Abstract
Rationale
A growing number of patients with coronary disease have refractory angina. Preclinical and early-phase clinical data suggest that intramyocardial injection of autologous CD34+ cells can improve myocardial perfusion and function.
Objective
Evaluate the safety and bioactivity of intramyocardial injections of autologous CD34+ cells in patients with refractory angina who have exhausted all other treatment options.
Methods and Results
In this prospective, double-blind, randomized, phase II study (ClinicalTrials.gov identifier: NCT00300053), 167 patients with refractory angina received 1 of 2 doses (1×105 or 5×105 cells/kg) of mobilized autologous CD34+ cells or an equal volume of diluent (placebo). Treatment was distributed into 10 sites of ischemic, viable myocardium with a NOGA mapping injection catheter. The primary outcome measure was weekly angina frequency 6 months after treatment. Weekly angina frequency was significantly lower in the low-dose group than in placebo-treated patients at both 6 months (6.8±1.1 versus 10.9±1.2, P=0.020) and 12 months (6.3±1.2 versus 11.0±1.2, P=0.035); measurements in the high-dose group were also lower, but not significantly. Similarly, improvement in exercise tolerance was significantly greater in low-dose patients than in placebo-treated patients (6 months: 139±151 versus 69±122 seconds, P=0.014; 12 months: 140±171 versus 58±146 seconds, P=0.017) and greater, but not significantly, in the high-dose group. During cell mobilization and collection, 4.6% of patients had cardiac enzyme elevations consistent with non-ST segment elevation myocardial infarction. Mortality at 12 months was 5.4% in the placebo-treatment group with no deaths among cell-treated patients.
Conclusions
Patients with refractory angina who received intramyocardial injections of autologous CD34+ cells (105 cells/kg) experienced significant improvements in angina frequency and exercise tolerance. The cell-mobilization and -collection procedures were associated with cardiac enzyme elevations, which will be addressed in future studies.
Keywords: angiogenesis, endothelial progenitor cells (EPC) myocardial ischemia, myocardial regeneration, stem cells
Angina pectoris is chest discomfort experienced by patients with obstructive coronary artery disease (CAD) when the demand for oxygenated blood exceeds the supply. First-line therapies for symptoms consist of lifestyle modifications, such as weight loss and smoking cessation, and medications, including antiplatelet therapy, β-blockers, calcium channel blockers and nitrates, which act primarily by reducing demand. If these measures fail to sufficiently alleviate anginal symptoms, then revascularization via percutaneous coronary interventions (PCI), such as angioplasty and stenting, or coronary artery bypass grafting (CABG), can be considered in order to improve blood supply.
As therapies for CAD and acute myocardial infarction (MI) have successfully reduced mortality, the population of patients with refractory angina has grown.1-4 Current estimates indicate that 850,000 patients in the United States have refractory angina.1,5-7 Affected individuals have exhausted the conventional therapeutic armamentarium, yet continue to experience disabling angina and are left with limited therapeutic options. Typically, revascularization is no longer possible because of a lack of suitable conduit vessels or the diffuse nature of the coronary disease. Therefore, new therapies for refractory angina are urgently needed.
Emerging evidence indicates that disease of the coronary microcirculation can contribute independently to symptoms and dysfunction in patients with CAD and, therefore, that the microcirculation is a suitable therapeutic target for treatment of ischemic disease.4,8,9 Preclinical studies have shown that human CD34+ cells can stimulate neovascularization in ischemic tissue,10-12 thereby increasing capillary density and improving function in models of acute and chronic myocardial ischemia. A phase I/IIa study of 24 patients provided early evidence of the feasibility, safety, and bioactivity of autologous CD34+ cells when administered by percutaneous, intramyocardial injection and supported further clinical development of this treatment strategy.13
Methods
The ACT34-CMI study was a prospective, double-blind, randomized, controlled clinical trial conducted at 26 centers in the United States. The primary objective of this phase II clinical trial was to test the hypothesis that intramyocardial injection of autologous CD34+ cells will reduce the frequency of angina episodes in subjects with chronic severe refractory angina. The institutional review board at each center approved the protocol, and all patients provided written informed consent. Baxter Healthcare sponsored the study and was responsible for the conduct of the investigation, with oversight provided by the principal investigator and the scientific advisory board. Safety data were monitored by an independent Data Safety Monitoring Board (Online Appendix I, available at http://circres.ahajournals.org), and a Clinical Endpoints Committee adjudicated major adverse cardiovascular endpoints (MACE). The principal investigator had full access to the raw data.
Study Population
Entry criteria included patients ages 21 to 80 years with Canadian Cardiovascular Society (CCS) class III–IV chronic refractory angina despite optimum medical management, including maximally tolerated doses of β-blockers, nitrates, and calcium-channel blockers, and with no suitable revascularization options. Each patient’s case and most recent angiogram was reviewed by an interventional cardiologist and cardiac surgeon who were not part of the study team to verify the lack of revascularization options. Single photon emission computed tomography (SPECT) imaging was required to document the presence of reversible ischemia. Patients were required to walk a minimum of 3 minutes but no longer than 10 minutes on a modified Bruce protocol exercise tolerance test (ETT) and had to experience angina or their angina equivalent during exercise testing. Exclusion criteria included left ventricular ejection fraction <25%, predominant congestive heart failure symptoms, MI within 60 days (creatine kinase-MB >3 times normal) of study entry, a successful or partially successful coronary revascularization procedure within the previous 6 months, placement of a biventricular pacemaker for cardiac resynchronization therapy for heart failure in the previous 90 days, and others (Online Appendix II for full inclusion and exclusion criteria).
Study Design, Cell Mobilization, Collection, and Preparation
Under normal conditions, the number of circulating CD34+ cells is too low to achieve the desired doses of cells by peripheral collection. Accordingly, granulocyte colony stimulating factor (G-CSF) (Filgrastim/Neupogen®, Amgen, Thousand Oaks, CA) was administered to increase the number of circulating CD34+ cells for subsequent collection via leukapheresis. To maintain the double-blind design, all subjects (regardless of treatment group) underwent mobilization with 5 μg/kg per day doses of G-CSF administered subcutaneously for 4 or 5 days, and leukapheresis was performed on the 5th day. On the following day, the mononuclear cell preparation collected during leukapheresis was enriched for CD34+ cells by using a commercially available device (Isolex 300i Magnetic Cell Selection System, Baxter Healthcare, Deerfield, IL). Lot release testing was performed on the final cell preparation to document sterility (Gram’s stain and subsequent culture), viability (7-AAD apoptosis staining),14 and purity (fluorescence-activated cell sorting for CD34+ cells).
Patients were randomly assigned to 1 of the 3 treatment groups via telephone call-in and an interactive voice-response system (IVRS). The cell-processing laboratory at each center was responsible for making the randomization call and preparing the CD34+ cells or control injection accordingly. Syringes containing cells or the control solution were identical in appearance.
Cell Injection Procedure
When all lot-release criteria were met (negative Gram’s stain, viability ≥70%, CD34+ ≥50%), subjects were brought to the catheterization laboratory, where electromechanical endocardial mapping15-17 was performed with the NOGA® Map system (Biologics Delivery Systems, Diamond Bar, CA) as previously described13 to identify viable, ischemic areas of the myocardium where CD34+ cells or placebo treatment would be delivered. Patients were randomized in a 1:1:1 ratio to receive 1×105 CD34+ cells/kg body weight, 5×105 (±10%) CD34+ cells/kg body weight (adjusted to a maximum of 100 kg), or placebo injection, which consisted of the identical diluent used for delivery of the CD34+ cells. The total cell dose was diluted in 2 cc of 0.9% NaCl (saline) plus 5% autologous plasma and was delivered via intramyocardial injection into 10 distinct sites (0.2 cc/site) with a NOGA Myostar® catheter.
Endpoints
The prespecified primary efficacy end point was angina frequency 6 months after treatment. Angina frequency was documented by interactive voice responsive system (IVRS) on a daily basis for 28 days at baseline and at the 3-, 6-, and 12-month follow-up visits. Patients contacted the IVRS by telephone daily for 28 days prior to each visit and received a reminder call if they failed to call in. Patients who missed more than 3 calls during baseline screening would have been dropped from the study, but no patients were withdrawn for this reason. Secondary efficacy endpoints included exercise tolerance testing, use of antianginal medication, CCS functional class, health-related quality of life (Seattle Angina Questionnaire, Short Form-36 Survey, Dyspnea Questionnaire, and the Euro 5 Questionnaire; details are provided in Online Appendix III), the combined rate of major adverse cardiac events events, SPECT, and cardiac magnetic-resonance imaging (in a substudy). Safety endpoints included adverse event reporting, chest X-ray and echo-cardiography (to assess for complications related to the injection procedure), and laboratory screening.
Statistical Analysis
Statistical analysis was performed by statisticians employed by Baxter Healthcare; the raw data were also transferred to the investigators for independent analysis. The targeted enrollment of 150 patients was based on the results of the phase I/IIa study and was calculated to provide 90% power to detect a difference in angina frequency of 3 to 6 (0.75 standard deviations) episodes per week. The primary analysis was performed according to the intention to treat principle. Angina frequencies are displayed as least squares means of the number of angina episodes per week ± the standard error of the least squares means. Other continuous variables are displayed as mean±SD. The primary efficacy end point was a decline in angina frequency from baseline to month 6; summaries of both 6- and 12-month results are presented here. Log-linear modeling (Poisson regression) was performed on the frequency of angina at 6 and 12 months. Initially, the raw baseline value was used as the covariate. On review of the distribution of angina counts, it became clear that a more appropriate analysis was to use the log of the baseline value as a covariate, and the results of this analysis are presented here. The independent parameters in the model were treatment group, visit (6 and 12 months), and the interaction of treatment and visit. Exercise testing was evaluated using analysis of variance with repeated measures. The independent parameters in the model were treatment group, visit (6 and 12 months), and the interaction of treatment and visit. The raw baseline value was used as covariate. Robust standard errors for a repeated-measures analysis of both the angina frequency data and exercise tolerance times were used to safeguard against possible misspecification of the assumed correlation structure among repeated measurements. Categorical variables were compared with Fisher’s exact test. Additional details are provided in Online Appendix II.
Results
Enrollment and Patient Disposition
Between April 2006 and March 2008, 26 centers across the United States enrolled 321 patients. At the end of the screening period, 147 patients failed to meet eligibility criteria and were withdrawn. The remaining 174 subjects began cell mobilization with G-CSF. Of these, 6 subjects withdrew before randomization for a variety of reasons (Figure 1).
Figure 1. Study design, eligibility, randomization, and follow-up.
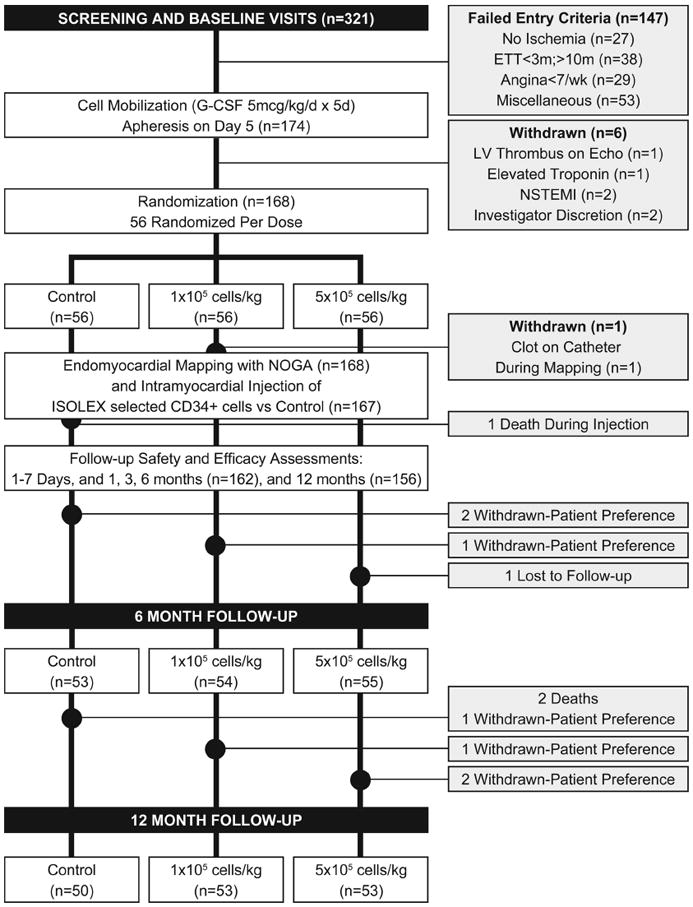
Of the 168 subjects who were randomized, 1 subject was withdrawn because a thrombus was observed on a pigtail catheter on removal, and 1 subject died after cardiac perforation and tamponade during the injection procedure. Thus, 166 subjects were available for follow-up, of whom 162 and 156 subjects completed the 6- and 12-month follow-up visits, respectively (Figure 1). During the course of the study, site monitoring raised questions in regard to certain data at 1 study site. It was decided to exclude the 7 subjects enrolled at this site from the analyses of the efficacy data, while retaining all safety information. This decision did not change the overall study results nor materially alter the statistical significance of the analyses.
Baseline Characteristics
Patient baseline characteristics were similar in all 3 treatment groups (Table 1). The total patient population included 22 (13%) females and 145 (87%) males with a mean age of 61±8.9 (range 41 to 91) years. Previous CABG had been performed in 93% of subjects, and the mean number of CABG operations per subject was 1.4±0.6 (range 0 to 4). Previous PCI had been performed in 83% of patients, and the mean number of prior PCI procedures was 2.9±3.1 (range 0 to 23) per subject. The study groups were similar with regard to age, weight, gender, race, smoking history, diabetic status, and cardiac status, although the control group had a higher percentage of subjects with a history of congestive heart failure.
Table 1.
Baseline Characteristics of the Study Population
| Control (n=56) | 1×105 cells/kg (n=55) | 5×105 cells/kg (n=56) | P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (mean±SD) | 61.8 (8.5) | 61.3 (9.1) | 59.8 (9.2) | 0.471* |
| Female, % | 10.7 | 16.4 | 12.5 | 0.668** |
| Cardiovascular risk factors | ||||
| HTN, % | 94.6 | 94.5 | 94.6 | 1.000† |
| Smoker, % | 73.2 | 74.6 | 71.4 | 0.933† |
| Diabetes, % | 55.4 | 47.3 | 55.4 | 0.617** |
| Medical history | ||||
| Prior MI, % | 75.0 | 78.2 | 80.4 | 0.798† |
| Prior CABG, % | 96.4 | 92.7 | 89.3 | 0.343† |
| Prior PCI, % | 83.9 | 87.3 | 78.6 | 0.464† |
| Prior CHF, % | 41.1 | 21.8 | 28.6 | 0.091† |
| Medications | ||||
| Beta blocker, % | 98.2 | 90.9 | 92.9 | 0.243† |
| Nitrate, % | 73.1 | 65.5 | 71.4 | 0.647† |
| Ca++ blocker, % | 51.8 | 41.8 | 50.0 | 0.535† |
| ASA, % | 96.4 | 87.3 | 96.4 | 0.081† |
| Clopidogrel, % | 69.6 | 72.7 | 78.6 | 0.553† |
| Statin, % | 69.6 | 76.4 | 76.8 | 0.625† |
| ACE-inh/ARB, % | 76.8 | 76.4 | 75.0 | 0.974† |
| Cardiovascular condition | ||||
| LVEF (mean±SD) | 59.8 (14.5) | 58.9 (14.2) | 60.6 (13.3) | 0.820* |
| Angina episodes/week (mean±SE) | 24.6 (3.0) | 22.9 (2.1) | 26.4 (2.8) | 0.653* |
| Blood pressure, systolic (mean±SD) | 122.0 (19.3) | 123.0 (16.4) | 124.8 (16.2) | 0.689* |
| Blood pressure, diastolic (mean±SD) | 68.5 (11.0) | 68.5 (9.9) | 71.9 (7.9) | 0.103* |
Baseline values are for treated subjects only. HTN, hypertension; MI, myocardial infarction; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; CHF, congestive heart failure; ACE-inh, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blocker; LVEF, left ventricular ejection fraction.
Analysis of variance, based on the model including treatment effect.
Pearson c2 test used for testing differences among treatment groups.
Fisher exact test used for testing differences among treatment groups.
Cell Mobilization and Apheresis
Administration of G-CSF was associated with bone pain in 20.1% of subjects, with angina in 17.4%, and with congestive heart failure in 2 patients. Eight subjects had troponin elevations that were consistent with a non-ST segment elevation MI by the new universal definition of MI18,19; 3 of these subjects were withdrawn from the study, including 1 subject whose troponin level was 1.2 times the upper limit of normal. One subject was withdrawn because of a left-ventricular thrombus noted on cardiac echo, 1 subject underwent coronary revascularization, 1 was hospitalized for an acute coronary syndrome, and 2 were withdrawn at the discretion of the local investigator.
CD34+ cell selection resulted in a cell product with the following composition: CD34+83.0%±14.6%, B cells 11.3%±12.5% and T cells 1.0%±2.3%.
Intramyocardial Injection Procedure
Endocardial mapping was performed in 168 patients. In 1 subject, a thrombus was observed on the mapping catheter tip when it was removed from the patient, and this patient was withdrawn from the study by the investigator (as noted under Enrollment and Patient Disposition). Intramyocardial injection procedures were safely accomplished in 165 of the remaining 167 subjects. Two subjects experienced an apparent myocardial perforation: 1 resulted in hemothorax that was treated successfully, and the 2nd resulted in cardiac tamponade. In the 2nd case, a pericardiocentesis procedure was unsuccessful, and the patient died.
A total of 47 (28%) subjects had elevated troponin levels (mean 1.27±4.73) at some point during the mobilization and injection period, all of which were minor and subclinical except for those mentioned above.
Primary Outcome
At 6 months, the frequency of angina was significantly lower in patients treated with the low dose of CD34+ cells than in the control group (6.8±1.1 versus 10.9±1.2 episodes per week, P=0.020) (Figure 2). Angina was also less frequent in the high-dose group (8.3±1.1 episodes per week) than in the control group, but the difference was not statistically significant (P=0.167). At 12 months, the frequency of angina remained significantly lower in the low-dose group than in the control group (6.3±1.2 versus 11.0±1.2 episodes per week, P=0.035) and lower (7.2±1.1), but not significantly (P=0.181), in the high-dose group than in the control group. At 6- and 12-months follow-up, the antianginal regimen was unchanged in 86.8% and 84.5% of control and 90.4% and 85.8% of treated patients, increased in 4.3% and 5.5% control and 6.0% and 8.2% treated and decreased in 8.9% and 10% control and 3.7% and 5.9% treated patients. There was no statistical interaction between changes in any medication and changes in angina frequency or ETT time.
Figure 2. Weekly angina incidence at 6 and 12 months.
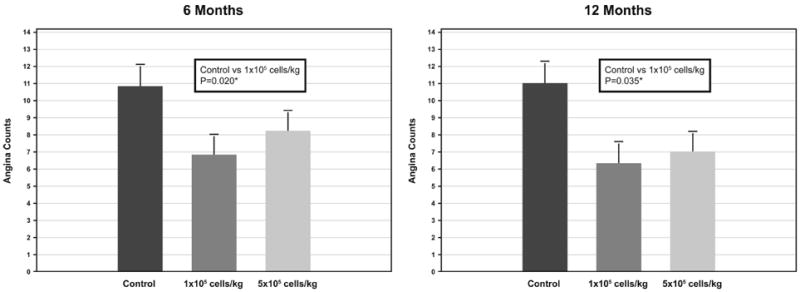
Least squares means and standard errors. *Probability values from pairwise comparisons of ratios from Poisson regression (log of baseline used as covariate).
Exercise Tolerance Testing
Improvement in total exercise time at 6 months was significantly greater in the low-dose group than in the control group (139±151 versus 69±122 seconds, P=0.014) (Figure 3). The high-dose group also had greater improvement than was observed in the control group (110±155 seconds), but the difference was not statistically significant (P=0.097). At 12 months, the low-dose group continued to show significantly greater improvement in total ETT time in comparison with the control group (140±171 versus 58±146 seconds, P=0.017), and improvement in the high-dose patients (103±162 seconds) remained greater, but not significantly, than did improvement in the control group (P=0.134).
Figure 3. Change in exercise time at 6 months and 12 months.
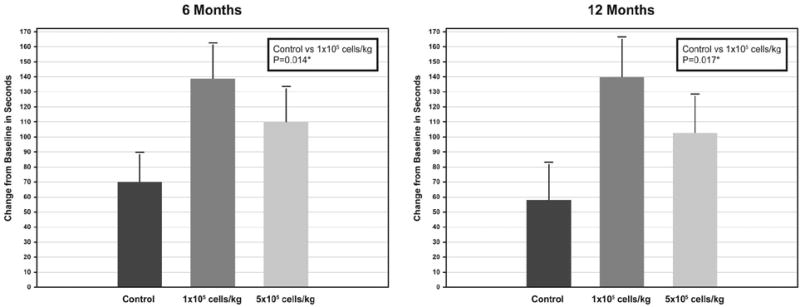
Means and standard errors. *Probability values from analysis of covariance with repeated measures (baseline value used as covariate).
All subjects had angina at baseline, as required by the inclusion criteria. At 6 months, the increase in time to the onset of angina during treadmill exercise was greater, but not significantly, in the low-dose group than in the control group (161±179 versus 87±208 seconds, P=0.235). Change in time to angina onset in the high-dose group (87±208 seconds) and in the control group were similar. At 12 months, the difference between the low-dose and control groups was greater than at 6 months, but did not reach statistical significance (139±148 versus 57±168 seconds, P=0.08).
Seattle Angina Questionnaire
At 6 months, the mean change in the Angina Stability Scale was 13.8±31.9 in control subjects, 29.8±30.1 in the low-dose group and 25.5±30.3 in the high-dose group (P=0.290). The percentage of subjects who improved was 40.8 in the control group, 69.2 in the low-dose group (P=0.005 versus the control group), and 67.3 in the high-dose group (P=0.010 versus the control group). At 12 months, the mean change in the Angina Stability Scale was 14.4±32.4 in the control group, 24.0±32.3 in the low-dose group, and 21.0±36.2 in the high-dose group.
The Angina Frequency Scale showed only minor differences between control and treatment groups at 6 months (control: 26.0; low dose: 28.3; high dose: 21.7) and 12 months (control: 27.9; low dose: 29.0; high dose: 22.6).
CCS Class, Antianginal Medications
Trends in CCS class and antianginal medication use all favored the subjects treated with CD34+ cells. CCS class worsened from baseline to 6 months in 12.8% of control subjects, in comparison with 2.0% of low-dose subjects and 3.8% of high-dose subjects. At 6 months, the percentage of patients with an improvement in CCS class was greater in the treated groups (low dose: 62.8; high dose: 60.7) than in control subjects (53.1). At 12 months, the percentage of subjects whose CCS class had worsened from baseline remained greater in the control group (8.7) than in the low-dose (3.9) and high-dose (5.8) groups, whereas the percentage of subjects who experienced a ≥2-class improvement was greater in the low-dose (23.1) and high-dose (25.0) groups than in the control subjects (15.2). Similarly, the mean reduction in nitroglycerine (NTG) tablet usage was greater in the treated groups than in control subjects at 6 months (low dose: −6.3±8.1; high dose: −7.3±9.9; control: −4.2±8.8 NTG tablets per day), although the differences between treated and control groups did not reach statistical significance; results were similar at 12 months.
SPECT Imaging
Adenosine SPECT imaging was performed at baseline and 6 and 12 months after injection. At 6 months, total severity score stress images showed a significant improvement in the low-dose group than in the control group (−117.4±221.2 versus +0.1±161.1, P=0.002). The remaining standard SPECT imaging parameters revealed no significant differences between treated and control groups.
Major Adverse Cardiovascular Events
There were 3 deaths in the study population, all of which occurred in the control group. Myocardial infarction occurred in 7 (12.5%) control-group patients, 3 (5.5%) low-dose-group patients, and 3 (5.4%) high-dose-group patients. When urgent revascularization, worsening congestive heart failure, and acute coronary syndrome were included as MACEs, there continued to be no evidence of harm associated with the intramyocardial injection of autologous CD34+ cells, and trends toward lower event rates were observed (Table 2).
Table 2.
Major Adverse Cardiac Events
| MACE | Control N=56 (%) | 1×105 cells/kg N=55 (%) | 5×105 cells/kg N=56 (%) | P Value* |
|---|---|---|---|---|
| Death | 3 (5.4) | 0 (0) | 0 (0) | 0.107 |
| MI | 7 (12.5) | 3 (5.5) | 3 (5.4) | 0.305 |
| Death, MI | 10 (17.9) | 3 (5.5) | 3 (5.4) | 0.058 |
| Death, MI, urgent revascularization | 11 (19.6) | 5 (9.1) | 4 (7.1) | 0.106 |
| Death, MI, urgent revascularization, worse CHF, ACS | 15 (26.8) | 7 (12.7) | 7 (12.5) | 0.093 |
| Stroke | 1 (1.8) | 0 (0) | 2 (3.6) | 0.774 |
| Cardiac hospitalization or ER visita | 21 (37.5) | 16 (29.1) | 18 (32.1) | 0.357 |
| Hospitalization for CHF | 4 (7.1) | 2 (3.6) | 1 (1.8) | 0.407 |
All MACE from start of mobilization to the end of the 12-month follow-up. MI, myocardial infarction; worse CHF, worsening congestive heart failure; ACS, acute coronary syndrome.
Includes all hospitalizations or emergency room (ER) visits that were cardiac related. This is based on adjudicated hospitalization and ER visit.
P values calculated using Fisher exact test.
Discussion
This phase II study, in which 167 “no-option” patients with refractory angina were enrolled, provides an opportunity to make observations regarding the feasibility, safety, and efficacy for a strategy of intramyocardial injection of autologous CD34+. The primary findings of this study are that intramyocardial injection of autologous CD34+ cells was associated with a significant decrease in angina frequency and a significant improvement in exercise tolerance in patients with optimally managed but refractory angina. The significant benefit of the low dose of CD34+ cell therapy observed at the primary end point of 6 months was preserved and increased slightly in magnitude at 12 months, contrasting with prior studies of angiogenic therapies in which placebo “catch-up” was observed.20 The overall improvement in low-dose-treated patients was also supported by positive trends in time to onset of angina, quality of life testing, nitroglycerine use, and CCS classification. Our findings are consistent with our pilot study13 as well as other pilot trials of intramyocardial bone marrow cell injection in the setting of chronic myocardial ischemia.21,22 Notably, however, this is the first randomized, controlled trial of stem-cell therapy in patients with refractory angina to achieve significant improvements in both anginal frequency and exercise tolerance.
Another important observation that should not be overlooked is the demonstration of feasibility of this treatment strategy in this multicenter investigation. Each treatment required that cell mobilization, collection by leukapheresis, CD34+-cell enrichment, and lot release testing, as well as NOGA-guided intramyocardial injection, be performed locally at the investigative site. Evidence that this complex protocol could be successfully implemented at multiple treatment centers was necessary before this therapeutic approach could be considered for use on a larger scale. Successful maintenance of the study blind was also critical for the development of a phase III study.
As with any new therapy, particularly in patients with advanced cardiovascular disease, safety is a key consideration. The overall occurrence of major adverse cardiac events was no higher in patients treated with CD34+ cells than in placebo-treated patients, and key safety indices tended to favor CD34+-cell–treated subjects. The study was not powered to detect differences between groups in safety events, and consequently, these observations indicate only that there is currently no evidence for an increased risk of adverse cardiac events associated with intramyocardial CD34+ cell injection. As expected, G-CSF administration and the apheresis procedure were associated with adverse events in this patient population.23 It is worth noting that the rate of enzyme elevation consistent with the new “universal” definition of MI (4.6%) observed in this study is substantially lower than that observed in 3 large meta-analyses of patients undergoing routine percutaneous intervention.24-26
Five subjects were withdrawn from the study following mobilization and apheresis because of events potentially, but not conclusively, attributable to these procedures. Future studies may be designed to determine whether alternate strategies for the collection of CD34+ cells, including the addition of rapidly acting mobilizing agents,27 altered apheresis protocols, or bone marrow aspiration, are available and warranted. The safety of the intramyocardial injection procedure itself also requires careful consideration. The occurrence of 2 (1.2%) myocardial perforation events during the injection procedure is consistent with previous studies.28 It is possible that the rate of adverse procedural events could decrease as the technique is used more routinely; nevertheless, this will remain an area of careful scrutiny as therapies requiring intramyocardial delivery of therapeutics are developed.
This study was not powered to detect differences in efficacy between the low-dose and high-dose groups. Nevertheless, patients who received the lower dose appeared to experience greater improvement in several end points. This lack of a definitive dose-dependent response was not anticipated, but is far from unprecedented, and is consistent with the results from preclinical studies of cell therapy for myocardial ischemia29 and with an extensive body of literature indicating that the response to biological manipulations of angiogenesis is often biphasic.30 Perhaps the most notable recent clinical example is a phase II study of the antiangiogenic drug bevacizumab31 for treatment of metastatic colon cancer. The low-dose treatment, which was half the magnitude of the high-dose treatment, was associated with higher response rates and survival, and the subsequent successful phase III trial, which led to FDA approval of the drug for the condition studied, used the low dose.32 No mechanism for the inverted dose–response relationship associated with bevacizumab has been described, and the mechanism responsible for the potentially more robust effect observed in patients treated with a lower dose of intramyocardially injected CD34+ cells is also unknown. One possible explanation is that the higher dose exceeded the (as yet unidentified) optimum cell density required for promoting paracrine effects within the confined space of the myocardium.
Perfusion imaging was performed in an attempt to quantify changes in blood flow. One SPECT assessment showed improvement in low-dose patients at 6 months, and the remaining SPECT parameters did not identify significant differences between treatment groups. Does this negate the possibility that changes in blood flow were responsible for the clinical effects observed? Not necessarily. SPECT imaging was designed and validated as a tool for detecting relative reductions in blood flow that result from the obstruction of epicardial coronary arteries, whereas the mechanism responsible for the benefit of CD34+-cell therapy is believed to involve increases in capillary density and improved micro-circulation in the area around the injection sites. These improvements are likely spread around throughout the ischemic zone of the myocardium, crossing the boundaries of the standard 17-segment SPECT map; thus, the ability to detect relative changes in blood flow would be, at best, limited. Newer methods for measuring perfusion, such as quantitative positron-emission tomography imaging, may be required to document changes in absolute blood flow at the microvascular level.33
The limitations of this investigation include its status as a phase II study; thus, the findings of improvement following intramyocardial injection of autologous CD34+ cells must be replicated in a phase III investigation before definitive conclusions regarding efficacy can be made. Furthermore, the study was not powered to detect differences between doses, so observations regarding the apparently greater potency of the lower cell dose do not provide a conclusive assessment of the potential dose–response relationship.
In summary, the results from this phase II study support the safety and efficacy of intramyocardially injected autologous CD34+ cells for symptom reduction and improved exercise capacity in “no-option” patients with refractory angina. Larger-scale studies are warranted to verify these effects and to refine the methods for collecting and administering CD34+ cells to patients with disabling angina symptoms.
Novelty and Significance.
What Is Known?
Human CD34+ cells are well known as hematopoietic stem cells used for stem cell transplants in patients who have bone marrow ablation by chemotherapy or radiation therapy.
The CD34+ cells can differentiate into hematopoietic lineage cells and reconstitute the bone marrow. In addition, they have also been shown to have endothelial lineage potential in vitro and in vivo.
Preclinical studies in models of myocardial or limb ischemia show that local delivery of human CD34+ cells improves perfusion and function in ischemic tissue.
What New Information Does This Article Contribute?
In a double-blind, randomized, controlled clinical trial, direct intramyocardial injection of autologous CD34+ cells was associated with an improvement of exercise tolerance and with reductions in angina frequency in 167 “no-option” patients with refractory angina.
In addition to yielding data on safety and efficacy, the study also provides evidence that the strategy of mobilizing, collecting, purifying, and delivering autologous CD34+ cells in patients with severe cardiovascular disease is feasible at a large number of centers.
As interventions for acute myocardial infarction have reduced mortality in patients with coronary artery disease, and because medical treatment has also improved long-term outcomes, a growing population of patients with refractory ischemia is emerging. These individuals have exhausted medical, interventional, and surgical options and have persistent, lifestyle-limiting ischemic symptoms. Epicardial revascularization is no longer possible, often because of extensive disease or chronic total occlusion. In addition to the loss of major conduit vessels in these subjects, however, the attenuation of the microcirculation is also thought to contribute to the decrease in overall myocardial perfusion. The CD34+ cell population has been shown to be enriched in cells with the ability to stimulate neovascularization, both by contributing directly to vessel formation and by secreting proangiogenic factors. This report provides evidence from a double-blind study that direct injection of CD34+ cells into the ischemic myocardium reduces chest pain and significantly increases exercise tolerance in patients with refractory angina.
Acknowledgments
The authors gratefully acknowledge Andrea Hunt, Paroo Uppal, Tina Thorne, Meredith Millay, Robin Reynolds, Ruth Stallard, Deborah Livingston, Mary Molter, Ani Grigorian, Candice Junge, Suzann Hammel, Dr. Paul-Andre de Lame, Ashley Peterson, Kari Krueger, and W. Kevin Meisner for their roles in the planning and execution of this study, analysis of study data, and preparation of this manuscript.
Sources of Funding Baxter Healthcare.
Non-standard Abbreviations and Acronyms
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- CCS
Canadian Cardiovascular Society
- ETT
exercise tolerance test
- G-CSF
granulocyte colony stimulating factor
- IVRS
interactive voice-response system
- MACE
major adverse cardiovascular endpoints
- MI
myocardial infarction
- PCI
percutaneous coronary interventions
- SPECT
single photon emission computed tomography
Appendix 1
Investigators and Clinical Sites
Tim Henry, MD
Jay Traverse, MD
Rachel Olson, RN
Karen Harvey, RN
Patti Mitchell, RN
Minneapolis Heart Institute
Minneapolis-St. Paul, MN
Theodore Bass, MD
Marco Costa, MD
Shirley Morden, RN
Shands Jacksonville Medical Center
Jacksonville, FL
Joon Sup Lee, MD
Lisa Baxendell, RN
UPMC
Pittsburgh, PA
Richard Schatz, MD
Heather Catchpole, RN
Scripps Clinic
La Jolla, CA
Gary Schaer, MD
Poorna Nagarajan, RN
Rush University Medical Center
Chicago, IL
Andrew Taussig, MD
Leanne Goodwin, RN
Florida Hospital
Orlando, FL
Alan Niederman, MD
Terri Kellerman, RN
Holy Cross Hospital
Fort Lauderdale, FL
Philip Horwitz, MD
Mark Anderson, MD
Neal Weintraub, MD
Amy Ollinger, RN
University of Iowa Healthcare
Iowa City, IA
Neal Weintraub, MD
M. Sue Huseman, RN
University of Cincinnati Medical Center
Cincinnati, OH
Steven Port, MD
Deb Waller, RN
St. Luke’s Medical Center
Milwaukee, WI
Carl Pepine, MD
Tempa Curry, RN
University of Florida
Gainesville, FL
David Fortuin, MD
Jacklyn Gentry, RN
Mayo Clinic
Scottsdale, AZ
Peter Soukas, MD
Melissa Antonellis, RN
St. Elizabeth’s Medical Center
Boston, MA
Dean Kereiakes, MD
Kathy Buszek, RN
The Lindner Clinical Trial Center
Cincinnati, OH
Amish Raval, MD
Cassondra Vander Ark, RN
University of Wisconsin
Madison, WI
Farrell Mendelsohn, MD
Susan Deramus, RN
Cardiology P.C.
Birmingham, AL
Alan Yeung, MD
Maria Perlas, RN
Yvonne Strawa, RN
Stanford University Hospital
Stanford, CA
Ken Rosenfield, MD
Cristina Brueggeman, RN
Massachusetts General Hospital
Boston, MA
Ron Waksman, MD
Petros Okubagzi, RN
Washington Hospital Center
Washington, DC
Warren Sherman, MD
Jeanie Sohn, RN
New York Presbyterian Hospital
New York, NY
Nicholas Chronos, MD
Rebecca Allen, RN
St. Joseph’s Research Institute
Atlanta, GA
Chiu Wong, MD
Dolores Reynolds, RN
Cornell University, Weill College of Medicine
New York, NY
Charles Davidson, MD
Sherrie Wolf, RN
Northwestern Memorial Hospital
Chicago, IL
Daniel Simon, MD
Valerie Cwiklinski, RN
University Hospitals of Cleveland
Cleveland, OH
Robert Strumpf, MD
Zaki Lababidi, MD
Nabil Dib, MD
Candice Kelly, RN
Arizona Heart
Phoenix, AZ
Paul Huang, MD
Jennifer Hudachek, RN
Swedish Seattle Hospital
Seattle, WA
Data Safety Monitoring Board
Jeffrey Brinker, MD, Chair
The Johns Hopkins Hospital
Baltimore, MD
Kenneth Ellenbogen, MD
Virginia Commonwealth University
Richmond, VA
Armand Keating, MD
Princess Margaret Hospital
Toronto, Canada
George Vetrovec, MD
Virginia Commonwealth University
Richmond, VA
James Dziura, MD
Yale University
New Haven, CT
Clinical Endpoint Committee Brigham and Women’s Hospital
Marc Pfeffer, MD
Akshay Desai, MD
Peter Finn, MD
Boston, MA
Core Labs Exercise Treadmill Time (ETT)
Ernest Gervino, MD
Harvard Clinical Research Institute
Boston, MA
SPECT
James Udelson, MD
Tufts Medical Center
Boston, MA
Magnetic Resonance Imaging / PERFUSE
Evan Appelbaum, MD
Harvard Clinical Research Institute
Boston, MA
Quality of Life
David Cohen, MD
Saint Lukes Mid America Heart Hospital
Kansas City, MO
Statistical Analysis Duke Clinical Research Institute
Robert Harrington, MD
Karen Pieper, M.S.
Robert Clare, M.S.
Duke University
Durham, NC
Appendix 2
Protocol Detail
| Therapeutic | Autologous CD34+ Cells (Auto-CD34+ cells) |
|
| |
| Study Phase | Phase II |
|
| |
| Protocol Title | A double blind, prospective, randomized, placebo-controlled study to determine the tolerability, efficacy, safety and dose range of intramyocardial injections of G-CSF mobilized Auto-CD34+ cells for reduction of angina episodes in subjects with refractory chronic myocardial ischemia. (ACT34-CMI) |
|
| |
| Objective | Primary Objective: The primary objective of the Phase 2 clinical trial is to demonstrate reduction in angina episodes, evaluate the efficacy, tolerability and safety of two doses of Auto-CD34+ cells administered via intramyocardial injection to subjects with refractory chronic myocardial ischemia (CMI). |
|
| |
| Study Design | This is a prospective, randomized, double blind, placebo-controlled study to evaluate the efficacy, tolerability, and safety of two doses of Auto-CD34+ cells when delivered by intramyocardial injection. A single administration of Auto-CD34+ cells will be dosed at either 1 × 105 or 5 × 105 (±10%) cells/kg body weight, up to a maximum of 100 kg, and compared to subjects receiving placebo. There will be 50 subjects per group. |
All subjects will receive subcutaneous injections of Granulocyte Colony Stimulating Factor (G-CSF) at a dose of 5 μg/kg/day for 5 days to mobilize CD34+ cells from the bone marrow to the peripheral blood. Prior to undergoing apheresis, complete blood counts will be performed on days 1, 2, 3, 4 and 5. Fluorescent-activated cell sorter (FACS) analysis will be performed on days 4 and 5, to determine the number of CD34+ cells in the circulation. On day 5, apheresis will be performed using the Amicus Blood Cell Separator (Fenwal) or an alternative approved apheresis system (e.g. Cobe Spectra), according to the manufacturer’s instructions for mononuclear cell collection. The endpoint of each collection will be the processing of up to 2-5 total blood volumes (TBV) and will be based on the circulating CD34+ cell count on the day of apheresis and subject tolerance for the apheresis procedure.
| |
|
| |
| On the day of cell injection, the apheresis product will be enriched for CD34+ cells using the Isolex 300i Magnetic Cell Selection System (Baxter Healthcare). Quality control testing will be performed on the apheresis product and on the final selected product. After the final selected product is tested and determined to meet release specifications, subjects will be randomly assigned to 1 × 105, 5 × 105 (± 10%) CD34+ cells/kg body weight (up to 100 kg) or to placebo (0.9% NaCl (saline) plus 5% autologous plasma) at this point in the trial. The subject will undergo cardiac catheterization with the NOGA™ electromechanical mapping system. This system is used to identify ischemic but viable regions of the myocardium as targets for cell delivery. CD34+ cells will be delivered in 10 intramyocardial injections of 0.2 mLs each into the target areas of myocardial ischemia using the MyoStar injection catheter (Biosense Webster, Inc., a Johnson & Johnson company). | |
|
| |
| Number of subjects | 150 |
|
| |
| Study population | Male or female subjects who are 21-80 years of age with refractory chronic myocardial ischemia on maximal therapy who are not suitable candidates for conventional revascularization |
|
| |
| Inclusion Criteria |
|
|
| |
| Exclusion Criteria |
|
|
| |
| Treatment Groups | Test: 1 × 105 (±10%) or 5 × 105 (±10 %) CD34+ cells / kg body weight (up to a maximum of 100 kg) |
| Control: placebo (0.9 % NaCl (saline) plus 5% autologous plasma) Mode of Administration: Intramyocardial injection | |
|
| |
| Duration of Treatment | Mobilization with G-CSF is 5 days. |
| Apheresis is performed in one day on day 5 of mobilization CD34+ selection is performed on day 6 | |
| Mapping and injection of stem cells or placebo is a 2-3 hour procedure performed on day 6. | |
| Subjects will be hospitalized for 24-hour observation after cell injection. | |
| The subject will then be followed for 12 months. | |
| In a separate protocol, subjects will be followed for 12 months after ending participation in this protocol. | |
|
| |
| Efficacy Variable | Primary Efficacy variable is frequency of angina episodes per week, when comparing subjects receiving injection of CD34+ cells to placebo. |
| Secondary Efficacy variables are divided into two categories, symptom relief and myocardial perfusion, and function measurement endpoints. | |
| Symptom Relief: ETT, anti-anginal medication, pedometer measurements, CCS functional class and QOL [SAQ, SF-36, Dyspnea Questionnaire, Euro 5 Questionnaire], and the combined rate of MACE events. | |
| Myocardial perfusion and function measurements: SPECT and cardiac MRI. | |
|
| |
| Safety Variable | Adverse event reporting, MACE, physical examination, vital signs, ECHO, laboratory parameters, revascularization procedures (as defined by new diseased vessel or progression of disease in a vessel not believed to be the cause of baseline angina), hospitalization rates for cardiac related admissions and Emergency Department/Acute Care Service visits for cardiac related admissions will assess safety. |
|
| |
| Pharmacoeconomics | Pharmacoeconomics will be evaluated by assessment of hospitalization rates, emergency room visits, revascularization procedures (as defined by a new diseased vessel or progression of disease in a vessel not believed to be the cause of baseline angina), and changes in medication. |
|
| |
| Statistical Methods | A log linear model (Poisson regression) will be performed on the frequency of angina at baseline and six months. The independent parameters in the model will be treatment group (as randomized) and visit (baseline, 6 months), and the interaction between treatment group and visit. The baseline value will be used as a covariate. Contrasts will be constructed on the difference between 6 months and baseline. Since this analysis is done on the log scale, these contrasts will take the form of relative risks. Missing data will be imputed using last value carried forward. The primary analysis will be with the intent-to-treat (ITT) population. |
| A secondary analysis will be done using the actual dose of cells the subject received. The independent parameters in the model will be dose of cells the subject actually received, visit (baseline, 6 months) and their interaction. The baseline value will be used as a covariate. | |
| Similar analyses will be performed using all available visits. Repeated measures analyses will be used including all visits. Data will be summarized by visit for each treatment group. The difference between the treated subjects and control subjects (as randomized) will also be summarized at each visit. Missing data will not be imputed for this analysis. | |
| One interim analysis is planned. This is for administrative purposes only and will not affect the conduct of the study. An independent committee will conduct the analysis. All study personnel will remain blinded until the end of the study. | |
| For the secondary efficacy parameters, analysis of variance with repeated measures will be performed on continuous data. Generalized linear models will be used to analyze ordinal and categorical data. The independent parameters in the model will be treatment group and visit (baseline, 6 months) and the interaction between treatment group and visit. The baseline value will be used as a covariate. | |
| Similar analysis will be performed using all available visits. | |
| Adverse events will be listed by subject and summarized in tabular format by and within standard of care (SOC) and treatment group. Listings and tables will be provided for treatment-emergent adverse events (defined for an individual subject as an event not present prior to beginning study medication, or, if present prior to beginning study medication, an event that increases in intensity, is considered related to the study medication, or becomes serious during the treatment or follow-up phases of the study). Separate listings and/or tables will also be provided for adverse events by maximum intensity, drug-related adverse events, serious adverse events, adverse events resulting in discontinuation of study medication, and deaths. | |
| Vital Signs assessment of the significance of the mean changes from baseline to each follow-up evaluation point will be made within treatment groups, using paired t-tests. Comparisons between treatment groups with respect to mean changes from baseline will be made using ANOVA/Categorical – Chi-square, or Fisher’s Exact as appropriate. | |
| Laboratory results will be summarized (summary statistics for quantitative assays, contingency tables for qualitative assays) by treatment group in tabular format. Assessment of the significance of the mean changes from baseline to each follow-up visit will be made within treatment groups for quantitative assays, using paired t-tests. Comparisons between treatment groups with respect to mean changes from baseline in selected quantitative assays will be made using analysis of variance (ANOVA). Individual subject values identified as abnormal (outside the lab normal ranges) and substantially abnormal will be listed. Frequency tables will be produced for selected assays summarizing shifts from pretreatment to the last assessment while on treatment and the final visit. | |
Appendix 3
Handling of Quality of Life Data
Handling of Seattle Angina Questionnaire (SAQ)
The SAQ consists of 11 questions. Question 1 consists of 9 sub-questions. These 9 sub-questions make up the Physical Limitations Scale. Question 2 comprises the Angina Stability Scale. Questions 3 and 4 make up the Angina Frequency Scale. Questions 5 through 8 make up the Treatment Satisfaction Scale. Questions 9 through 11 make up the Disease Perception Scale. There is no summary score for the Seattle Angina Questionnaire.
The Seattle Angina Questionnaire responses are ordinal values. Items that correspond to the lowest level of functioning are assigned a value of 1, while items that corresponded to higher functioning levels are assigned a higher ordinal value. These scores will be converted to a 0-100 scale score by subtracting the lowest possible scale score, dividing it by the response range, and multiplying it by 100. The following equations will be used to calculate the score for each scale.
| equation 1 |
-
Where SumQ is the sum of the responses to the questions for that scale
NQ is the number of non-missing responses to the questions for that scale
Range is largest possible response
If more than half of the responses for a given scale are missing then the score is considered to be missing. For example, for the Physical Limitation Score there are 9 questions, so if more than four responses are missing for these 9 questions then the Physical Limitation Score will be missing.
Handling of Short Form-36 Survey (reference 1 and 2)
The SF-36 consists of 11 questions. Table 1 has the name of the scale, the questions that correspond to that scale, the lowest and highest possible raw scores, and the possible raw score range.
Table 1.
SF-36 Definitions of Scales
| Scale | Corresponding Questions | Lowest and Highest Possible Raw Scores | Possible Raw Score Range |
|---|---|---|---|
| Physical Functioning | 3a through 3j | 10, 30 | 20 |
| Role-Physical | 4a through 4d | 4, 8 | 4 |
| Bodily Pain | 7 and 8 | 2, 12 | 10 |
| General Health | 1, 11a through 11d | 5, 25 | 20 |
| Vitality | 9a, 9e, 9g, and 9i | 4, 20 | 16 |
| Social Functioning | 6 and 10 | 2, 10 | 8 |
| Role Emotional | 5a, 5b, and 5c | 3, 6 | 3 |
| Mental Health | 9b, 9c, 9d, 9f, and 9h | 5, 25 | 20 |
| Health Transition | 2 | 1,5 | 4 |
The score for each of the scales was transformed to a 0-100 scale using the coded values and the following equation.
| equation 2 |
Equation 2 was adjusted for missing values similar to equation 1.
Handling of the Dyspnea Questionnaire
Using the same strategy as in the SF-36, the four questions in the Dyspnea Questionnaire will be combined to form an overall score. A Yes answer will be scored as a 1 and a No answer as a 2. The sum of the four questions will then be calculated. The overall score will be derived as follows.
| equation 3 |
If the subject answers Yes to all four questions then their score will be a 0, and if they answer No to all four questions their score will be 100. Equation 3 will be adjusted for missing values similar to equation 1.
Handling of Questions on Health Perception, (EuroQOL Questionnaire, EQ-5D)
The 5 domains of the EQ-5D were converted into utility weights using a published algorithm based on the US population. Details of this approach are provided in reference 3.
Reference 1 :. Ware JE, Loinski M, Dewey JE. How to score Version 2 of the SF-36® Health Survey, Lincoln, RI: QualityMetric Incorporated, 2000.
Reference 2: Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and Evaluation of the Seattle Angina Questionnaire: A New Functional Status Measure for Coronary Artery Disease, J Am Coll Cardiol. 25: 333-41, 1995
Reference 3: Shaw, JW, Johnson, JA, Coons, SJ. US Valuation of the EQ-5D Health States, Evaluation and Testing of the D1 Valuation Model. Medical Care. 2005;43: 203-220
Footnotes
Disclosures Douglas W. Losordo was previously a paid consultant to Baxter Healthcare. Timothy D. Henry, Joon Sup Lee, Carl J. Pepine, Thomas J. Povsic, Robert A. Harrington, and Richard A. Schatz are paid consultants to Baxter Healthcare. David Amrani, Bruce M. Ewenstein, Norbert Riedel, and Kenneth Story are employed by Baxter Healthcare. Kerry Barker was previously employed by Baxter Healthcare. Charles Davidson, Marco A. Costa, Theodore Bass, MD, Farrell Mendelsohn, F. David Fortuin, and Jay H. Traverse have no disclosures.
References
- 1.Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, Follath F, Hellemans I, Herlitz J, Luscher T, Pasic M, Thelle D. The problem of chronic refractory angina: report from the esc joint study group on the treatment of refractory angina. Eur Heart J. 2002;23:355–370. doi: 10.1053/euhj.2001.2706. [DOI] [PubMed] [Google Scholar]
- 2.Jolicoeur EM, Granger CB, Henry TD, Holmes DJ, Pepine CJ, Mark D, Chaitman BR, Gersh BJ, Ohman EM. Clinical and research issues regarding chronic advanced coronary artery disease: part i. Contemporary and emerging therapies. Am Heart J. 2008;155:418–434. doi: 10.1016/j.ahj.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Jolicoeur EM, Ohman EM, Temple R, Stockbridge N, Smith S, Mark D, Califf RM, Henry TD, Chaitman BR, Granger CB. Clinical and research issues regarding chronic advanced coronary artery disease: part ii. Trial design, outcomes, and regulatory issues. Am Heart J. 2008;155:435–444. doi: 10.1016/j.ahj.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee D, Bhatt DL, Roe MT, Patel V, Ellis SG. Direct myocardial revascularization and angiogenesis—how many patients might be eligible? Am J Cardiol. 1999;84:598–600. doi: 10.1016/s0002-9149(99)00387-2. [DOI] [PubMed] [Google Scholar]
- 7.Lenzen M, Scholte op Reimer W, Norekval TM, De Geest S, Fridlund B, Heikkila J, Jaarsma T, Martensson J, Moons P, Smith K, Stewart S, Stromberg A, Thompson DR, Wijns W. Pharmacological treatment and perceived health status during 1-year follow up in patients diagnosed with coronary artery disease, but ineligible for revascularization. Results from the euro heart survey on coronary revascularization. Eur J Cardiovasc Nurs. 2006;5:115–121. doi: 10.1016/j.ejcnurse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Erbs S, Linke A, Schachinger V, Assmus B, Thiele H, Diederich KW, Hoffmann C, Dimmeler S, Tonn T, Hambrecht R, Zeiher AM, Schuler G. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the Doppler substudy of the reinfusion of enriched progenitor cells and infarct remodeling in acute myocardial infarction (repair-ami) trial. Circulation. 2007;116:366–374. doi: 10.1161/CIRCULATIONAHA.106.671545. [DOI] [PubMed] [Google Scholar]
- 9.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 11.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. Cd34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T. Dose-dependent contribution of cd34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 13.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, Olson RE, Flanagan J, Sodano D, Murayama T, Kawamoto A, Kusano KF, Wollins J, Welt F, Shah P, Soukas P, Asahara T, Henry TD. Intramyocardial transplantation of autologous cd34+ stem cells for intractable angina: a phase i/iia double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 14.Lecoeur H, de Oliveira-Pinto LM, Gougeon ML. Multiparametric flow cytometric analysis of biochemical and functional events associated with apoptosis and oncosis using the 7-aminoactinomycin d assay. J Immunol Methods. 2002;265:81–96. doi: 10.1016/s0022-1759(02)00072-8. [DOI] [PubMed] [Google Scholar]
- 15.Kornowski R, Hong MK, Gepstein L, Goldstein S, Ellahham S, Ben-Haim SA, Leon MB. Preliminary animal and clinical experiences using an electromechanical endocardial mapping procedure to distinguish infarcted from healthy myocardium. Circulation. 1998;98:1116–1124. doi: 10.1161/01.cir.98.11.1116. [DOI] [PubMed] [Google Scholar]
- 16.Kornowski R, Hong MK, Leon MB. Comparison between left ventricular electromechanical mapping and radionuclide perfusion imaging for detection of myocardial viability. Circulation. 1998;98:1837–1841. doi: 10.1161/01.cir.98.18.1837. [DOI] [PubMed] [Google Scholar]
- 17.Kornowski R, Hong MK, Leon MB. Images in cardiovascular medicine. Left ventricular electromechanical mapping of myocardial ischemia. Circulation. 1999;99:2708. doi: 10.1161/01.cir.99.20.2708. [DOI] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez-Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 20.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, Hillegass WB, Rocha-Singh K, Moon TE, Whitehouse MJ, Annex BH. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the traffic study): a randomised trial. Lancet. 2002;359:2053–2058. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 21.Tse HF, Thambar S, Kwong YL, Rowlings P, Bellamy G, McCrohon J, Thomas P, Bastian B, Chan JK, Lo G, Ho CL, Chan WS, Kwong RY, Parker A, Hauser TH, Chan J, Fong DY, Lau CP. Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (protect-cad trial) Eur Heart J. 2007;28:2998–3005. doi: 10.1093/eurheartj/ehm485. [DOI] [PubMed] [Google Scholar]
- 22.van Ramshorst J, Bax JJ, Beeres SL, Dibbets-Schneider P, Roes SD, Stokkel MP, de Roos A, Fibbe WE, Zwaginga JJ, Boersma E, Schalij MJ, Atsma DE. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 23.Wilson RF, Henry TD. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor: double-edged swords. J Am Coll Cardiol. 2005;46:1649–1650. doi: 10.1016/j.jacc.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Nienhuis MB, Ottervanger JP, Bilo HJ, Dikkeschei BD, Zijlstra F. Prognostic value of troponin after elective percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc Interv. 2008;71:318–324. doi: 10.1002/ccd.21345. [DOI] [PubMed] [Google Scholar]
- 25.Testa L, Van Gaal WJ, Biondi Zoccai GG, Agostoni P, Latini RA, Bedogni F, Porto I, Banning AP. Myocardial infarction after percutaneous coronary intervention: a meta-analysis of troponin elevation applying the new universal definition. QJM. 2009;102:369–378. doi: 10.1093/qjmed/hcp005. [DOI] [PubMed] [Google Scholar]
- 26.De Labriolle A, Lemesle G, Bonello L, Syed AI, Collins SD, Ben-Dor I, Pinto Slottow TL, Xue Z, Torguson R, Suddath WO, Satler LF, Kent KM, Pichard AD, Lindsay J, Waksman R. Prognostic significance of small troponin i rise after a successful elective percutaneous coronary intervention of a native artery. Am J Cardiol. 2009;103:639–645. doi: 10.1016/j.amjcard.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 27.Devine SM, Vij R, Rettig M, Todt L, McGlauchlen K, Fisher N, Devine H, Link DC, Calandra G, Bridger G, Westervelt P, Dipersio JF. Rapid mobilization of functional donor hematopoietic cells without g-csf using amd3100, an antagonist of the cxcr4/sdf-1 interaction. Blood. 2008;112:990–998. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- 28.Kastrup J, Jorgensen E, Ruck A, Tagil K, Glogar D, Ruzyllo W, Botker HE, Dudek D, Drvota V, Hesse B, Thuesen L, Blomberg P, Gyongyosi M, Sylven C. Direct intramyocardial plasmid vascular endothelial growth factor-a165 gene therapy in patients with stable severe angina pectoris a randomized double-blind placebo-controlled study: the euroinject one trial. J Am Coll Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 29.Dixon JA, Gorman RC, Stroud RE, Bouges S, Hirotsugu H, Gorman JH, 3rd, Martens TP, Itescu S, Schuster MD, Plappert T, St John-Sutton MG, Spinale FG. Mesenchymal cell transplantation and myocardial remodeling after myocardial infarction. Circulation. 2009;120:S220–S229. doi: 10.1161/CIRCULATIONAHA.108.842302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 31.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase ii, randomized trial comparing bevacizumab plus fluorouracil (fu)/leucovorin (lv) with fu/lv alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 32.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 33.Johnson NP, Gould KL. Clinical evaluation of a new concept: resting myocardial perfusion heterogeneity quantified by markovian analysis of pet identifies coronary microvascular dysfunction and early atherosclerosis in 1,034 subjects. J Nucl Med. 2005;46:1427–1437. [PubMed] [Google Scholar]


