Abstract
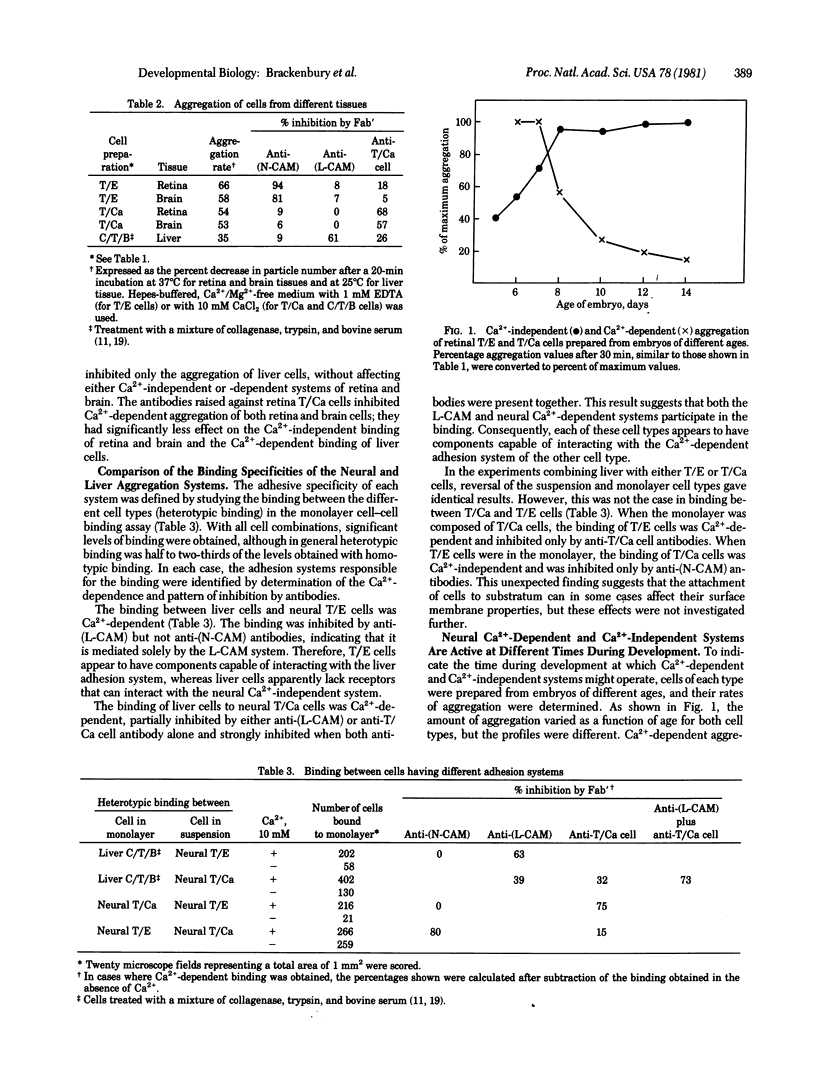
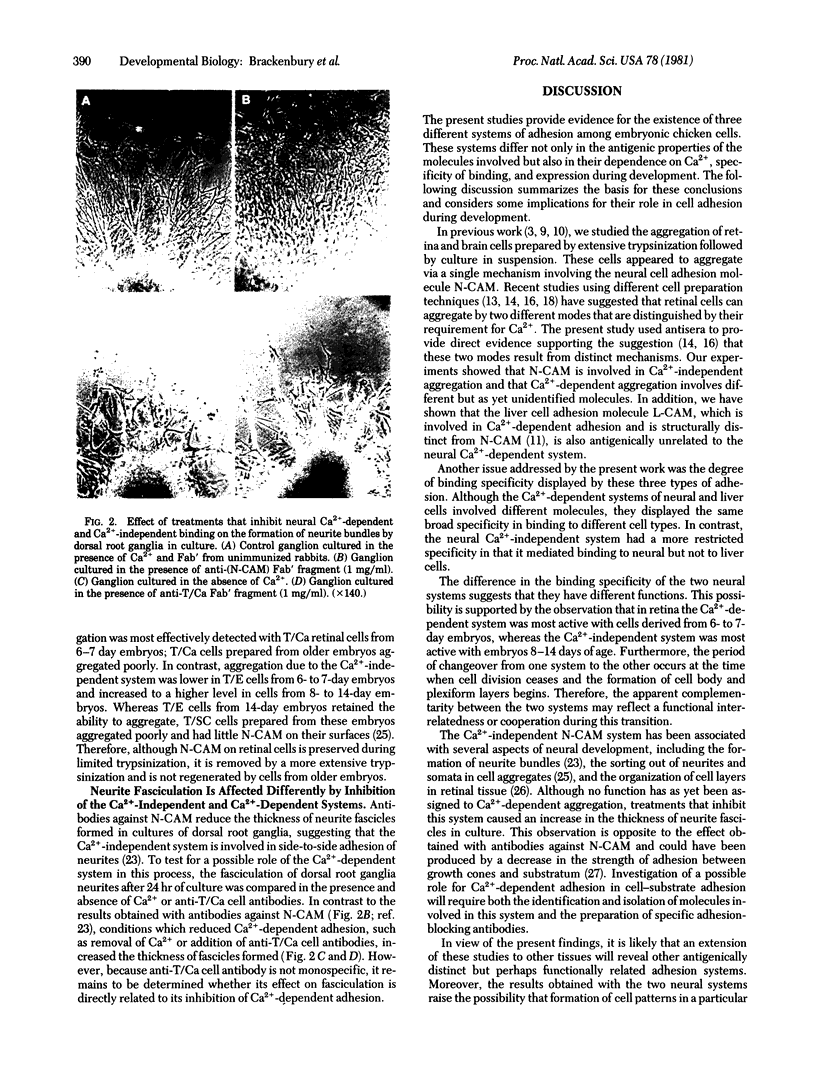
Three criteria have been used to distinguish among different systems of embryonic cell adhesion: dependence on Ca2+, involvement of particular cell-surface molecules, and binding specificity. The characterization of the adhesion with respect to cell-surface molecules was carried out by using specific antibodies against the neural and liver cell adhesion molecules (N-CAM and L-CAM) and antibodies raised against retinal cells prepared by limited trypsinization in the presence of Ca2+ (called "T/Ca cells"). Aggregation of cells prepared from retina or brain without Ca2+ did not require Ca2+ and was inhibited by anti-(N-CAM) antibodies but not by anti-(L-CAM) or anti-T/Ca cell antibodies. In contrast, cells obtained from the same tissues in the presence of Ca2+ did require Ca2+ to aggregate. This aggregation was inhibited by anti-T/Ca cell antibodies but not by anti-(N-CAM) or anti-(L-CAM) antibodies. Hepatocyte aggregation also required Ca2+ and was inhibited only by anti-(L-CAM) antibodies. These results define three antigenically distinct cell adhesion systems in the embryo and raise the possibility that additional systems will be found. The neural Ca2+-independent system displayed a limited tissue specificity, mediating binding to neural but not liver cells. In contrast, the Ca2+-dependent systems of both neural and liver cells caused binding to all cell types tested. The Ca2+-dependent system was most active in retinal cells from 6-7 day embryos, whereas the Ca2+-independent system was most active at later times during development. In addition, treatments that inhibited the Ca2+-independent or Ca2+-dependent systems had very different effects on the fasciculation of neurites from dorsal root ganglia. All of the results suggest that Ca2+-independent and Ca2+-dependent adhesion systems play different functional roles during embryogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balsamo J., Lilien J. Functional identification of three components which mediate tissue-type specific embryonic cell adhesion. Nature. 1974 Oct 11;251(5475):522–524. doi: 10.1038/251522a0. [DOI] [PubMed] [Google Scholar]
- Bertolotti R., Rutishauser U., Edelman G. M. A cell surface molecule involved in aggregation of embryonic liver cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4831–4835. doi: 10.1073/pnas.77.8.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury R., Thiery J. P., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J Biol Chem. 1977 Oct 10;252(19):6835–6840. [PubMed] [Google Scholar]
- Buskirk D. R., Thiery J. P., Rutishauser U., Edelman G. M. Antibodies to a neural cell adhesion molecule disrupt histogenesis in cultured chick retinae. Nature. 1980 Jun 12;285(5765):488–489. doi: 10.1038/285488a0. [DOI] [PubMed] [Google Scholar]
- Coon H. G. Clonal stability and phenotypic expression of chick cartilage cells in vitro. Proc Natl Acad Sci U S A. 1966 Jan;55(1):66–73. doi: 10.1073/pnas.55.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Grunwald G. B., Geller R. L., Lilien J. Enzymatic dissection of embryonic cell adhesive mechanisms. J Cell Biol. 1980 Jun;85(3):766–776. doi: 10.1083/jcb.85.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman R. E., Moscona A. A. Isolation of retina-specific cell-aggregating factor from membranes of embryonic neural retina tissue. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3594–3598. doi: 10.1073/pnas.73.10.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoi E. R., Marchase R. B. Ligatin from embryonic chick neural retina. J Cell Biol. 1979 Mar;80(3):642–650. doi: 10.1083/jcb.80.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire E. J. Intercellular adhesive selectivity. II. Properties of embryonic chick liver cell-cell adhesion. J Cell Biol. 1976 Jan;68(1):90–100. doi: 10.1083/jcb.68.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell R., Gottlieb D. I., Glaser L. Embryonal cell surface recognition. Extraction of an active plasma membrane component. J Biol Chem. 1975 Jul 25;250(14):5655–5659. [PubMed] [Google Scholar]
- Rutishauser U., Edelman G. M. Effects of fasciculation on the outgrowth of neurites from spinal ganglia in culture. J Cell Biol. 1980 Nov;87(2 Pt 1):370–378. doi: 10.1083/jcb.87.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Gall W. E., Edelman G. M. Adhesion among neural cells of the chick embryo. IV. Role of the cell surface molecule CAM in the formation of neurite bundles in cultures of spinal ganglia. J Cell Biol. 1978 Nov;79(2 Pt 1):382–393. doi: 10.1083/jcb.79.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Thiery J. P., Brackenbury R., Edelman G. M. Adhesion among neural cells of the chick embryo. III. Relationship of the surface molecule CAM to cell adhesion and the development of histotypic patterns. J Cell Biol. 1978 Nov;79(2 Pt 1):371–381. doi: 10.1083/jcb.79.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Thiery J. P., Brackenbury R., Sela B. A., Edelman G. M. Mechanisms of adhesion among cells from neural tissues of the chick embryo. Proc Natl Acad Sci U S A. 1976 Feb;73(2):577–581. doi: 10.1073/pnas.73.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela B. A., Lis H., Sharon N., Sachs L. Isolectins from wax bean with differential agglutination of normal and transformed mammalian cells. Biochim Biophys Acta. 1973 May 17;310(1):273–277. doi: 10.1016/0005-2795(73)90030-5. [DOI] [PubMed] [Google Scholar]
- Steinberg M. S., Armstrong P. B., Granger R. E. On the recovery of adhesiveness by trypsin-dissociated cells. J Membr Biol. 1973;13(2):97–128. doi: 10.1007/BF01868223. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977 Nov;75(2 Pt 1):464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M., Ozaki H. S., Tokunaga K., Okada T. S. Experimental manipulation of cell surface to affect cellular recognition mechanisms. Dev Biol. 1979 May;70(1):195–205. doi: 10.1016/0012-1606(79)90016-2. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Brackenbury R., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. II. Purification and characterization of a cell adhesion molecule from neural retina. J Biol Chem. 1977 Oct 10;252(19):6841–6845. [PubMed] [Google Scholar]
- Urushihara H., Ozaki H. S., Takeichi M. Immunological detection of cell surface components related with aggregation of Chinese hamster and chick embryonic cells. Dev Biol. 1979 May;70(1):206–216. doi: 10.1016/0012-1606(79)90017-4. [DOI] [PubMed] [Google Scholar]
- Urushihara H., Takeichi M. Cell-cell adhesion molecule: identification of a glycoprotein relevant to the Ca2+-independent aggregation of Chinese hamster fibroblasts. Cell. 1980 Jun;20(2):363–371. doi: 10.1016/0092-8674(80)90622-4. [DOI] [PubMed] [Google Scholar]
- Walther B. T., Ohman R., Roseman S. A quantitative assay for intercellular adhesion. Proc Natl Acad Sci U S A. 1973 May;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]



