunized WT or dko mutant mice at day 0pi, 14pi, and 21pi. The results are the mean ±SD for 5 wells (each containing pooled cells from 3 mice) per group in A and C and for 3 mice per group in (B) in a single experiment and are representative of those in three independent experiments. **p<0.001 by the one-way ANOVA test using ProStat Ver 5.5.
Th17 cells in AM dko mice exhibit normal responses to IRBP1-20 induction
Since the Th17 cell has been demonstrated to be associated with several autoimmune disorders (11–13), we tested the Th17 response to IRBP1-20 immunization in both WT and AM dko mice. Consistent with the notion that immunization with IRBP1-20 in CFA mainly provokes a Th17 cell response (41, 46, 47), Th17 cells were induced in both WT and dko mice (Fig. 2B and D), suggesting that the Th17 response is normal in dko mice. To further test Th17 cell polarization during IRBP1-20 immunization, we isolated and cultured CD4 T cells from WT and dko mice immunized with IRBP1-20 at day 0 and days14pi and 21pi in the presence of IL-23, anti-IFN-γ and anti-IL-4 antibodies and γ-irradiated APCs from the same immunized mice for 48 h, then measured IL-17A released into the culture medium by ELISA and found that IL-17A levels were increased by IRBP1-20 immunization, with a peak at day 14pi and a gradual decrease thereafter by day 21pi (Fig. 3C). These data indicate that AM dko mice have a normal Th17 response to autoantigen immunization.
High levels of regulatory T cells are induced in dko mice immunized with IRBP1-20 at day 21pi
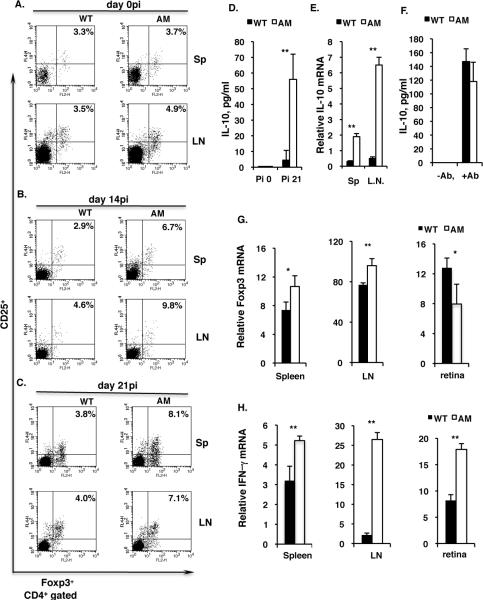
Given the facts that TAM triple knockout mice develop spontaneous autoimmune disorders (49, 51) and that regulatory T (Treg) cells inhibit autoimmunity (20–22), we then examined whether the Treg cell frequency in IRBP1-20-immunized dko mice was decreased compared to that in WT mice. However, surprisingly, after immunization by day 14pi and 21pi, the dko mice produced more CD4+CD25+Foxp3+ regulatory T cells than the WT controls (Fig. 4A–C). Consistent with this, dko mice, as well as the CD4+ T cells prepared from dko mice, produced increased amounts of IL-10 protein (Fig. 4D) and Il-10 mRNA (Fig. 4E), implying there was an increased frequency of Treg cells in the dko mice at day 21pi. To serve as a control, CD4+ T cells isolated from naïve WT and dko mice at 4–5 weeks of age were activated by anti-CD3 and anti-CD28 antibodies and IL-10 levels in the culture medium were measured by ELISA, and the cells from the two types of mice were found to produce similar amounts of IL-10 (Fig 4F). An increased frequency of Treg cells in the dko mouse spleen and lymph node might possibly inhibit the CD4+ T helper response. However, we noted previously (60) that, at day 21 after immunization with a low dose (100 μg) of IRBP1-20, a large number of CD3-positive T cells was still present in the mutant retina, so we therefore measured Foxp3 mRNA levels by qPCR in the WT and dko retina. As shown in Figure 4G, there was less Foxp3 transcript in the dko retina than in the WT retina, although increased Foxp3 mRNA levels were detected in dko spleens and lymph nodes compared to the WT (Fig. 4H, right). In contrast, increased IFN-γ mRNA levels were detected in both retina and lymphoid tissues in the dko mice compared to the WT (Fig. 4G–H).
Figure 4. IRBP1-20 immunization induces more regulatory T cell (Treg) production in dko mice than in the wild-type.
A–C, Flow cytometric analysis of the CD4+/CD25+/Foxp3+ Treg cell subset in the pooled splenic and lymph node T cells prepared from WT and dko mice at day 0pi (A), day 14pi (B) and day 21pi (C) with IRBP1-20. The result is one representative of 4–6 mice in each group. Sp, spleen; LN, lymph node. D, ELISA measurement of IL-10 in the culture medium after two days co-culture of CD4+ T cells and syngeneic APCs in the presence of IRBP1-20. E. qPCR quantification of IL-10 mRNA in the spleen and lymph nodes of WT and dko mice at day21 pi. F, Pooled spleen and lymph node CD4+ T cells from WT or AM dko mice were plated on a 96-well plate bound with anti-CD3 antibody in the presence of soluble anti-CD28 antibody (+Ab). The control cells were plated on a 96-well plate in the absence of both anti-CD3 and -CD28 antibodies (−Ab). After two days of culture, levels of IL-10 in the culture medium were measured by ELISA (eBioscience). G and H, qPCR measurement of Foxp3 mRNA (G) and IFN-γ (H) levels in the spleen (Sp), lymph node (L.N.), and retina of WT and dko mice at day 21pi. The results in (D and E) are the mean ±SD for 5 wells per group in one experiment and are representative of those obtained in three independent experiments. The results in (F, G and H) are the mean ±SD of 3 mice in a single experiment and are representative of those in three independent experiments. *p<0.01, **p<0.001 in the one-way ANOVA test using ProStat Ver 5.5.
AM dko APCs have a greater ability to drive T cells to develop into the Th1 lineage
DCs, as a major APC cell type, play a critical role in driving adaptive immune responses. In the immune system, the TAM family of receptor tyrosine kinases is expressed mainly on myeloid cells and monocytes and their derivatives and not on T and B lymphocytes (49, 61, 62). We therefore examined whether the aberrant T cell activation in dko mice was caused by dysfunctional APCs. We tested CD4 T cell polarization in an in vitro culture system in which purified CD4+ T cells from day 14pi mice were co-cultured with CD11c+ DCs purified from either WT or AM dko mice in the presence of IRBP1-20 under either Th1 (Fig. 5A) or Th17 (Fig. 5B) polarizing conditions. In agreement with the cytokine-release data presented above, CD4+ T cells from dko mice produced more IFN-γ than WT cells using either type of DCs (Fig. 5A). In addition, both WT and dko CD4 T cells produced more IFN-γ in the presence of dko APCs than in the presence of WT APCs (Fig. 5A). This increase was mainly limited to the IFN-γ-releasing Th1 population, since there was little difference in IL-17A release into the culture medium between the different combinations of T cells and DCs, even under Th17 culture conditions (Fig. 5B).
Figure 5. AM dko DCs are more potent than wild-type DCs at driving Th1 differentiation.
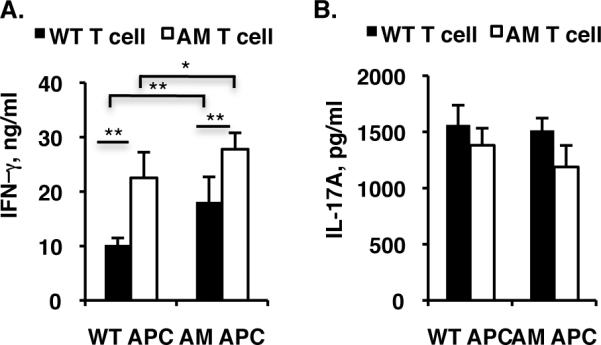
Pooled spleen and lymph node CD4+ T cells from WT or dko mice were plated onto γ-irradiated WT or dko mice APCs in 96-well plates and cultured for 72 h in the presence of IRBP1-20 under Th1 (A) or Th17 (B) differentiation conditions, then IFN-γ (A) or IL-17A (B) in the culture medium was measured by ELISA. The results are the mean ±SD and are representative of those obtained in three independent experiments. * p<0.05, **p<0.001
Macrophages and DCs from AM dko mice produce high levels of proinflammatory cytokines that drive Th1 polarization
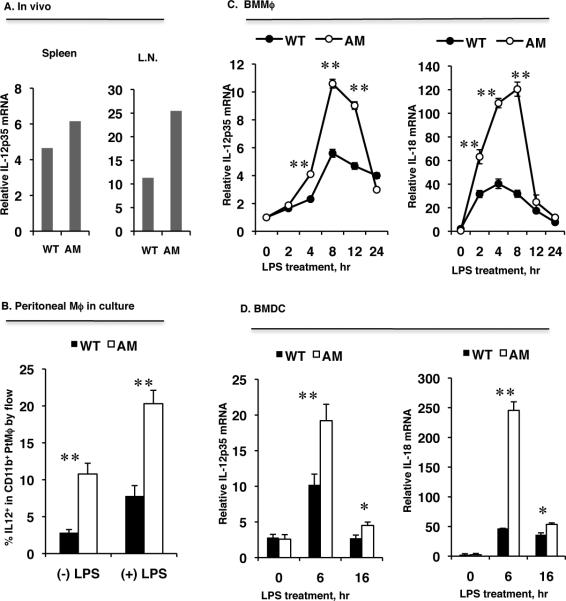
It has been well demonstrated that the APC-derived cytokines IL-12 and IL-18 are key players in the development of the Th1 response and in driving Th1-mediated autoimmunity (29, 32, 33, 63, 64). Given our results that dko mice produced more Th1 cells than WT mice, we speculated that dko DCs or macrophages might secrete more IL-12 and IL-18 upon activation. Increased levels of IL-12p35 mRNA were demonstrated in dko spleen and lymph node (LN) by real-time qPCR compared to WT (Fig. 6A) and increased IL-12 protein production was confirmed by flow cytometric analysis of peritoneal macrophages after 4 hours of LPS-stimulation in vitro (Fig. 6B). To further examine IL-12 and IL-18 production elicited by LPS stimulation, we quantified mRNA levels by qPCR in both WT and dko macrophages and DCs derived from bone marrow progenitors and found that LPS stimulation caused a rapid increase in both IL-12p35 and IL-18 with a peak at 6 h of treatment, followed by a drop to basal level within 24 h in both macrophages (Fig. 6C) and DCs (Fig. 6D). As expected, LPS stimulation induced a greater IL-12p35 and IL-18 response from the bone marrow-derived dko macrophages (Fig. 6C) and DCs (Fig. 6D) compared to WT. These data suggest that overproduction of IL-12 and IL-18 might be, at least in part, responsible for favoring Th1 polarization in AM dko mice.
Figure 6. dko DCs and macrophages produce more IL-12 and IL-18 in response to LPS treatment than wild-type cells.
A. IL-12p35 mRNA levels in total splenic and lymph node RNA prepared and pooled from three naïve WT and three dko mice measured by qPCR. B, Thioglycollate-induced peritoneal macrophages (Mϕ) were treated with LPS for 4 h, then IL-12 expression on the CD11b-gated macrophages was analyzed by FACS. The results are the mean ± SD for n=3 mice. C and D, IL-12p35 and IL-18 mRNA levels in bone marrow-derived macrophages (BMMϕ) (C) or bone marrow-derived DCs (BMDC) (D) during LPS treatment measured by qPCR. The results are the mean ±SD for 3 wells per group in one experiment and are representative of those obtained in three experiments **p<0.05, **p<0.001 by the one-way ANOVA test using ProStat Ver 5.5.
Discussion
Previous studies have shown that mice lacking TAM receptors develop spontaneous autoimmune disorders (49, 59) due to unrestricted activation of cytokine production by DCs or macrophages (51, 54). Moreover, TAM triple knockout mice generate autoantigen-specific CD4 T cells and have a much higher susceptibility to development of autoimmune disease on immunization with a retina-specific antigen (60). We, in the present study, further showed that AM double knockout mice also exhibited spontaneous lymphocyte infiltration into the degenerating retina and produce retina antigen-specific CD4 T cells. This is consistent with previous published observation that AM dko as well as Mertk single knockout mice shows autoimmune disorders, although they are not as severe as the TAM triple knockout mice (49, 59). Since T cells do not express TAM receptors (49, 61, 62), abnormal T cell differentiation is considered to be caused mainly by DCs, a potent APC, that normally only express Axl and Mertk but not Tyro3 (51). To investigate the T cell effector response to retinal autoantigen in these mutant mice, we therefore focused on the AM dko mice and studied CD4+ T cell polarization following IRBP1-20 immunization. Our results showed that mice lacking AM receptors responded to IRBP1-20 immunization with activation of both the Th1 and Th17 pathways, but the Th1 response was more dominant and hyperreactive. The CD4 T cell response to immunization is generally considered to reach a peak in two weeks. At this time (day 14pi), the number of Th1 T cells was twice as high in the dko mice as in the WT mice and this dominant Th1 population was still present at day 21pi, at which time the Th1 phenotype was near to basal levels in the WT controls. Such excessive Th1 response was not unique to IRBP immunization, CD4+ T cells from naïve dko mice generated increased numbers of Th1 cells and released more IFN-γ into culture medium upon activation by plate-bound anti-CD3 and soluble anti-CD28 antibodies even at the absence of the conditioning cytokines. Preferential polarization into the Th1 lineage may be due to the presence of IL-12 secreting cells, such as DC, in the cell preparation. However, neutralizing antibodies against IL-12 and IL-18 failed to completely restore the IFN-γ-secreting Th1 cells to the wild-type level, suggesting that those naïve CD4 T cells from mutants might contain higher frequency of Th1 committed progenitors or memory T cells. The memory T cells have been demonstrated to be more susceptible to anti-CD3 and -CD28 activation in production of cytokines and T cell polarization (65, 66). We have previously shown that TAM knockout mice contained more memory T cells (60), which might give to Th1 cells upon antibody activation.
Th17 cells specific for self-antigens are responsible for a severe autoimmune response in several animal models (11–13), including EAU (41, 46, 47). In agreement with these results, we observed a normal Th17 response in the mutant mice, although the mutant mice showed a slightly increased response at day 14pi. It has been well established that both Th1 and Th17 effector responses can be induced by immunization with IRBP and that the dominance of one response over the other is dependent on the method of immunization (39, 41). In WT mice, immunization with IRBP1-20 in CFA elicits a dominant Th17 response, while transfer of IRBP1-20-specific CD4+ T cells or IRBP1-20-pulsed DCs leads to a dominant Th1 response (41, 48). However, in the present study, we demonstrated that immunization with the retinal autoantigen, IRPB1-20, in CFA elicited a dominant Th1 effector response in AM dko mice. This difference is probably due to overproduction of IFN-γ in the mutants (49), which is able to inhibit commitment to the Th17 phenotype (12).
The differentiation of naïve CD4+ T cells into different lineages of effectors is largely dependent upon the production of cytokines by activated APCs. The cytokines secreted by APCs create an environment that is critical for directing the differentiation of activated antigen-specific lymphocytes into a distinct effector T cells subset. Since T cells do not express TAM receptors (49, 61, 62), the abnormalities in the T cell responses in the AM double mutant mice might be due to DCs or macrophages lacking this family of receptors. Both Axl and Mertk play negative regulatory roles in DCs and macrophage upon pathogen stimulation or apoptotic cell encounter, by upregulation of the inhibitory SOCSs expression (51) or by inhibition of NF-κB activation (58) and constraining DCs activation and maturation (57), respectively. Without Axl and Mertk, DCs produce increased level of proinflammatory cytokines (51, 58). We therefore hypothesized that AM receptors negatively regulate the expression of distinct cytokines that drive Th1 polarization and that APCs lacking Axl and Mertk receptors produce altered levels or a different profile of cytokines that drive a specific reaction for induction of effector T cell polarization. We focused our study on two cytokines, IL-12 and IL-18, as these cytokines are secreted by APCs and, in turn, activate Th1 development by upregulating the synthesis of inflammatory IFN-γ [30, 31, 63], while mice deficient in IL-12 and IL-18 are resistant to experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (67, 68) and show reduced Th1 cell development. IL-12 is considered to play a major pathogenic role in autoimmune diseases, such as EAU and EAE, by promoting the generation of IFN-γ-producing Th1 effector cells (63, 64). Increased inflammatory IFN-γ production is seen in naïve dko mice (49). The data presented in this study showed that both DCs and macrophages lacking Axl and Mertk receptors were hyperreactive to LPS stimulation in terms of production of both IL-12 and IL-18. This result was in contradiction of what was observed on the nonobese diabetic (NOD) mice lacking Mertk expression, in which the NOD-Mertk−/− DCs produced equal amount of IL-12 as the control cells after 72 hours of LPS treatment, unless the DCs had been previously treated with apoptotic cells (AC) (57). Such difference may come from the duration of LPS treatment and the genetic background from which the DCs were derived. Interestingly, AC-induced inhibition of IL-12 production during pathogen-stimulated DC activation is indeed mediated by Mertk signaling (57). It is conceivable that AM dko mice, that have accumulated massive apoptotic cell debris caused by a defective phagocytic activity in the phagocytes, produce elevated level of IL-12 and Il-18, perhaps other cytokines too, due to loss of Mertk inhibition of DC activation and maturation. These increased levels of IL-12 and IL-18 are probably responsible for the increased Th1 response in the AM dko mice. These results suggest that AM receptors regulate APC cytokine production that drives the Th1 effector response.
In addition, we observed less IL-4 secretion by dko CD4+ T compared to WT cells after in vitro activation by anti-CD3 and anti-CD28 antibodies, which may result from a higher percentage of IFN-γ-secreting Th1 cells in the total CD4 T cell population.
The fourth subset of CD4 T cells studied in this investigation was regulatory T cells. The frequency of CD4+CD25+Foxp3+ Treg cells was increased in AM dko mice at days 14pi and 21pi compared to WT mice. This much higher Treg cell population after self-antigen immunization may simply reflect overreaction to immunization in general or may due to the increased secretion of TGF-β in the mutants. It is well known that the regulatory functions mediated by Treg cells, as well as Treg cell active proliferation, are dependent on stimulation with antigens, including tissue-specific self-antigens (69–72). Deposition of retinal self-antigens during photoreceptor degeneration or overreaction to IRBP1-20 immunization in the dko mice may contribute to the increased frequency of Treg cells. Interestingly, although we observed an increased Treg cells population in mutant peripheral lymph tissues, we indeed found a decreased level of Treg-specific Foxp3 in the mutant retina. Many clinical observations show that patients with autoimmune diseases have lower level of circulating Treg cells (73). Whether or not the mutant mice are resistant to secondary immunization by the same antigen or the Treg cells in the mutants are not functionally active remain for further investigation.
Supplementary Material
Acknowledgements
The authors thank Dr. Douglas Dean for valuable discussion and Research to Prevent Blindness, Inc for general support. QLU is a 2010 RPB William and Mary Greve Special Scholar investigator.
Footnotes
This work was supported by grants NIH R01-EY018830, P20-RR017702 and The Research-to-Prevent-Blindness special award (QLU); QLI was supported by grants NIH R01-EY01989 and P20-RR018733. Abbreviations: TAM, Tyro3, Axl, Mertk; AM dko, Axl and Mertk double knockout; Gas6, growth-arrest-specific 6; IRBP, interphotoreceptor retinoid-binding protein; DC, dendritic cells; EAU, experimental autoimmune uveoretinitis.
References
- 1.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat Immunol. 2001;2:487–492. doi: 10.1038/88678. [DOI] [PubMed] [Google Scholar]
- 4.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 5.Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J Immunol. 2002;169:6842–6849. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- 6.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 7.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 8.Dong C, Flavell RA. Th1 and Th2 cells. Curr Opin Hematol. 2001;8:47–51. doi: 10.1097/00062752-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 10.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR. Autoimmune inflammation from the Th17 perspective. Autoimmun Rev. 2007;6:169–175. doi: 10.1016/j.autrev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 15.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 16.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of selftolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 17.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 18.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crispin JC, Alcocer-Varela J, de Pablo P, Martinez A, Richaud-Patin Y, Alarcon-Segovia D. Immunoregulatory defects in patients with systemic lupus erythematosus in clinical remission. Lupus. 2003;12:386–393. doi: 10.1191/0961203303lu368oa. [DOI] [PubMed] [Google Scholar]
- 23.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 24.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 26.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 29.Micallef MJ, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferongamma production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFNgamma production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 31.Chang JT, Segal BM, Nakanishi K, Okamura H, Shevach EM. The costimulatory effect of IL-18 on the induction of antigen-specific IFN-gamma production by resting T cells is IL-12 dependent and is mediated by up-regulation of the IL-12 receptor beta2 subunit. Eur J Immunol. 2000;30:1113–1119. doi: 10.1002/(SICI)1521-4141(200004)30:4<1113::AID-IMMU1113>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 33.Boraschi D, Dinarello CA. IL-18 in autoimmunity: review. Eur Cytokine Netw. 2006;17:224–252. [PubMed] [Google Scholar]
- 34.Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Kolls JK, Joosten LA, van den Berg WB. Induction of cartilage damage by overexpression of T cell interleukin-17A in experimental arthritis in mice deficient in interleukin-1. Arthritis Rheum. 2005;52:975–983. doi: 10.1002/art.20885. [DOI] [PubMed] [Google Scholar]
- 35.Wang D, Drenker M, Eiz-Vesper B, Werfel T, Wittmann M. Evidence for a pathogenetic role of interleukin-18 in cutaneous lupus erythematosus. Arthritis Rheum. 2008;58:3205–3215. doi: 10.1002/art.23868. [DOI] [PubMed] [Google Scholar]
- 36.Calvani N, Richards HB, Tucci M, Pannarale G, Silvestris F. Up-regulation of IL-18 and predominance of a Th1 immune response is a hallmark of lupus nephritis. Clin Exp Immunol. 2004;138:171–178. doi: 10.1111/j.1365-2249.2004.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong CK, Ho CY, Li EK, Tam LS, Lam CW. Elevated production of interleukin-18 is associated with renal disease in patients with systemic lupus erythematosus. Clin Exp Immunol. 2002;130:345–351. doi: 10.1046/j.1365-2249.2002.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park MC, Park YB, Lee SK. Elevated interleukin-18 levels correlated with disease activity in systemic lupus erythematosus. Clin Rheumatol. 2004;23:225–229. doi: 10.1007/s10067-004-0867-x. [DOI] [PubMed] [Google Scholar]
- 39.Caspi RR, Silver PB, Luger D, Tang J, Cortes LM, Pennesi G, Mattapallil MJ, Chan CC. Mouse models of experimental autoimmune uveitis. Ophthalmic Res. 2008;40:169–174. doi: 10.1159/000119871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caspi RR, Roberge FG, Chan CC, Wiggert B, Chader GJ, Rozenszajn LA, Lando Z, Nussenblatt RB. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–1495. [PubMed] [Google Scholar]
- 41.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rizzo LV, Silver P, Wiggert B, Hakim F, Gazzinelli RT, Chan CC, Caspi RR. Establishment and characterization of a murine CD4+ T cell line and clone that induce experimental autoimmune uveoretinitis in B10.A mice. J Immunol. 1996;156:1654–1660. [PubMed] [Google Scholar]
- 43.Sanui H, Redmond TM, Kotake S, Wiggert B, Hu LH, Margalit H, Berzofsky JA, Chader GJ, Gery I. Identification of an immunodominant and highly immunopathogenic determinant in the retinal interphotoreceptor retinoid-binding protein (IRBP) J Exp Med. 1989;169:1947–1960. doi: 10.1084/jem.169.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 45.Taneja V, David CS. HLA class II transgenic mice as models of human diseases. Immunol Rev. 1999;169:67–79. doi: 10.1111/j.1600-065x.1999.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 46.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 47.Peng Y, Han G, Shao H, Wang Y, Kaplan HJ, Sun D. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–4161. doi: 10.1167/iovs.07-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang J, Zhu W, Silver PB, Su SB, Chan CC, Caspi RR. Autoimmune uveitis elicited with antigen-pulsed dendritic cells has a distinct clinical signature and is driven by unique effector mechanisms: initial encounter with autoantigen defines disease phenotype. J Immunol. 2007;178:5578–5587. doi: 10.4049/jimmunol.178.9.5578. [DOI] [PubMed] [Google Scholar]
- 49.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 50.Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, Earp HS, Goff SP, Lemke G. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 51.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 52.Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, Akira S, Yamanishi K, Kawase I, Nakanishi K, Kishimoto T. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/s1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 53.Hanada T, Yoshida H, Kato S, Tanaka K, Masutani K, Tsukada J, Nomura Y, Mimata H, Kubo M, Yoshimura A. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19:437–450. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 54.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanada T, Tanaka K, Matsumura Y, Yamauchi M, Nishinakamura H, Aburatani H, Mashima R, Kubo M, Kobayashi T, Yoshimura A. Induction of hyper Th1 cell-type immune responses by dendritic cells lacking the suppressor of cytokine signaling-1 gene. J Immunol. 2005;174:4325–4332. doi: 10.4049/jimmunol.174.7.4325. [DOI] [PubMed] [Google Scholar]
- 56.Evel-Kabler K, Song XT, Aldrich M, Huang XF, Chen SY. SOCS1 restricts dendritic cells' ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J Clin Invest. 2006;116:90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallet MA, Sen P, Flores RR, Wang Y, Yi Z, Huang Y, Mathews CE, Earp HS, Matsushima G, Wang B, Tisch R. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205:219–232. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sen P, Wallet MA, Yi Z, Huang Y, Henderson M, Mathews CE, Earp HS, Matsushima G, Baldwin AS, Jr, Tisch RM. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-{kappa}B activation in dendritic cells. Blood. 2006 doi: 10.1182/blood-2006-04-017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 60.Ye F, Li Q, Ke Y, Lu Q, Han L, Kaplan HJ, Shao H. Tam Receptor Knockout Mice Are Susceptible to Retinal Autoimmune Induction. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.10-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemke G, Lu Q. Macrophage regulation by Tyro 3 family receptors. Curr Opin Immunol. 2003;15:31–36. doi: 10.1016/s0952-7915(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 62.Graham DK, Bowman GW, Dawson TL, Stanford WL, Earp HS, Snodgrass HR. Cloning and developmental expression analysis of the murine c-mer tyrosine kinase. Oncogene. 1995;10:2349–2359. [PubMed] [Google Scholar]
- 63.Caspi RR. Th1 and Th2 responses in pathogenesis and regulation of experimental autoimmune uveoretinitis. Int Rev Immunol. 2002;21:197–208. doi: 10.1080/08830180212063. [DOI] [PubMed] [Google Scholar]
- 64.Xu H, Rizzo LV, Silver PB, Caspi RR. Uveitogenicity is associated with a Th1-like lymphokine profile: cytokine-dependent modulation of early and committed effector T cells in experimental autoimmune uveitis. Cell Immunol. 1997;178:69–78. doi: 10.1006/cimm.1997.1121. [DOI] [PubMed] [Google Scholar]
- 65.Chen Z, Tato CM, Muul L, Laurence A, O'Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 67.McIntyre KW, Shuster DJ, Gillooly KM, Warrier RR, Connaughton SE, Hall LB, Arp LH, Gately MK, Magram J. Reduced incidence and severity of collagen-induced arthritis in interleukin-12-deficient mice. Eur J Immunol. 1996;26:2933–2938. doi: 10.1002/eji.1830261219. [DOI] [PubMed] [Google Scholar]
- 68.Wei XQ, Leung BP, Arthur HM, McInnes IB, Liew FY. Reduced incidence and severity of collagen-induced arthritis in mice lacking IL-18. J Immunol. 2001;166:517–521. doi: 10.4049/jimmunol.166.1.517. [DOI] [PubMed] [Google Scholar]
- 69.Scully R, Qin S, Cobbold S, Waldmann H. Mechanisms in CD4 antibody-mediated transplantation tolerance: kinetics of induction, antigen dependency and role of regulatory T cells. Eur J Immunol. 1994;24:2383–2392. doi: 10.1002/eji.1830241019. [DOI] [PubMed] [Google Scholar]
- 70.Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J Exp Med. 1999;189:877–882. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigendependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117:289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.