Summary
The current study considered the influence of perceived discrimination on the diurnal cortisol rhythm of 50 African American older adults and a matched comparison groups of 100 Whites (Mage =56.6; 58% female). The role of socioeconomic status (SES) as a moderator of the effects of discrimination on the diurnal decline was also considered for each group. In support of the idea that perceptions of unfair treatment take on a unique meaning for stigmatized minority groups, results suggest that perceived discrimination is associated with a flatter (less healthy) diurnal slope among Whites but a steeper (more healthy) diurnal slope among African Americans. Perceived discrimination was also found to be more strongly associated with a steepening of the diurnal slope among lower SES African Americans than higher SES African Americans.
Keywords: Perceived Discrimination, Diurnal Cortisol Rhythm, Race, African American, HPA Axis Dysregulation, Physical Health Disparities
In recent years, the role of discrimination as a potential mechanism for explaining mental and physical health outcomes in racial health disparities has received increased attention. While discrimination has been consistently linked to negative mental health outcomes, results for associations between discrimination and physical health have been much less consistent (Paradies, 2006; Williams et al., 2003). Furthermore, few studies have considered the possibility of a unique association between perceived discrimination and physical health for stigmatized minorities, as compared to Whites, despite evidence suggesting that discrimination takes on a very different meaning for minority groups (Major et al., 2002). Additionally, only one study has considered perceived discrimination in the context of the diurnal cortisol rhythm, despite the importance of these rhythms as a biomarker of chronic stress (Adam and Kumari, 2009). Addressing these gaps in the literature, the current study considers perceived daily discrimination as a predictor of the diurnal cortisol rhythm among African Americans and Whites. Based on previous research (e.g., Krieger and Sidney, 1996), socioeconomic status is also considered as a moderator of this relationship.
Cortisol, Stress, and Ethnicity
The hypothalamic-pituitary-adrenal (HPA) axis is one important biological pathway through which stressful experiences are linked to physical disease (McEwen, 1998). Specifically, chronic stressors may modulate the release of cortisol, which regulate a host of cardiovascular, metabolic, homeostatic, and immunologic functions. Although levels of cortisol vary over the course of the day (typically peaking in the early morning and falling from afternoon to evening), chronically stressful situations have been shown to lead to dysregulation of this pattern, which may lay the groundwork for pathogenic processes (Adam and Kumari, 2009).
While little research has systematically explored differences in the cortisol diurnal rhythm across racial/ethnic groups, some studies suggest that non-Hispanic Whites have higher waking levels of cortisol and steeper diurnal cortisol slopes than African Americans (Cohen et al., 2006; DeSantis et al., 2007). Even after controlling for a range of psychosocial factors and health behaviors, African Americans exhibit a flatter diurnal cortisol rhythm (Cohen et al., 2006), which has been linked to chronic stress (Adam et al., 2006), poorer health outcomes (Sephton et al., 2000), and increased risk of mortality (Matthews et al., 2006). Chronic exposure to racial discrimination among African Americans is one plausible explanation for these observed differences in the diurnal cortisol slope across racial/ethnic groups.
Discrimination and Health Outcomes
Prejudice and discrimination lead to structural and institutional barriers that limit resources such as employment, housing, and medical access (Fix and Struyk, 1993; Pager and Shepherd, 2008; Smedley et al., 2003). Inability to access these resources threaten both the physical and psychological well-being of stigmatized minority groups (Clark et al. 1999). In addition to structural and institutional discrimination, African Americans also suffer various forms of daily discrimination such as being ignored, avoided, excluded, belittled, or treated with less courtesy and respect (Ong et al., 2009; Sellers and Shelton, 2003). Since a host of research has demonstrated that inclusion (Leary, 1990), affiliation (Bowlby, 1969) and positive valuation by others (Pyszcynski et al., 1997) are essential precursors to well-being, most theories have argued that perceived discrimination or prejudice would tend to lead to negative health outcomes (Clark et al., 1999; Mays et al., 2007).
Consistent with the conceptualization of perceived discrimination as a stressor, research on discrimination and mental health suggests that perceived discrimination is positively correlated with depression and anxiety (Banks et al., 2006; Schulz et al., 2006), and is predictive of increases in negative affect and psychological distress over time (Gibbons et al., 2004; Brody et al., 2006). Some evidence also suggests that perceived discrimination is associated with poorer physical health. For example, studies suggest that discrimination is positively associated with coronary artery calcification (Lewis et al., 2006), and inversely associated with a self-report measure of overall physical condition (Ryan et al., 2006). Moreover, studies suggest that, after controlling for SES and health behaviors, African American mothers who report higher levels of discrimination are more likely to have preterm or low birth weight deliveries (Collins et al., 2006; Mustillo et al., 2004).
However, it is also important to note that many published studies have found no significant association between perceived discrimination and physical health (Williams and Mohammed, 2009). Furthermore, although most researchers have conceptualized perceived discrimination as a stressor, other perspectives have emphasized that the extent to which individuals report experiences of discrimination also reflects how they are interpreting their social reality. For example, researchers have pointed out that, for stigmatized groups such as African Americans, acknowledging the existence of discrimination in daily life may be an important component of a well-adjusted psyche (Cross, 1991; Helms, 1995; Sellers et al., 1998; Spencer and Markstrom-Adams, 1990). Furthermore, evidence suggests that because instances of racial discrimination are likely to be occurring on a regular basis for members of stigmatized groups, acknowledging the presence of discrimination may be necessary for effective coping and adjustment (Major et al., 2002). On the other hand, a lack of reported discrimination may be indicative of avoidance, denial, or suppression which has been associated with a host of negative health outcomes (Jorgensen and Thibodeau, 2007). Therefore, although members of stigmatized minority groups would be expected to show poorer overall physical health outcomes (due to institutional barriers and greater exposure to daily discrimination), one would not necessarily expect minority individuals who report more discrimination to have worse health as compared to those who report little or no discrimination. In fact, if perceptions of discrimination are indicative of a healthy acknowledgement of racial bias in society, it is plausible that individuals reporting higher levels of daily discrimination would be more well-equipped to deal with discrimination when it occurs, and thus more likely to demonstrate resilience with respect to health outcomes.
Supporting this perspective, in an early social psychology framework, Gurin and colleagues suggested that an orientation towards external control, or system blame, may be beneficial for members of stigmatized minority groups whose lives are heavily influenced by external forces (Gurin et al., 1978). Similarly, Crocker and Major (1989) proposed the “attributional ambiguity” hypothesis, arguing that perceptions of discrimination can serve as protective factor for those targeted by prejudice and discrimination, allowing negative outcomes to be attributed to faults in the other, rather than one’s own shortcomings. In one study, for example, African Americans who were led to believe that negative treatment was not due to race, experienced more decreases in self-esteem than those who were led to believe that race was a factor (Crocker et al., 1991).
Studies examining physical health have also found similar results. In a study considering mortality rates, researchers found that African Americans who reported experiencing racism and endorsed a system-blaming orientation were more likely to be alive 13 years later than self-blamers who did not report experiencing discrimination (LaVeist et al., 2001). This finding held even after controlling for possible confounding variables such as health status at baseline, smoking, and income. The authors argue that attributing negative events to external factors rather than the self may be particularly adaptive for negatively stigmatized groups.
While studies considering the association between discrimination and blood pressure have yielded mixed results (Brondolo et al., 2003), some research on cardiovascular outcomes has also found evidence that perceived discrimination may be protective. In particular, Krieger (1990) found that African American women who reported no discrimination were more than twice as likely to have high blood pressure as those who reported one or more instances of discrimination. Additionally, in a larger sample of African American men and women, Krieger and Sidney (1996) found a U-shaped association between discrimination and blood pressure such that blood pressure was highest among those reporting no racial discrimination and lowest among those reporting moderate levels of discrimination. In line with the theories discussed above, the authors argued that the elevated blood pressure among those who reported no discrimination may reflect a form of internalized racism, whereby individuals who are part of a stigmatized group may in some sense perceive negative treatment as “deserved”.
The Current Study
Despite the known importance of cortisol in the stress response, only one study (Cohen et al., 2006) has considered perceived discrimination in the context of the diurnal cortisol rhythm. In particular, Cohen and colleagues (2006) considered whether differences in levels of reported discrimination between Whites and African Americans accounted for differences in the diurnal rhythm, and found that it did not. However, neither this study, nor to our knowledge any other, has considered the unique association between perceived discrimination and cortisol rhythms among negatively stigmatized groups, as compared to the majority group. This is surprising given that discrimination is thought to take on a very different meaning for stigmatized groups than for majority group members (Major et al., 2002; Schmitt and Branscombe, 2002). In order to address this gap in the literature, the current study will consider the relationship between perceived discrimination and cortisol diurnal rhythm for both African Americans and Whites. Based on the research discussed, we expect that, overall, African Americans will exhibit a less healthy (flatter) diurnal slope than Whites. We also predict that for Whites, discrimination will be associated with a flatter (less healthy) diurnal cortisol slope. Because of conflicting interpretations regarding the meaning of perceived discrimination among African Americans, as well as mixed findings on the association between discrimination and physical health, we make no explicit hypotheses regarding the role of perceived discrimination on HPA-axis functioning for African Americans.
The current study will also consider the moderating role of socioeconomic status (SES). SES is an important contextual variable, which may have a substantial influence on the relationship between discrimination and physical health. However, there is a dearth of empirical work on this topic, and conflicting perspectives exist on the direction of the effect. One perspective on the role of SES is that individuals from less advantaged backgrounds would have less coping resources available to them and therefore be more vulnerable to stressful experiences (Birch et al., 2000; Krueger and Chang, 2008). However, in light of research suggesting that attributions to prejudice may be protective (Crocker et al., 1991; Major et al., 2003), another perspective is that making attributions of daily stressful events to discrimination may be particularly protective for individuals in less advantaged contexts. Along these lines, Krieger and Sidney (1996) found that perceived discrimination was more strongly associated with indicators of cardiovascular health among lower SES African Americans. Adding to the small body of research on this topic, the current study will consider the moderating role of socioeconomic status in the relationship between perceived discrimination and the diurnal cortisol rhythm for African Americans and Whites.
Methods
Data
The current study draws on data from the second wave of the National Survey of Midlife in the United States (MIDUS II) and the National Study of Daily Experiences (NSDE II) (Almeida et al., 2009). Phone interviews and self-administered questionnaires for MIDUS II were conducted in 2004-2006. A random sub-sample of MIDUS II respondents were then recruited into the NSDE II. These individuals were asked to complete nightly telephone interviews as well as provide four cortisol samples each day on four consecutive days (16 cortisol samples per person). All procedures were carried out with the adequate understanding and written consent of study participants. A total of 2,022 individuals completed the NSDE II interviews (a response rate of 78%), and of these 1,736 provided saliva samples (86%).
Sample
Analyses for the current study focused on individuals with cortisol data who self-identified as Black or African American (n = 50) as well as a matched comparison group of Whites (n = 100)1. The comparison group was selected to match the African American participants on age, gender, and SES from 1,311 White participants with cortisol data. A larger comparison group of Whites (twice the size of the African American group) allows for increased statistical power to test for group differences and is commonly used when a greater number of possible comparison participants are available (Henry and McMillan, 1993; Ong et al., 2010; Shadish et al., 2002). Age was coded into five categories for the purposes of matching (35-44 years; 44-54; 55-64; 65-74; 75-84). The SES measure is described below. The final sample consisted of 150 adults between the ages of 35 and 81 (Mage= 56.7; SD = 11.7). Descriptive statistics for African American and White participants are presented in Table 1.
Table 1. Means and Standard Deviations for African Americans and Whites.
| African American n = 50 |
White n = 100 |
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| Age | 56.70 | 11.61 | 56.55 | 11.64 |
| Gender | .42 | .50 | .42 | .50 |
| Socioeconomic Status | 4.89 | 1.51 | 4.90 | 1.50 |
| Smoker | .16 | .37 | .14 | .35 |
| Cort. Medication | .10 | .30 | .07 | .26 |
| Other Medication | .20 | .40 | .23 | .42 |
| Discrimination | 1.94 | .62 | 1.35 | .49 |
| Log (Waking Cortisol) | 2.47 | .46 | 2.67 | .52 |
| Log (30min Post-waking Cortisol) | 2.79 | .52 | 2.95 | .54 |
| Log (Before Lunch Cortisol) | 2.04 | .45 | 1.94 | .50 |
| Log (Bedtime Cortisol) | 1.60 | .63 | 1.17 | .53 |
Note. Gender (female=0; male=1); Smoker (0=non-smoker; 1=smoker); Cort. Medication (0=no corticosteroid medication use; 1=corticosteroid medication use); Other Medication (0=no other medication use; 1=other medication use). Means for dichotomous variables can be interpreted as proportions (e.g., 42 percent male). Original units for cortisol are nmol/liter. A log transformed cortisol value (x) can be converted back to the original scale using the following formula: ex − 1.
Salivary Cortisol Assessment
Participants in the NSDE II were each mailed a cortisol collection kit containing numbered and color-coded salivettes approximately one week prior to giving their first saliva samples. Saliva samples were collected immediately upon waking, 30 min after waking, before lunch, and at bedtime using salivette collection devices (Sarstedt, Nümbrecht, Germany). The time of data collection for each sample was also recorded. Concentrations of cortisol in each sample were measured using a commercially available luminescence immunoassay (IBL, Hamburg, Germany). Intra-assay and inter-assay coefficients of variation were below 5 percent. Cortisol collection and assay procedures are reported in detail elsewhere (Almeida et al., 2009).
Compliance was monitored on 25% of participants who provided saliva samples using a “smart box” procedure. The boxes contained a computer chip that recorded the time respondents opened and closed the box. Correlations between self-reported sample times and times obtained from the “smart box” ranged from 0.75 for the evening occasion to 0.95 for the morning occasion (Almeida et al., 2009).
Measures
Daily Discrimination
Perceptions of daily discrimination was assessed using a 9-item measure administered in the MIDUS II questionnaire (Kessler et al., 1999). Participants were asked how often on a day-to-day basis they experience different types of discrimination. For example, participants were asked how often “people act as if they think you are not smart”, how often “you are treated with less respect than other people”, and how often “people act as if they are afraid of you”. Response options were on a four point scale: (1) never, (2) rarely, (3) sometimes, and (4) often. Items were averaged such that higher scores on the scale indicated higher levels of perceived daily discrimination (α = .91). Since the raw discrimination variable was positively skewed, it was log transformed to eliminate outliers. The log transformed version of the discrimination variable was used in all of our analyses (MBlack = .611, SD = .334; MWhite = .246, SD = .313; MFull Sample = .368, SD = .363).
Following the discrimination measure, participants were also asked to indicate the main reason for discrimination. Approximately 90% of African Americans in the MIDUS II study (as well as in the current sample) reported that race was the main reason for the instances of discrimination reported in the questionnaire (Kessler et al., 1999). Conversely, only three of the White participants in the current sample reported that the discrimination they experienced was due to race. Instead White participants cited gender (21%), age (19%), religion (11%), and height/weight (10%) as the main reasons for the unfair treatment they experienced.
Demographics
Gender was reported in the MIDUS II telephone interview. Age was calculated by subtracting each participant’s date of birth from the date of their MIDUS II telephone interview. Participant’s reports of their own and their spouse’s level of education were averaged as an indicator of household SES. Level of education was coded on the following 10-point scale: eighth grade or less (1), some high school (2), GED (3), graduated from high school (4), some college but no degree (5), graduated from 2 year college or vocational school (6), graduated from four or five year college (7), some graduate school (8), masters degree (9), PhD, MD, JD, or other professional degree (10). For unmarried participants (35%), just their own level of education was used.
Control Variables
The influence of smoking and medication use was controlled due to known influence of these factors on the diurnal cortisol rhythm (Badrick et al., 2007; Granger et al., 2009; Steptoe and Ussher, 2005). In the MIDUS II interview participants were asked to report whether they currently smoke cigarettes, and in the NSDE II daily interviews participants also reported the number of cigarettes they smoked each day. Smoking status was coded as 1 if participants reported being a current smoker or reported smoking any cigarettes across the daily study, and was otherwise coded as 0.
As part of the NSDE II telephone interviews, participants also reported on their current use of medications that may have some influence on diurnal cortisol rhythms (e.g., steroid medications, medications or creams containing cortisone, birth control pills, other hormonal medications) (Granger et al., 2009). A dichotomous variable was created indicating whether or not participants were using any corticosteroid medications (0 = no corticosteroid medication use; 1 = corticosteroid medication use) and whether or not they were using other medications (0 = no other medication use; 1 = other medication use).
Analysis Strategy
The diurnal cortisol rhythm was parameterized using a three-level multilevel model (Raudenbush and Bryk, 2002). In the context of the current study, level 1 variables are those that vary across occasions of measurement within a day (e.g., cortisol), level 2 variables are those that vary across days but do not change across measurements within a day (e.g., time of awakening), and level 3 variables are characteristics of individuals in that they do not change across occasions or days (e.g., gender). The level 1 equation for all of the models presented in this paper is as follows:
The intercept (π0), linear slope (π1), quadratic effect (π2), and awakening response (π3) are all latent variables, which together define the shape of an individual’s cortisol trajectory. Variance components are estimated for all level 1 parameters, such that each is allowed to vary across individuals and the intercept and slope are also allowed to vary across days.
Time since waking was coded in hours, and the awakening response was coded as 1 for the second cortisol sample (taken approximately 30 minutes after waking) and as 0 for the other samples. Coding time and the awakening response in this manner defines the intercept as the time of waking and allows for the diurnal slope to be unaffected by the magnitude of the awakening response (Adam et al., 2006). This modeling strategy is in line with current conceptualizations of the cortisol diurnal rhythm (Adam and Kumari, 2009; Wilhelm et al., 2007) and has been used successfully in prior research (e.g., Adam et al., 2006; Doane and Adam, 2010).
Control and substantive variables were entered as level 2 and 3 predictors in order to test our hypotheses of interest. Following subscript conventions used in recent research on the cortisol rhythm (Adam et al., 2006), the form of the level 2 and level 3 equations are as follows:
Parameters at higher levels contain subscripts that link them to lower level parameters. For example, the first subscript on the level 2 parameter β30 indicate that it defines the level 1 parameter π3, and the first and second subscripts on the level 3 parameter γ300 together indicate that it defines to the level 2 parameter β30. The subscript i relates to level 1 (occasion), j relates to level 2 (day), and k to level 3 (person). When specified, the subscripts i, j, and k uniquely define each parameter.
At level 2, time of awakening was person-centered and was included in all models as a predictor of the awakening response. This allows for the influence of within-person deviations from their average wake-up time to be accounted for (Almeida et al., 2009). Approximately 90% of the samples relating to the awakening response (the second saliva sample given each day) were provided within a window from 15 to 60 minutes after waking. In order to account for variability due to early or late sampling, dichotomous control variables indicating which samples were provided less than 15 minutes and more than 60 minutes after awakening were included in our models as predictors of the awakening response. A dichotomous variable—indicating whether or not a given day of data was collected on a weekend—was also included in all models as a predictor of the awakening response (Scholtz et al., 2004).
At level 3, smoking and medication use were controlled because of their known influences on the diurnal cortisol rhythm (Badrick et al., 2007; Granger et al., 2009). Age, gender, and SES were also included as level 3 predictors in all models. Dichotomously coded variables were all uncentered (gender, smoker, medication use). Age and SES were both grand-mean centered. SES was also divided by the sample standard deviation (z-scored) to simplify interpretation of the model parameters.
Addressing specific hypotheses
Two sets of models were estimated in order to test the hypotheses of interest. The first set of models considered race differences in cortisol rhythms (model 1), and looked at levels of perceived discrimination as a possible mediator of these differences (model 2). We then added a discrimination by race interaction in order to test the hypotheses that perceived discrimination will be associated with more healthy cortisol rhythms for African Americans but not for Whites.
The second set of models looked at African Americans and Whites separately in order to consider moderators of discrimination for each group. In particular, we tested the hypothesis that the influence of perceived discrimination on the diurnal slope would be conditioned by SES for African Americans, but not for Whites. Model 1 established the main effect of discrimination, and model 2 added the discrimination by SES interaction term.
Results
Means and standard deviations for study variables are shown in Table 1 for African Americans and Whites. Independent samples t-tests were conducted to test for differences on continuous variables between groups. As expected, perceived discrimination was higher among African Americans than Whites, t(148) = 6.58, p < .001. Additionally, in line with previous research (Cohen et al., 2006), cortisol levels were lower at waking, t(148) = −2.36, p = .019, and higher at bedtime, t(148) = 4.36, p < .001, for African Americans than for Whites. No other differences were found between groups on any of the other study variables. Based on previous research (Paradies, 2006), we also considered the associations between perceived discrimination and SES for African Americans and Whites, but did not find them to be significant (African Americans: r = .193, p = .180; Whites: r = −.050, p = .621).
Cortisol Diurnal Rhythms for African Americans and Whites
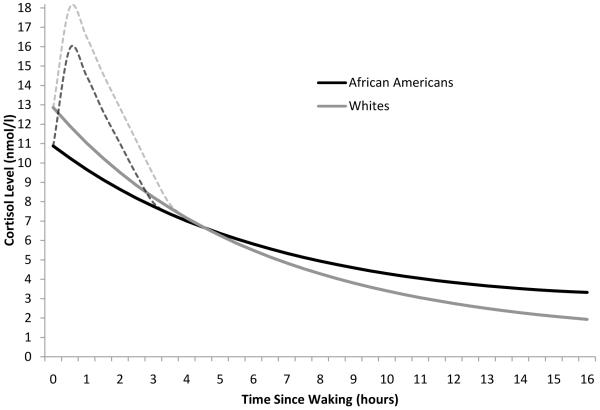
Our first set of analyses established an average diurnal rhythm for African Americans and Whites, and looked at the influence of discrimination for each group. Models relating to these analyses are shown in Table 2. Model 1 tested for race differences in the diurnal rhythm controlling for age, gender, SES, smoking, and medication use. In line with previous research (DeSantis et al., 2007; Cohen et al., 2006), parameter estimates for model 1 indicated that African Americans had a 15% lower waking cortisol levels than Whites (p = .049), and a 31% flatter diurnal slope (p < .001)2. The intercept was also recoded to 16 hours post-waking in order to test for differences in bedtime cortisol levels across groups. In line with findings from the raw data, bedtime levels were found to be 73% higher among African Americans than Whites (Estimate = .393, SE = .098, p < .001). No differences were found between groups in the awakening response. Figure 1 depicts the average diurnal cortisol rhythms for African Americans and Whites.
Table 2. Three-level Model Parameter Estimates Showing Differential Effects of Perceived Discrimination for African Americans and Whites.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Fixed Effects | Estimate | SE | Estimate | SE | Estimate | SE |
| Wake-up cortisol, π0 | ||||||
| Average wake-up level, β00 | ||||||
| Intercept, γ000 | 2.629*** | .064 | 2.626*** | .065 | 2.590*** | .069 |
| Age, γ001 | .004 | .003 | .004 | .003 | .005 | .003 |
| Gender, γ002 | .129 | .075 | .130 | .076 | .137 | .074 |
| SES, γ003 | .066* | .034 | .067* | .034 | .059 | .033 |
| Smoker, γ004 | −.078 | .140 | −.074 | .137 | −.065 | .134 |
| Cort. medication, γ005 | .079 | .106 | .079 | .105 | .114 | .107 |
| Other medication, γ006 | −.011 | .106 | −.110 | .106 | −.100 | .105 |
| Race, γ007 | −.154* | .076 | −.148 | .080 | −.211** | .072 |
| Discrimination, γ008 | -- | -- | −.006 | .033 | −.081 | .048 |
| Discrimination*Race, γ009 | -- | -- | -- | -- | .205** | .073 |
| Time since waking, π1 | ||||||
| Average linear slope,β10 | ||||||
| Intercept, γ100 | −.144*** | .011 | −.143*** | .011 | −.139*** | .011 |
| Age, γ101 | .001* | .000 | .001* | .000 | .001* | .000 |
| Gender, γ102 | −.000 | .006 | −.000 | .006 | −.001 | .006 |
| SES, γ103 | −.005 | .003 | −.005 | .003 | −.004 | .003 |
| Smoker, γ104 | .020* | .010 | .019 | .010 | .019 | .010 |
| Cort. medication, γ105 | .005 | .012 | .005 | .010 | .002 | .011 |
| Other medication, γ106 | .008 | .008 | .008 | .008 | .007 | .007 |
| Race, γ107 | .034*** | .006 | .032*** | .008 | .038*** | .008 |
| Discrimination, γ108 | -- | -- | .002 | .004 | .009* | .005 |
| Discrimination*Race, γ109 | -- | -- | -- | -- | −.020** | .007 |
| Time since waking squared, π2 | ||||||
| Average curvature, β20 | ||||||
| Intercept, γ200 | .0029*** | .0005 | .0029** | .0005 | .0029*** | .0005 |
| Awakening response, π3 | ||||||
| Average awakening response, β30 | ||||||
| Intercept, γ300 | .389*** | .055 | .374*** | .060 | .386*** | .063 |
| Age, γ301 | −.002 | .003 | −.002 | .003 | −.002 | .003 |
| Gender, γ302 | .056 | .067 | .059 | .068 | .057 | .068 |
| SES, γ303 | −.054 | .030 | −.053 | .030 | −.051 | .031 |
| Smoker, γ304 | −.079 | .118 | −.062 | .126 | −.064 | .126 |
| Cort. medication, γ305 | −.150 | .131 | −.150 | .128 | −.161 | .126 |
| Other medication, γ306 | .244** | .079 | .245** | .078 | .242** | .078 |
| Race, γ307 | .019 | .062 | .047 | .068 | .065 | .067 |
| Discrimination, γ308 | -- | -- | −.028 | .033 | −.004 | .045 |
| Discrimination*Race, γ309 | -- | -- | -- | -- | −.062 | .065 |
| Waking time, β31 | −.049 | .038 | −.050 | .038 | −.050 | .038 |
| Weekend day, β32 | −.143* | .060 | −.142* | .060 | −.142* | .060 |
| < 15 minutes from waking, β33 | −.173 | .144 | −.182 | .147 | −.187 | .146 |
| > 60 minutes from waking, β34 | −.269** | .078 | −.263** | .079 | −.261** | .079 |
p<.05
p<.01
p<.001.
Figure 1.
Average diurnal cortisol rhythms for African Americans and Whites. N = 150. Note. The peak of the awakening response is estimated in the model; the rest of the awakening response (dashed line) is illustrative, and is included in the figure for visual completeness.
Model 2 added perceived discrimination as a predictor of the waking level, slope, and awakening response. Replicating previous research (Cohen et al., 2006), discrimination did not account for a substantial portion of the difference between African Americans and Whites in the waking level or diurnal slope (see Table 2). Furthermore, in model 2, perceived discrimination did not predict the waking level, diurnal slope, or awaking response.
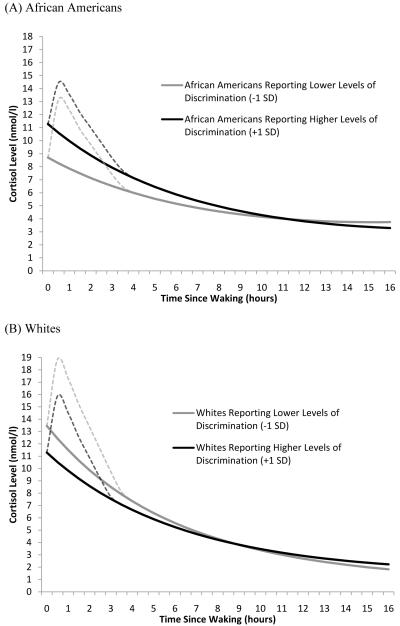
Model 3 added an interaction term allowing the influence of discrimination to vary across races. Findings from this model showed that the influence of discrimination varied with race (p = .008). In particular, higher levels of perceived discrimination were associated with an 11% flatter diurnal slope for Whites, but a 33% steeper slope for African Americans. Furthermore, the influence of discrimination on waking levels also varied with race (p =.006): higher levels of perceived discrimination (+1 SD) were associated with 16% higher waking levels for African Americans, but 8% lower waking levels for Whites. Figure 2 illustrates the relationship between perceived discrimination and the diurnal cortisol slope for African Americans (Figure 2A) and Whites (Figure 2B). Simple slopes analysis revealed that the main effect of discrimination on both the waking level and slope was significant for African Americans (waking level: Estimate = .126, SE = .049, p = .011; slope: Estimate = .011, SE = .006, p = .048). Since Whites are the reference group, the influence of perceived discrimination on the diurnal slope for Whites is shown in model 3 (p = .035). The main effect of discrimination on the waking level was not, however, significant for Whites (p = .130).
Figure 2.
Fitted interaction plot showing the influence of perceived discrimination on the diurnal cortisol rhythm for (A) African Americans and (B) Whites. Higher and lower levels of perceived discrimination were defined as plus and minus one SD from the sample mean. In original scale units this translates to scores of 1.0 and 2.1. N=150.
SES as a Moderator of the Influence of Perceived Discrimination
Our second set of models looked at African Americans and Whites separately in order to consider SES as a moderator of the relationship between perceived discrimination and the diurnal cortisol rhythm for each group. Models for African Americans are shown in Table 3 (N = 50). The first model included the main effects of discrimination and SES as well as the control and demographic variables. This model replicated the findings reported above, showing that perceived discrimination was associated with a steeper diurnal slope for African Americans (p = .008). Model 1 also indicated that African American males had a steeper slope than females (p = .052).
Table 3. Three-level Model Parameter Estimates for African Americans Showing the Moderating Influence of Socioeconomic Status.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Fixed Effects | Estimate | SE | Estimate | SE |
| Wake-up cortisol, π0 | ||||
| Average wake-up level, β00 | ||||
| Intercept, γ000 | 2.389*** | .069 | 2.381*** | .074 |
| Age, γ001 | .004 | .005 | .003 | .005 |
| Gender, γ002 | .102 | .123 | .117 | .128 |
| SES, γ003 | .030 | .049 | .029 | .050 |
| Smoker, γ004 | .175 | .232 | .176 | .231 |
| Cort. medication, γ005 | .022 | .117 | .019 | .116 |
| Other medication, γ006 | .098 | .152 | .102 | .154 |
| Discrimination, γ007 | .100 | .051 | .101 | .051 |
| Discrimination*SES, γ008 | -- | -- | .013 | .039 |
| Time since waking, π1 | ||||
| Average linear slope,β10 | ||||
| Intercept, γ100 | −.086*** | .017 | −.090*** | .017 |
| Age, γ101 | −.000 | .000 | −.001 | .000 |
| Gender, γ102 | −.021 | .011 | −.015 | .011 |
| SES, γ103 | −.006 | .005 | −.006 | .005 |
| Smoker, γ104 | .024 | .013 | .026 | .013 |
| Cort. medication, γ105 | −.000 | .017 | −.003 | .017 |
| Other medication, γ106 | −.013 | .111 | −.012 | .011 |
| Discrimination, γ107 | −.013** | .005 | −.014** | .004 |
| Discrimination*SES, γ108 | -- | -- | .007* | .003 |
| Time since waking squared, π2 | ||||
| Average curvature, β20 | ||||
| Intercept, γ200 | .0023* | .0010 | .0023* | .0010 |
| Awakening response, π3 | ||||
| Average awakening response, β30 | ||||
| Intercept, γ300 | .499*** | .097 | .492*** | .108 |
| Age, γ301 | .002 | .004 | .002 | .004 |
| Gender, γ302 | −.080 | .108 | −.072 | .118 |
| SES, γ303 | −.076 | .058 | −.078 | .057 |
| Smoker, γ304 | −.135 | .158 | −.133 | .160 |
| Cort. medication, γ305 | −.017 | .260 | −.013 | .257 |
| Other medication, γ306 | .232 | .116 | .239* | .119 |
| Discrimination, γ307 | −.021 | .051 | −.023 | .051 |
| Discrimination*SES, γ308 | -- | -- | −.009 | .046 |
| Waking time, β31 | −.127** | .038 | −.129** | .037 |
| Weekend day, β32 | −.173 | .117 | −.168 | .119 |
| < 15 minutes from waking, β33 | −.279** | .090 | −.275** | .086 |
| > 60 minutes from waking, β34 | −.378** | .108 | −.388** | .104 |
p<.05
p<.01
p<.001.
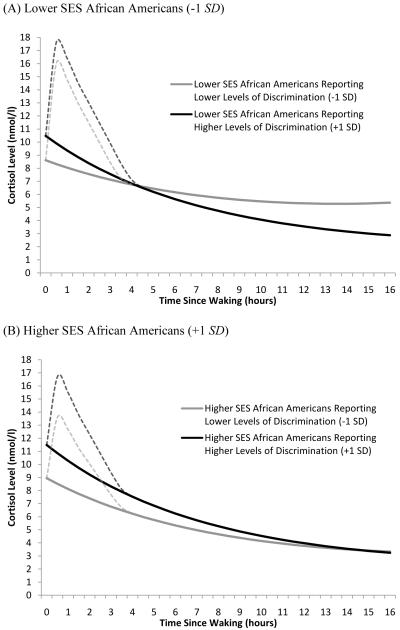
Model 2 added the discrimination by SES interaction. In line with our hypotheses, findings suggested that the association between perceived discrimination on the diurnal slope was stronger for lower SES African Americans (p = .035). In particular, whereas for higher SES African Americans (+1 SD) lower levels of perceived discrimination (−1 SD) were associated with a 19% flatter diurnal cortisol slope, for lower SES African Americans (−1 SD), lower levels of perceived discrimination were associated with a 40% flatter diurnal slope. Figure 3 illustrates the influence of perceived discrimination for lower SES African Americans (Figure 3A) and higher SES African Americans (Figure 3B).
Figure 3.
Fitted interaction plot showing the influence of perceived discrimination on the diurnal cortisol rhythms for (A) lower SES African Americans and (B) higher SES African Americans. Higher and lower SES were defined as plus and minus one SD from the mean for African Americans. This translates to scores of 3.4 and 6.4 on the SES scale. Higher and lower levels of perceived discrimination were also defined as plus and minus one SD from the mean for African Americans. In original scale units this translates to scores of 1.3 and 2.6. N=50.
In a separate model, the potential moderating influence of SES was also explored for Whites. However, the discrimination-by-SES interaction did not influence the linear slope (Estimate = −.005, SE = .004, p = .235). The interaction also had no influence on the waking level or awakening response.
Discussion
To the best of our knowledge this study is the first to demonstrate an association between perceived discrimination and the diurnal cortisol rhythm, as well as the first to demonstrate that this association may be different for African Americans than for Whites. In particular, the results suggest that higher levels of perceived discrimination are associated with a flatter (less healthy) diurnal slope for Whites, but a steeper (more healthy) diurnal slope for African Americans. These findings are in line with theoretical perspectives suggesting that an awareness of racism in daily life may serve as an important protective mechanism for negatively stigmatized minority groups (Cross, 1991; Helms, 1995; Sellers et al., 1998; Spencer and Markstrom-Adams, 1990). Furthermore, the findings add to existing evidence that an awareness of discrimination may have a positive impact on the physical health of African Americans (e.g., Krieger and Sidney, 1996; LaVeist et al., 2001).
The current study also replicated previous research on differences in the diurnal cortisol rhythm between African Americans and Whites (Cohen et al., 2006). In particular, findings suggest that African Americans tend to have a lower waking cortisol level and a flatter diurnal slope than Whites, even after controlling for SES. These patterns of diurnal cortisol are considered to be indicative of HPA axis dysregulation, and have been associated with chronic stress exposure and worse health outcomes (Adam and Kumari, 2009). With at least three studies now showing these effects, it will be important for future research to discern specific explanatory variables that account for these disparities in health.
Another important focus of this study was to consider the influence of SES as a moderator of the relationship between perceived discrimination and health. Findings on this topic suggest that the positive association between perceived discrimination and the diurnal cortisol slope is stronger for lower SES African Americans than for their higher SES counterparts. Specifically, results showed that lower SES African Americans reporting low levels of discrimination had the flattest (least healthy) diurnal slope. This corroborates existing research on the relationship between discrimination and health (Krieger and Sidney, 1996), and extends these findings to show that, with respect to the healthy functioning of the HPA axis, an awareness or acknowledgement of discrimination may be particularly important for African Americans living in less advantaged socioeconomic contexts.
On the flip side, these findings could also be interpreted to suggest that perceiving discrimination may be less protective for higher SES African Americans. This may be because acknowledging discrimination may have more challenging interpersonal consequences for higher SES African Americans, who are more likely to be in contact with Whites in their work environments, and thus more likely to be faced with the challenge of determining how to work with individuals who are treating them differently because of their race. This interpretation is also consistent with the research suggesting that African Americans may benefit less (with respect to their health) from increases in SES than do Whites, because of the racial challenges that come with interacting in work settings that are often dominated by Whites (Gee, 2002; Pamuk et al., 1998; Yen et al., 1999).
Limitations and Future Directions
Several limitations of the current study should be noted. Because the study was conducted on a moderately sized sample of older adults, findings should not be generalized beyond this age group and should be considered preliminary until replicated. In addition to corroborating the findings among older adults, it will also be important for research to consider whether differential relationships across ethnic groups are also evident during adolescence and early adulthood. It is possible, for example, that perceptions of discrimination may be more strongly associated with negative health outcomes during early adolescence, when the self-concept (e.g., racial identity) is still in its early stages of development.
Secondly, although it appears that reporting discrimination is not associated with diurnal cortisol dysregulation among African Americans, this does not necessarily suggest that perceived discrimination is not having any negative influence on health. It may, for example, be the case that perceptions of discrimination are associated with internal blame and shame or loneliness among Whites (which have been linked to diurnal cortisol dysregulation), but with external blame and anger among African Americans. Although anger has not been linked to dysregulation of the diurnal cortisol slope, it has been consistently linked to cardiovascular health outcomes (Smith, 2006). Therefore, although perceived discrimination does not seem to be linked to HPA-axis pathology, it may be linked to cardiovascular health outcomes for African Americans. While findings relating to the effects of discrimination on blood pressure among African Americans are currently mixed (Brondolo et al., 2003; Williams and Mohammed, 2008), a closer examination how an individual responses to experiences of discrimination may influence different health systems is called for.
While the current study established differential associations between perceived discrimination and the cortisol diurnal rhythm for African Americans and Whites, it was not within the scope of this study to test the specific mechanisms behind these differences. It will therefore be fruitful for future research to consider variables that may explain the relationship between perceived discrimination and physical health. For example, given existing evidence relating to the importance of racial/ethnic identity in the lives of stigmatized minority groups (Cross, 1991; Helms, 1995; Sellers et al., 1998; Spencer and Markstrom-Adams, 1990; Wong et al, 2003), we believe that implicit and explicit measures of racial/ethnic identity may offer promising potential as explanatory variables. Moreover, research which further considers how attributions of specific negative experiences to discrimination influence physiological reactivity will also be helpful in piecing together an understanding of how daily experiences accumulate to influence physical health over longer periods of time (e.g., Mendes et al, 2008). Finally, types of stigma and their association with perceived control may have differential consequences for health. For example, it is possible that discrimination based on uncontrollable characteristics such as race or gender may be more easily discounted and therefore less harmful than discrimination on the basis of characteristics such as religion or weight, factors that are more likely to be perceived as within an individual’s control (Quinn and Crocker, 1999).
The assertion that perceptions of discrimination may reflect a healthy awareness of racism among members of stigmatized minority groups also brings up the issue of how the injurious influence of discrimination can be effectively studied. Along these lines, we believe that person-centered daily experience designs (Bolger et al., 2003) will be important for progress in this area of research. Such studies are able to consider average reported levels of discrimination alongside within-person deviations across days, and therefore, within a single dataset, are better able to parse the influence of person-specific reporting tendencies from specific noxious events. Furthermore, experimental designs which manipulate both exposure to discrimination as well as the subjectivity of the experience would cast light on how perceptions and interpretations of discrimination may influence the individual.
Most studies examining the role of discrimination have considered the recipients as passive, rather than active, and thus have not adequately considered how individuals interpret, make meaning of, and cope with the experience. Because models of stress and resilience emphasize the importance of both the appraisal and coping process (Lazarus, 2000; Lazarus and Folkman, 1984; Pearlin et al., 1981), in understanding how stress affects well-being, it is imperative for research to consider how experiences of discrimination are interpreted and managed. For example, it is possible that a tendency not to report discrimination may reflect a form of suppression for stigmatized minority groups (Krieger and Sidney, 1996). Findings from the current study highlight the importance of considering the meaning of reporting, or not reporting, discrimination among members of stigmatized minority groups, as compared to majority group members. We hope that our findings will stimulate innovations in basic and applied research that will lead to a more accurate understanding of how daily experiences of discrimination influence disparities in health.
Acknowledgements
The authors thank Dr. Anthony Ong and Dr. Anthony Burrow for helpful comments on earlier versions of this analysis, and Ms. Adrienne Meltzer, who assisted with preparation and proof-reading of the manuscript.
Role of the Funding Source
Support for this research was provided by the National Institute on Aging Grants RC2 AG036780-01. The MIDUS (Midlife in the United States) study data collection was supported by the National Institute on Aging Grants P01 AG020166 and R01 AG019239.
Footnotes
Racial categories were created from questions in the MIDUS II telephone interview. The “African American” sample was defined as those who indicated “Black and/or African American” as the main category or only category that describes their racial background, and did not report being of Hispanic origin. White was defined as individuals who indicated “White” as the only category that describes their racial background, and did not report being of Hispanic origin.
Effect sizes were calculated by converting log transformed estimates from the model back to the original units (nmol/liter) and then calculating the percent difference in the awaking level and slope based on these values.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proc. Natl. Acad. Sci. U.S.A. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography Soc. Biol. 2009;55:220–238. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Piazza JR, Stawski RS. Interindividual differences in intraindividual variability in the cortisol awakening response: An examination of age and gender. Psychol. Aging. 2009;24:819–827. doi: 10.1037/a0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J. Clin. Endocrinol. Metab. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- Banks KH, Kohn-Wood LP, Spencer M. An examination of the African American experience of everyday discrimination and symptoms of psychological distress. Community Ment. Hlt. J. 2006;42:555–570. doi: 10.1007/s10597-006-9052-9. [DOI] [PubMed] [Google Scholar]
- Birch S, Jerrett M, Eyles J. Heterogeneity in the determinants of health and illness: the example of socioeconomic status and smoking. Soc. Sci. Med. 2000;51:307–317. [PubMed] [Google Scholar]
- Bolger N, Davis A, Rafaeli E. Diary methods: Capturing life as it is lived. Annu. Rev. Psychol. 2003;54:579–616. doi: 10.1146/annurev.psych.54.101601.145030. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Disruption of affectional bonds and its effects on behavior. Canada’s Mental Health Supplement. 1969:59. [Google Scholar]
- Brody GH, Chen YF, Murry VM, Ge X, Simons RL, Gibbons FX, Gerrard M, Cutrona C. Perceived discrimination and the adjustment of African American youths: A five-year longitudinal analysis with contextual moderation effects. Child Dev. 2006;77:1170–1189. doi: 10.1111/j.1467-8624.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- Brondolo E, Rieppi R, Kelly KP, Gerin W. Perceived racism and blood pressure: A review of the literature and conceptual and methodolgical critique. Ann. Behav. Med. 2003;25:55–65. doi: 10.1207/S15324796ABM2501_08. [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans: A biopsychosocial model. Am. Psychol. 1999;54:805–816. doi: 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman TE. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom. Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Collins J, David R, Handler A, Wall S, Andes S. Very low birth weight in African American infants: The role of maternal exposure to interpersonal racial discrimination. Am. J. Public Health. 2004;94:2132–2138. doi: 10.2105/ajph.94.12.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J, Voelkl K, Testa M, Major B. Social stigma: The affective consequences of attributional ambiguity. J. Pers. Soc. Psychol. 1991;60:218–228. doi: 10.1037//0022-3514.64.1.60. [DOI] [PubMed] [Google Scholar]
- Cross WE., Jr. Shades of Black. Temple University Press; Philadelphia: 1991. [Google Scholar]
- DeSantis A, Adam EK, Doane L, Mineka S, Zinbarg R, Craske M. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J. Adolesc. Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Fix M, Struyk RJ. Clear and Convincing Evidence: Measurement of Discrimination in America. Urban Institute Press; Washington, D.C.: 1993. [Google Scholar]
- Gee GC. A Multilevel analysis of the relationship between institutional and individual racial discrimination and health status. Am. J. Public Health. 2002;92:615–623. doi: 10.2105/ajph.92.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons FX, Gerrard M, Cleveland MJ, Wills TA, Brody G. Perceived discrimination and substance use in African American parents and their children: A panel study. J. Pers. Soc. Psychol. 2004;86:517–529. doi: 10.1037/0022-3514.86.4.517. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Gurin P, Gurin G, Morison B. Personal and ideological aspects of internal and external control. Soc. Psychol. 1978;41:275–296. [Google Scholar]
- Helms JE. An update on Helm’s White and people of color racial identity model. In: Ponterotto JG, Casas JM, Suzuki LA, Alexander CM, editors. Handbook of multicultural counseling. Sage; Thousand Oaks, CA: 1995. pp. 181–198. [Google Scholar]
- Henry GT, McMillan JH. Performance data: Three comparison methods. Eval. Rev. 1993;17:643–652. [Google Scholar]
- Jorgensen RS, Thibodeau R. Defensive avoidance of disapproval: The relationship of a defensive style to physical and mental health. Harv. Rev. Psychiatry. 2007;15:9–17. doi: 10.1080/10673220601183923. [DOI] [PubMed] [Google Scholar]
- Krieger N. Racial and gender discrimination: Risk factors for high blood pressure? Soc. Sci. Med. 1990;30:1273–1281. doi: 10.1016/0277-9536(90)90307-e. [DOI] [PubMed] [Google Scholar]
- Krieger N, Sidney S. Racial discrimination and blood pressure: The CARDIA study of young black and white adults. Am. J. Public Health. 1996;86:1370–1378. doi: 10.2105/ajph.86.10.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger PM, Chang VW. Being poor and coping with stress: health behaviors and the risk of death. Am. J. Public Health. 2008;98:889–96. doi: 10.2105/AJPH.2007.114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVeist TA, Sellers R, Neighbors HW. Perceived racism and self and system blame attribution: consequences for longevity. Ethn. Dis. 2001;11:711–721. [PubMed] [Google Scholar]
- Lazarus RS. Toward better research on stress and coping process. Am. Psychol. 2000;55:665–673. doi: 10.1037//0003-066x.55.6.665. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal and Coping. Springer; New York, NY: 1984. [Google Scholar]
- Leary MR. Responses to social exclusion: Social anxiety, jealousy, loneliness, depression, and low self-esteem. J. Soc. Clin. Psychol. 1990;9:221–229. [Google Scholar]
- Lewis T, Everson-Rose S, Powell LH, Matthews KA, Brown C, Karavolos K, et al. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: The SWAN Heart Study. Psychosom. Med. 2006;68:362–368. doi: 10.1097/01.psy.0000221360.94700.16. [DOI] [PubMed] [Google Scholar]
- Major B, Quinton WJ, McCoy SK. Antecedents and consequences of attributions to discrimination: Theoretical and empirical advances. In: Zanna MP, editor. Advances in experimental social psychology. Vol. 34. Academic Press; San Diego, CA: 2002. pp. 251–330. 2002. [Google Scholar]
- Major B, Quinton WJ, Schmader T. Attributions to discrimination and self-esteem: Impact of group identification and situational ambiguity. J. Exp. Soc. Psychol. 2003;39:220–231. [Google Scholar]
- Matthews K, Schwartz J, Cohen S, Seeman TE. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom. Med. 2006;68:657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination and health outcomes among African Americans. Annu. Rev. Psychol. 2007;58:201–225. doi: 10.1146/annurev.psych.57.102904.190212. doi: 10.1146/annurev.psych.57.102904.190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Mendes WB, Major B, McCoy S, Blascovich J. How attributional ambiguity shapes physiological and emotional responses to social rejection and acceptance. J. Pers. Soc. Psychol. 2008;94:278–291. doi: 10.1037/0022-3514.94.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustillo S, Krieger N, Gunderson EP, Sidney S, McCreath H, Kiefe CI. Self-reported experiences of racial discrimination and Black–White differences in preterm and low-birth weight deliveries: The CARDIA Study. Am. J. Public Health. 2004;94:2125–2131. doi: 10.2105/ajph.94.12.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong AD, Fuller-Rowell TE, Bonanno GA. Prospective Predictors of Positive Emotions Following Spousal Loss. Psychol. Aging. 2010;25:653–660. doi: 10.1037/a0018870. [DOI] [PubMed] [Google Scholar]
- Ong AD, Fuller-Rowell TE, Burrow AL. Racial discrimination and the stress process. J. Pers. Soc. Psychol. 2009;96:1259–1271. doi: 10.1037/a0015335. [DOI] [PubMed] [Google Scholar]
- Pager D, Shepherd H. The sociology of discrimination: Racial discrimination in employment, housing, credit, and consumer markets. Annu. Rev. Sociol. 2008;34:181–209. doi: 10.1146/annurev.soc.33.040406.131740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamuk E, Makuk D, Heck K, Reuben C. Socioeconomic status and health chartbook. National Center for Health Statistics; Hyattsville, MD: 1998. [Google Scholar]
- Paradies Y. A systematic review of empirical research on self-reported racism and health. Int. J. Epidemiol. 2006;35:888–901. doi: 10.1093/ije/dyl056. [DOI] [PubMed] [Google Scholar]
- Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J. Health Soc. Behav. 1981;22:337–356. [PubMed] [Google Scholar]
- Pyszczynski T, Greenberg J, Solomon S. Why do we need what we need? A terror management perspective on the roots of human social motivation. Psychol. Inq. 1997;8:1–20. [Google Scholar]
- Quinn DM, Crocker J. When ideology hurts: Effects of belief in the Protestant ethic and feeling overweight on the psychological well-being of women. J. Pers. Soc. Psychol. 1999;77:402–414. doi: 10.1037//0022-3514.77.2.402. [DOI] [PubMed] [Google Scholar]
- Ryan AM, Gee GC, Laflamme DF. The Association between self-reported discrimination, physical health and blood pressure: Findings from African Americans, Black immigrants, and Latino immigrants in New Hampshire. J. Health Care Poor U. 2006;17:116–132. doi: 10.1353/hpu.2006.0092. [DOI] [PubMed] [Google Scholar]
- Schmitt MT, Branscombe NR. The meaning and consequences of perceived discrimination in disadvantaged and privileged social groups. In: Stroebe W, Hewstone M, editors. European review of social psychology. Vol. 12. Wiley; Chichester, UK: 2002. pp. 167–200. [Google Scholar]
- Schlotz W, Hellhammer J, Schulz P, Stone AA. Perceived work overload and chronic worrying predict weekend-weekday differences in the cortisol awakening response. Psychosom. Med. 2004;66:207–214. doi: 10.1097/01.psy.0000116715.78238.56. [DOI] [PubMed] [Google Scholar]
- Schulz AJ, Gravlee CC, Williams DR, Israel BA, Mentz G, Rowe Z. Discrimination, symptoms of depression, and self-rated health among African American women in Detroit: Results from a longitudinal analysis. Am. J. Public Health. 2006;96:1265–1270. doi: 10.2105/AJPH.2005.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for general causal inference. Houghton Mifflin; Boston, MA: 2002. [Google Scholar]
- Sellers RM, Shelton JN. The role of racial identity in perceived racial discrimination. J. Pers. Soc. Psychol. 2003;84:1079–1092. doi: 10.1037/0022-3514.84.5.1079. [DOI] [PubMed] [Google Scholar]
- Sellers R, Smith M, Shelton J, Rowley S, Chavous T. Multidimensional model of racial identity: A reconceptualization of African American racial identity. Pers. Soc. Psychol. Rev. 1998;2:18–39. doi: 10.1207/s15327957pspr0201_2. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl. Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Smedley BD, Stith AY, Nelson AR, editors. Unequal treatment: Confronting racial and ethnic disparities in health care. National Academy Press; Washington, D.C.: 2003. [PubMed] [Google Scholar]
- Spencer MB, Markstrom-Adams C. Identity processes among racial and ethnic minority children in America. Child Dev. 1990;61:290–310. [Google Scholar]
- Steptoe A, Ussher M. Smoking, cortisol and nicotine. Int. J. Psychophysiol. 2006;59:228–235. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA. Discrimination and racial disparities in health: Evidence and needed research. J. Behav. Med. 2009;32:20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Neighbors HW, Jackson JS. Ethnic-racial discrimination and health: Findings from community studies. Am. J. Public Health. 2003;93:200–208. doi: 10.2105/ajph.93.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CA, Eccles JS, Sameroff A. The influence of ethnic discrimination and ethnic identification on African American adolescents’ school and socioemotional adjustment. J. Pers. 2003;71:1197–1232. doi: 10.1111/1467-6494.7106012. [DOI] [PubMed] [Google Scholar]
- Yen IH, Ragland DR, Greiner BA, Fisher JM. Workplace discrimination and alcohol consumption: findings from the San Francisco Muni Health and Safety Study. Ethn. Dis. 1999;9:70–80. [PubMed] [Google Scholar]