Abstract
Endogenous opioids are involved in the hedonic aspects of eating. Opioid impairments and alterations have been implicated in the pathophysiology of bulimia nervosa and binge eating disorder. Specific contributions by Bartley G. Hoebel have furthered the understanding how cyclical caloric restriction and intermittent optional access to sugar solutions results in opioid-like forebrain neural alterations and dependency in rodents. The present study sought to investigate caudal brainstem and nodose ganglion mu-opioid receptor mRNA alterations in a rodent model of dietary-induced binge eating of sweetened fat (vegetable shortening blended with 10% sucrose). Five groups (n = 7 or 8) of adult female Sprague Dawley rats were exposed to various dietary conditions for 6 weeks. As measured by in situ hybridization, there was reduced (approximately 25% from naive) mu-opioid receptor mRNA in the nucleus of the solitary tract (NTS) in the binge access group, which had intermittent calorie restriction and optional limited access to the sweetened fat. A similar reduction in expression was demonstrated in the continuous access group, which has unlimited optional sweetened fat and an obese phenotype. In the nodose ganglion, mu-opioid receptor mRNA was increased (approximately 30% from groups with sweetened fat access) in rats with intermittent caloric restriction alone. Our findings and the body of work from the Hoebel laboratory suggest that dietary-induced binge eating can consequentially alter opioidergic forebrain and hindbrain feeding-related neural pathways. Future work is needed to determine whether similar alterations are involved in the maintenance and progression of binge eating and other related eating pathologies.
Keywords: eating disorders, MOR1, naloxone, OPRM1
Introduction
Binge eating is a core criterion in the diagnosis of bulimia nervosa (BN) and binge eating disorder (BED)[1–3]. Disrupting the pattern of binge eating and normalizing eating behaviors are significant obstacles to the long-term effective treatment of these eating disorders [2,4]. A more complete understanding of the pathophysiology of eating disorders will enable the development of better treatment long-term outcomes. To this end, distinctions are often made between trait-related and state-related factors in investigating the sustaining neurobiology of psychiatric diseases[5,6]. Trait-related factors are inherited or acquired susceptibilities that are antecedent to the clinical manifestation of the disorder. State-related factors, in contrast, are typically transient and result from the conditional or behavior consequences of the disorder [5]. Investigations into the state-related consequences of binge-like dietary patterns on the neural and behavioral components of eating have been greatly advanced by the use of rodent models of dietary-induced binge eating [7–13]. This article will describe the significant contribution and impact of Bartley G. Hoebel on understanding the opioidergic consequences of dietary-induced binge eating using sugar solutions in rodents. In addition, we will highlight our findings in the hindbrain of female rats exposed to a dietary-induced binge eating paradigm using a sugar-fat mixture.
Human and rodent studies have demonstrated the involvement of endogenous opioids in positive affect associated with eating [14]. Opioid antagonists (e.g., naltrexone and naloxone) selectively reduce the intake and hedonic ratings of highly palatable foods [14–16]. Oral naltrexone (200–300 mg daily) in clinical settings has been demonstrated to reduce bingeing and purging frequency in BN [17–19], although concerns over adverse effects have limited its use in long-term treatment of BN and BED [20]. Moreover, circulating levels of beta-endorphin and cortical mu-opioid receptor binding have been correlated with eating pathologies [21,22]. Investigations into how dietary conditions, which promote and maintain binge eating, result in state-related opioidergic dysfunction would contribute to understanding of eating disorders.
The model of dietary-induced binge eating used by the Hoebel laboratory incorporates several dietary elements relevant to binge eating and eating pathologies [10,11]. First, the Hoebel paradigm uses optional access to aqueous sugar solutions (25% glucose or 10% sucrose). Sugar solutions are given at the same time as standard chow allowing rats to self-select their diet. Having optional access to the sugar solutions mimics a sweet dessert item (which are typically “binge” foods in humans) and provides an extra sources of calories [23,24]. Second, the sugar solutions are not available ad libitum, but are given intermittently. This has the apparent resemblance of the pulsatile eating pattern reported in eating disorder patients and serves to increase the salience of the highly palatable food [4,25]. Third and along the lines of the second item, bingeing animals in the Hoebel paradigm are calorie restricted. Rather than be restricted to a certain amount of food, they are time-restricted (12 h of food). This not only serves to increase the salience to promote rapid and excessive feeding in shorter time period, but entrains the animals and shifts their dietary patterns. As a consequence of this schedule rats show a steady escalation in intake of sugar solutions over days and ingest more of the sugar solution at the early phase of access period [10,11].
Several neurochemical (i.e., dopamine and acetylcholine) and behavioral changes (i.e., cross-sensitization) have been reported using the Hoebel model of sugar bingeing ([13,26] for review), but relevance here is given to the opioidergic alterations. Specific opioid alterations in sugar bingeing rats that occurred after 30 cycling days include increases in limbic forebrain receptor densities. Indeed, increased binding of the mu-opioid receptor specific ligand, [125I]DAMGO ([D-Ala, N-MePhe, Gly-ol]-enkephalin], was demonstrated in the nucleus accumbens shell, cingulate cortex, and hippocampus. Notably, mu-opioid receptor binding was decreased in the midbrain substania nigra pars reticulata [11]. In a follow-up experiment, sugar bingeing rats were tested for somatic signs of opiate dependency following either spontaneous (12h, 24h, or 36 h deprivation) or naloxone (20 mg/kg, IP) precipitated withdrawal. Sugar bingeing rats demonstrated increased teeth chattering, forepaw tremors, and head shakes following naloxone and 24 h of food deprivation[11]. Other studies in from the Hoebel laboratory and their collaborators have indicated reductions in striatal preproenkephalin mRNA in sugar bingeing rats [27]. Together, these findings suggest an opiate-like effect of sugar bingeing on rats.
In addition to their well-described action in the striatum, opioids have been demonstrated to be involved in feeding-related actions in other brain regions [28–32]. This raises the possibility of opioid dysfunction at multiple brain sites as a consequence of dietary conditions. Our studies investigated the involvement of the caudal brainstem opioid system in dietary-induced binge eating. In particular, the nucleus tractus solitarius (NTS) is a critical hindbrain neural structure involved in the integration and control of food intake [33,34]. Although circulating peripheral hormones (e.g., leptin, ghrelin, glucagon-like peptide-1) modulate caudal brainstem feeding responses and body weight, sensory information (i.e., mechanical, chemosensory) involved in the negative feedback control of meals and gastrointestinal input is conveyed predominantly via vagal afferents [35–40]. Increases in gastric capacity, alterations in meal-related hormones, and reductions in subjective satiety ratings have been reported in BN and BED [41–43]. Such findings suggest gastrointestinal integrative functions are likely to be altered in BN and BED to accommodate the overeating and reduce satiety.
Mu-opioid receptors are distributed throughout the NTS and vagus nerve [44–48]. Several studies have suggested that NTS opioid signaling engage forebrain regions to modulate food intake [49,50]. In order to further distinguish whether mu-opioid receptors alterations were located on neurons within the NTS or on vagal afferents terminals innervating this nuclei, in situ hybridization was used to measure mRNA levels. The dietary-induced binge eating paradigm used in this experiment is different from the sugar bingeing protocol used by the Hoebel laboratory, but does have several overlapping characteristics. These include optional access to the highly palatable food, an intermittent schedule, and cyclical periods of calorie restriction. Previously, we have demonstrated that a history of this binge access schedule to the sweetened fat produces a pattern of overeating whereby rats consume in 2 h an amount of calories that approximates the ad libitum 24 h calorie intake on standard chow. After 6 weeks, animals on this binge access schedule have greater meal-induced neuronal activation, as measured by immunoreactive c-Fos, in the medial region of the NTS [51]. The particular experiments to be presented here assessed whether engaging in bingeing induced by this schedule alters mu opioid receptor expression in this integrative nucleus within the dorsal hindbrain.
Material and Methods
Animals
A total of thirty-nine adult female Sprague Dawley rats (Harlan Laboratories, Frederick, MD), with an initial weight range of 200–225g were individually housed in stainless steel wire mesh hanging cages and placed on a 12/12 h light dark schedule (lights off @ 1230 h). All rats received ad libitum standard laboratory chow (Global Diet-2018, Harlan Teklad; 3.3 Kcal/g, fixed formula diet of 18% protein, 5% fat) unless otherwise noted. Water was available at all times during the experiment. All the procedures were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University.
Feeding schedules and experimental groups
The feeding protocols used in these experiments were similar to those previously described by our laboratory [51]. Briefly, the highly palatable food used was “sweetened fat”, which consisted of vegetable shortening, (Crisco®, a generous gift from J.M. Smucker, Co.; 1.5 g trans fat) blended with 10% sucrose; 8.6 Kcal/g. A sweetened fat pre-exposure was used to determine if the rats had initial differences in their dietary preference. All groups received a 24 h pre-exposure to the sweetened fat 3 days before beginning their respective feeding schedules. The rats were divided into five groups with an initial statistically similar body weight and sweetened fat preference. These groups were designated Continuous Access, Binge Access, Chow Restricted, Scheduled Access, or Naive groups (n=8 for all groups, except Chow Restricted n=7). The Continuous Access group had unlimited optional access to both standard chow and jars containing sweetened fat throughout the experiment. Jars containing sweetened fat were refreshed as needed and completely changed out every third day. The Binge Access and Chow Restricted group were restricted at the beginning of the dark cycle to 33% of the previous day’s chow calorie intake on Days 2 and 5 of each week. On restriction days animals were noted to consume the entire restricted amount of chow within the first 4 h. Hence, on the restriction days, this amounted to the equivalent of an approximate 20 h food deprivation prior to the re-feeding days. On subsequent days (days 3 and 6) 2 h into the dark cycle (1430 h) (total deprivation time ~ 22 hrs), the Binge Access group was given access to both standard chow and sweetened fat, whereas the Chow Restricted groups were re-fed with chow alone. The Binge Access group had access to the jars of sweetened fat only for the first 2 h of each re-feeding period. In this fashion, the Binge Access group was exposed to a repeated cycle that consisted of three no restriction days (days 1, 4, and 7), two weekly episodes of calorie restriction (days 2 and 5), and two weekly episodes of scheduled re-feeding starting with 2 h access to an optional highly palatable food (day 3 and 6). This schedule was chosen to provide the animals with combination of intermittent days of calorie restriction, palatable food access and ad libitum standard chow access within a 7-day period. A fourth group, Scheduled Access, received the 2 h access to the sweetened fat at the same time as the Binge Access groups, but did not undergo any repeated bouts of chow restriction. The fifth group, Naive was not exposed to the sweetened fat (except during the pre-exposure) or any bouts of re-feeding. The Naive group received standard chow ad libitum.
Palatable food and chow intake during the feeding schedules
Animals were maintained on each of these weekly feeding schedules (n= 8 per group) for 6 weeks. Food intake and spillage was recorded to nearest 0.1 g and measured separately for the 2 h re-feeding period and the 20 h following the re-feeding period on days 3 and 6 (i.e., re-feeding days or “binge days”) throughout the experiment. The 24 h caloric intake was also measured on days 1 and 4 (days before the calorie restriction for Binge Access and Chow Restricted groups). Intakes for Day 7 were not recorded.
Vaginal Cytology
The stage of estrus was assessed by vaginal cytology. This was done twice a week fifteen minutes before lights out (1215 h) on days 2 and 5 of the weekly schedule (i.e., re-feeding days for of the binge). Briefly the vaginal cavity of rats were lavaged with sterile saline (0.9%) and the cells were characterized by vaginal epithelial cell morphology [52].
Nodose ganglion and brain removal, fat pad weights, and plasma hormone assays
After a total of 6 weeks on the feeding schedule, all groups were food-restricted beginning at the onset of the dark cycle to 33% of the previous day’s caloric intake. Since the groups were on different feeding schedules, the uniform 33% calorie restriction before sacrifice was employed to eliminate the potential confounding effects of recent food intake. On the following day, 2 h into the dark cycle at the time of the expected re-feeding for Binge Access, Chow-Restricted, and Scheduled Access groups, rats were taken into a separate room and anesthetized with 1 ml/kg of a 4:3 mixture of ketamine (100 mg/ml) and xylazine (20 mg/ml). The nodose ganglion of the vagus (left side) was removed and immediately immersed in −40°C isopentane (2-methylbutane) and stored at −80°C. Animals were then decapitated and brains were removed and processed in an identical manner to the excised nodose tissue. Trunk blood (~4 ml) from each rat was collected into an EDTA vacutainer tube, 20 μl of which was removed for blood glucose assay (Freestyle, Abbott Laboratories), and maintained on ice until centrifugation at 3,000 rpm for 10 min. Standard radioimmunoassay kits (Millipore, St. Charles, MO) were used to determine plasma insulin (sensitivity; 0. 1 ng/ml), ghrelin (total) (sensitivity; 100 pg/ml), and leptin (sensitivity; 0.5 ng/ml) levels. Retroperitoneal and subcutaneous fat pads were dissected from carcasses and weighed to the nearest 0.1 g.
Tissue section and riboprobes
Brain tissue was sectioned at 14 μm in the coronal plane on a cryostat through the rostrocaudal extent of the nucleus of the solitary tract (NTS) and area postrema (AP). Sectioning commenced at the level of the obex (−5.6 mm from the interaural line) continuing rostral to the gelatinous subnucleus and the caudal aspect of the medial vestibular nucleus on the dorsal boundary (−3.6 mm from the interaural line) [53]. Nodose ganglion were embedded in brain paste (bovine) and sectioned at 14 μm in the longitudinal plane. Four sections per brain/nodose ganglion were mounted on mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and anatomically and group matched for each hybridization assay.
Plasmids (pgem 4Z) containing the rat mu-opioid receptor construct (744–1064 bp; accession number L22455, generous gift of S.J. Watson, University of Michigan) were linearized by recommended restriction enzymes. Antisense and sense riboprobes for each were labeled with [35S]UTP(Specific Activity; 1200 Ci/mmol, Amersham/GE Healthcare; Piscataway NJ) by using in vitro transcription systems with appropriate polymerases according to the manufacturer’s protocols (Promega; Madison, WI) and purification of the labeled probe with mini Quick Spin RNA Columns (Roche Applied Science, Indianapolis, IN) to yield a specific activity of 5 × 108 cpm/μg.
In situ hybridization
Frozen brain and nodose ganglion sections were removed from storage at −80°C and placed into 4% formaldehyde fo r 20 min (22°C) prior to processing for in situ hybridization. Slides were then rinsed in water, followed by 0.1 M triethanolamine, pH 8.0, and treated with a mixture of 0.1 M triethanolamine, pH 8.0, and acetic anhydride (400:1, v/v) with stirring for 10 min. The sections were rinsed again in water and dehydrated through graded alcohols, and allowed to air dry. Overnight incubated in hybridization buffer containing 50% formamide, 0.3 M NaCl, 10 mM Tris·HCl (pH 8.0), 1 nM EDTA (pH 8.0), 1x Denhardt’s solution (Eppendorf), 10% dextran sulfate, 10 mM DTT, 500 μg/ml yeast tRNA, and labeled ribroprobe at 55°C. The next day the sections had two 5-min rinses in 2 x SSC (300 mM sodium chloride, 30 mM sodium citrate, pH 7.2) at 55°C followed by a rinse with RNase A (2 00 μg/ml in 100 mM Tris, pH 8.0, and 0.5M NaCl) for 30 min at 37°C. Subsequently, sections were washed twice in 0.1x SSC at 55°C for 15 min. Slides were dehydrated through graded alcohol and exposed with BioMax XAR Film (Eastman Kodak Company, New York, NY) for 10 days (NTS) or 7 days (nodose ganglia).
Quantitative analysis
Images obtained by in situ hybridization were analyzed using the Scion Imaging software (Version 4.0.3.2, NIH, Bethesda, MD). Films were first scanned using an Epson Professional Scanner and then quantified with Scion Image software that utilized 14C-microscales (Amersham/GE Healthcare; Piscataway NJ) as a standard. Data for each animal were means of the product of hybridization area X optical density (OD) and this value was subtracted from the film background OD. There was no measurable OD of signal greater than film background in either the NTS or nodose ganglia in sections hybridized with the labeled sense probe. Data for each animal were normalized to those of Naive controls as 100%, and all of the data are expressed as means ± SE.
Statistical analyses
Food intakes and body weights for the 6-wk feeding schedules were analyzed using individual repeated measure ANOVA. Adipose tissue weights and Hormone and fat tissue measurements were analyzed with a one-way ANOVA. In situ hybridization measurements were analyzed separately for the NTS and nodose by one-way ANOVA. Post hoc comparisons were made when appropriate with a Newman-Keuls test. All statistical analyses were performed with Statistica software (version 7.1, StatSoft Inc., Tulsa OK), and significance was set at α = 0.05.
Results
Food intake during 6-week schedule
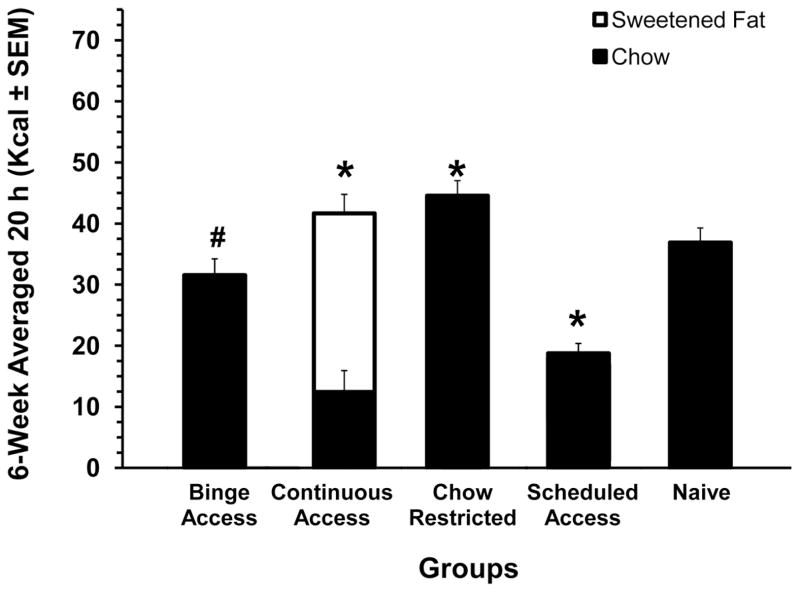
The total caloric intakes for the 2 h period (on days 3 and 6 when the sweetened fat was available) were significantly different among groups [F (4,73) = 142.2, P< 0.001] and there was a significant group X weeks interaction [F(20,366) = 1.9, P < 0.05]. The Binge Access group consumed the most total Kcals during the 2 h (p < 0.01). The total Kcal intakes were significantly different between all groups on weeks 2 and 5 (p < 0.05 for both), see figure 1A. There was also an overall difference among the Binge Access, Continuous Access and Scheduled Access [F(2, 45)=67.1, P < 0.001] for the sweetened fat intake during the 2 h with the Scheduled Access having the highest sweetened fat intake (p < 0.001). For chow consumption during this 2 h period there was a group difference [F(4, 73)= 122.5, P < 0.001] with Chow Restricted group having the highest chow intake (p <0.001). There were no differences in the chow consumed during the 2 h period between the Continuous Access and Scheduled Access, see figure 1B. The 20-h caloric intakes following the 2 h of re-feeding on days 3 and 6 differed significantly between groups [F(4, 73)=122.18, P< 0.001]. Intake of the Binge Access group during 20h approached that of the Naive group, but was still significantly less (p < 0.05). On the other hand, the Chow Restricted and Continuous Access groups consumed the most calories while the Scheduled Access group consumed the least compared with Naive controls (p < 0.001 for all), see figure 2. Compensatory feeding behaviors were demonstrated on days 1 and 4 of the feeding schedule by the Binge Access, and Chow-Restricted, and Scheduled Access group [F(4, 73) = 82.17, P < 0.001], with Scheduled Access group consuming the least on these days and the Chow Restricted consuming the most calories (p < 0.01), data not shown.
Figure 1. Total caloric intakes for the 2-h re-feeding period on days 3 and 6 of the 6-wk feeding schedule.
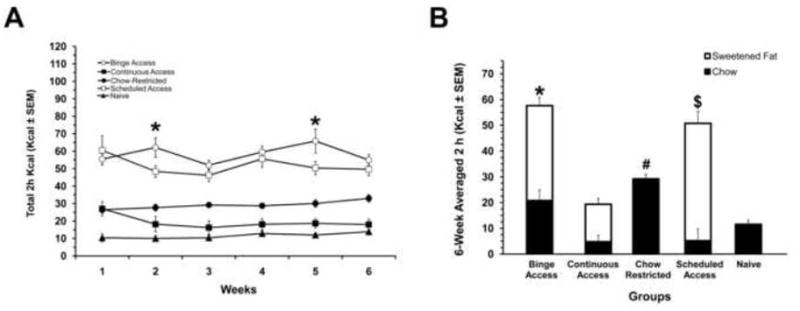
A: 6-wk total caloric intake for the 2-h period. All groups were significantly different from Naive, * indicates differences between Binge Access and Schedule Access (p < 0.05) at the respective weeks. B: 6-wk averaged intakes to illustrate the dietary contribution of calories. The Binge Access group consumed significantly more total calories (*, p < 0.001), Schedule Access group consumed more sweetened fat ($, p< 0.001), and the Chow Restricted more chow (#, p < 0.001). White bars are calories derived from sweetened fat; solid bars are calories derived from chow. Mean ± SE.
Figure 2. Averaged 6-week total caloric intake for the 20-h period following the 2-h re-feeding period on days 3 and 6.
All groups were significantly different from Naive (*, p < 0.001 and #, p < 0.05). White bars are calories derived from sweetened fat; solid bars are calories derived from chow. Mean ± SE.
Vaginal Cytology
Based on the cellular morphology, most animals were in phases of diestrus (87%) throughout the experiment prior to the re-feeding on days 3 and 6. In instances where animals were in estrus or proestrus (13%), there was no appreciable effect on food intake or body weight. For example, in the Binge Access animals, 2 h total intakes were 55± 3 Kcal for diestrus and 53 ± 7 Kcal for estrus/proestrus.
Body weights, fat pads, and plasma hormones
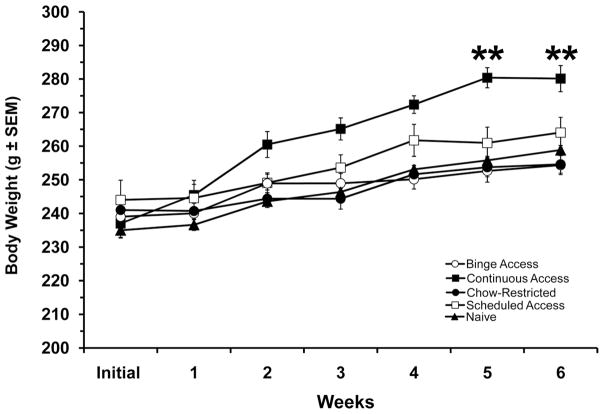
Body weights over the 6-weeks indicated differences among groups [F(4, 73) = 8.67, P < 0.001], weeks [F(5,365) = 127.30, P < 0.001] and the group x week interaction[F(20, 365) = 5.49, P<0.001]. The Continuous Access group demonstrated the most pronounced weight gain, which was significantly different from all other groups at weeks 5 and 6 of the feeding schedule (p < 0.001), see figure 3. Carcass fat pad dissection also revealed differences among groups for retroperitoneal [F(4,34) = 20.15, P < 0.001] and subcutaneous [F(4,34) = 15.47, P < 0.001] fat pad weights. For both fat depots, the Continuous Access animals had the highest weights compared with all groups (p < 0.01), see Table 1. Also, table 1 illustrates the blood glucose and hormone value at the time of sacrifice. Only leptin [F(4, 34)=10.15, P<0.001] and total ghrelin [F(4, 34)=7.56, P<0.001] were different between groups. Leptin values were highest in the Continuous Access group compared with all groups (p<0.001). Total ghrelin values, however, were lowest in Continuous Access group compared with all other groups (p<0.05). Total ghrelin levels for the Binge Access, Chow Restricted, and Scheduled Access groups were lower than those of the Naive controls (p< 0.05, for all).
Figure 3. Body weights over the 6-week feeding schedules.
Continuous Access group had a significantly increase in body weight, which were different from all groups at weeks 5 and 6 (**, p < 0.001). Mean ± SE.
Table 1.
Carcass fat and blood parameters after 6 weeks on feeding schedules
| Groups (n = 8 for all groups, except Chow Restricted n = 7).
|
|||||
|---|---|---|---|---|---|
| Binge Access | Continuous Access | Chow Restricted | Scheduled Access | Naive | |
| White Adipose Tissue | |||||
| Subcutaneous (g) | 3.5 ± 0.2 | 6.17 ± 0.4** | 3.6 ± 0.2 | 3.28 ± 0.3 | 3.11 ± 0.3 |
| Retroperitoneal (g) | 1.03 ± 0.1 | 2.33 ± 0.3** | 1.1 ± 0.1 | 0.88 ± 0.1 | 1.13 ± 0.1 |
| Glucose (mg/dL) | 88.9 ± 6.4 | 102 ± 4.3 | 92.2 ± 4.34 | 90 ± 6.5 | 88.0 ± 5.8 |
| Insulin (ng/ml) | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.17 ± 0.01 | 0.18 ± 0.01 |
| Leptin (ng/ml) | 1.07 ± 0 | 1.97 ± 0.3** | 1.07 ± 0.1 | 1.03 ± 0.1 | 0.9 ± 0.1 |
| Ghrelin(total;ng/ml) | 1.53 ± 0.1* | 0.91 ± 0.0** | 1.37 ± 0.1* | 1.39 ± 0.1* | 2.05 ± 0.3 |
p<0.05 significantly different from all groups
p<0.05 significantly different from Naive Controls
In situ hybridization for mu-opioid receptor expression
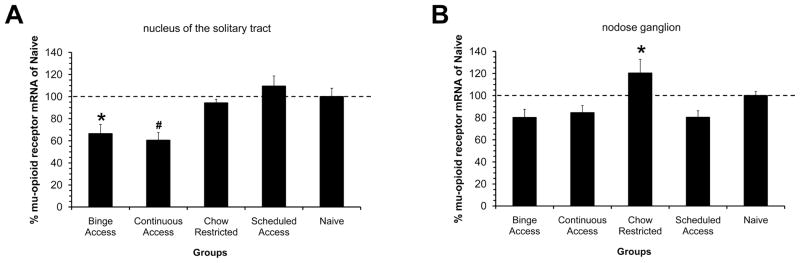
Mu-opioid receptor mRNA was distributed mainly in the medial subnucleus of the NTS with scattered or diffuse distribution in the centrally located commissural subnucleus. There was no or scant distribution of mu-opioid receptor mRNA in the area postrema and dorsal motor vagus nucleus. Along the anterior-posterior axis of the NTS, there was a gradient of expression with moderate levels emerging at the level of the area postrema and becoming denser in the rostral expanse (at the level of the gelatinous subnucleus). There were differences in mRNA levels of the mu-opioid receptor in the these regions of the NTS among groups [F(4, 27)=7.2, P<0.01]. Binge Access animals had reduced mu-opioid receptor expression compared with Scheduled Access and Naive controls (p < 0.05). A similar reduction in mu opioid receptor mRNA was also demonstrated in the Continuous Access group. Not only did the Continuous Access have reductions compared with Scheduled Access and Naive groups (p<0.05), but reductions were also demonstrated in comparison with Chow Restricted group (p<0.05), see figure 4A. In the nodose ganglion group differences were also apparent for mu-opioid mRNA levels [F(4, 19)=7.25, P<0.005]. The Chow Restricted group demonstrated increased mu-opioid receptor mRNA compared with Binge Access, Continuous Access, and Naive Controls (p < 0.05 for all), see figure 4B.
Figure 4. Mu-opioid receptor mRNA levels as measured by in situ hybridization in groups after 6 weeks on the feeding schedules.
Data is expressed as percentage of hybridization levels measured in Naive controls, represented as dotted line in both graphs. A: Percent hybridization measured in the nucleus of solitary tract at the level of the area postrema. Binge access group has reduced mRNA levels compared with Scheduled Access and Naive animals (*, p< 0.05). Continuous Access group had reduced mRNA levels compared with Chow Restricted, Schedule Access, and Naive groups (#, p < 0.05). B: Percent hybridization measured in the nodose ganglion of the vagus nerve. Chow Restricted group had increased mRNA levels compared with Binge Access, Continuous Access, and Schedule Access groups (*, p<0.05). Mean ± SE.
Discussion
The findings from this study demonstrate that when adult female rats are exposed to a binge access schedule, a cyclical pattern of calorie restriction with limited optional access to a sweetened fat mixture; they display a pattern of robust overeating. In comparison, the pattern of overeating on re-feeding days was different from that of the Schedule Access group, which received the sweetened fat at the same frequency as the Bing Access group without intermittent calorie restriction. That is, the Binge Access group consumed more total calories during the 2 h re-feeding and during the following 20 h intake period on re-feeding days (days 3 and 6). In fact, the Schedule Access group consumed the most sweetened fat during the 2 h re-feeding, but the least amount of standard chow during the following 20 h. This pattern of over-eating followed by periods of under-eating is similar to other dietary-induced binge eating paradigms that use fat or fat containing highly palatable foods [7,9,13,54–56]. The Hoebel laboratory has further examined this “self-restriction” behavior between binge-like episodes using optional access to a nutritionally complete sweet-fat diet (45% fat, 17% sucrose) [56]. One finding from that study was the “self-restriction” to the standard chow occurred to varying degrees in groups with either intermittent access (Monday/Wednesday/Friday) or daily access to the sweet-fat diet. In the present study the “self-restriction” or post-binge feeding suppression was reduced in the binge access group either because they consume less sweetened fat during 2 h access or rebounding from the calorie restriction or combination of both. Although an anticipatory contrast effect has been reported with brief access (10 min) sugar varying diets [57], the “self-restriction” to the standard chow has not been reported or characterized in dietary-induced models of binge eating using sugar solutions. Because high-fat foods compared with high sugar foods typically have less post-ingestive satiety [58], it would be interesting to determine whether the magnitude of the “self-restriction” using sugar compared with fat in comparable models of dietary-induced binge eating.
Several models of dietary-induced binge eating have reported a lack of a body weight difference between groups with different feeding conditions [9–12]. The lack of differences in body weights, fat pads, and hormone profiles of the Binge Access, Chow-Restricted, and Schedule Access compared with Naive animals, therefore, was not entirely surprising. Because of the excessive weight gain, elevated fat pad weight, increased plasma leptin, and decreased plasma total ghrelin levels, however, the Continuous Access rats were characterized as having an obese phenotype. The inclusion of this group allowed us to make comparisons between obese and bingeing animals.
Endogenous opioid signaling in the NTS is involved in the modulation of feeding mediated predominately by interconnections within forebrain regions [49,50,59–61]. Microinjection of naltrexone into the NTS has been demonstrated to significantly decrease the orexigenic response of hypothalamic paraventricular nucleus injections of NPY [50]. Reciprocally, chronic infusion (>13 day) of naltrexone into the medial NTS leads to decreased food intake and increases in amygdala pro-dynorphin mRNA [49]. Mu-opioid receptor signaling in the NTS is also important for neural circuits within the hindbrain [62,63]. The mu-opioid receptor selective ligand, DAMGO, inhibits glutamatergic input to the dorsal motor vagus nucleus through pre-synaptic mu-OR containing NTS neurons [64]. Furthermore, reduction in gastric tone are mediated by mu-opioid receptor in the NTS [65]. This suggests that reductions in mu-opioid receptor exhibited by the Binge Access and Continuous Access groups are not only the result of dysregulation of forebrain inputs, but also of gut to hindbrain vago-vagal reflexes. The increase in mu-opioid receptor mRNA in the nodose ganglion of the Chow Restricted relative to the Binge Access, Continuous Access and Scheduled Access groups suggest a peripherally mediated down-regulated of mu-opioid receptor mRNA resulting from palatable food access. The group differences in expression observed in the NTS (i.e., no difference in Schedule Access) could have resulted from feedback from reciprocal connections with forebrain regions. Taken together with our previous findings of increased neuronal activation in the NTS followed a meal of the Binge Access rats[51], the downregulation of mu-opioid receptor mRNA could likely have also resulted from increased activity as a consequence of the bingeing schedule. Further experiments, however, are needed to determine the extent of forebrain influence on opioidergic hindbrain activity.
A few factors limit the interpretations of our findings. First, although we focused our investigations on NTS regions that receive meal-related gastrointestinal input, we have not determined whether mu-opioid receptor mRNA changes are located on gastric responsive neurons. Second, these findings need to be supported by measuring protein levels and determine the function of mu-opioid receptors in NTS. Along these lines and given the observed reductions in mu-opioid receptor mRNA, it would be interesting to determine whether dietary-induced binge eating with sweetened fat results in opioid-like dependency. Findings reported in a review by the Hoebel laboratory suggest that dietary-induced binge eating models that use a predominately fat binge meal fail to result in an opioid-like dependency. Dependency was tested by measuring the somatic signs following prolonged deprivation or naloxone precipitated in a variety of different binge protocols [66]. While the neural pathways mediating the effects of sugar and fat bingeing and opioid-like dependency may diverge in part, it is not yet clear how they may produce different states of opioid signaling. This issue certainly warrants further study.
The work of Bart G. Hoebel has been instrumental in establishing models of sugar bingeing and disordered eating. He has certainly advanced our understanding of the overlapping neurobiology of feeding, motivation, and addiction.
Acknowledgments
This work was supported by National Institutes of Health Grants DK-19302 and DK-078484.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Hsu LK, Mulliken B, McDonagh B, Krupa Das S, Rand W, Fairburn CG, et al. Binge eating disorder in extreme obesity. Int J Obes Relat Metab Disord. 2002;26:1398–403. doi: 10.1038/sj.ijo.0802081. [DOI] [PubMed] [Google Scholar]
- 2.Elmore DK, de Castro JM. Meal patterns of normal, untreated bulimia nervosa and recovered bulimic women. Physiol Behav. 1991;49:99–105. doi: 10.1016/0031-9384(91)90238-j. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. American Psychiatric Association. Task Force on DSM-IV. [Google Scholar]
- 4.Mitchell JE, Pyle RL, Eckert E, Hatsukami D, Soll E. Bulimia nervosa with and without a history of overweight. J Subst Abuse. 1990;2:369–74. doi: 10.1016/s0899-3289(10)80008-2. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Bidwell LC, Norton D. Trait vs. State Markers for Schizophrenia: Identification and Characterization through Visual Processes. Curr Psychiatry Rev. 2006;2:431–8. doi: 10.2174/157340006778699729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- 7.Kinzig KP, Hargrave SL, Honors MA. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiol Behav. 2008;95:108–13. doi: 10.1016/j.physbeh.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- 9.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–53. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 10.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 11.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–88. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- 12.Avena NM, Rada P, Hoebel BG. Curr Protoc Neurosci. Unit9. Chapter 9. 2006. Sugar bingeing in rats; p. 23C. [DOI] [PubMed] [Google Scholar]
- 13.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002;26:713–28. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 15.Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Naloxone, an opiate blocker, reduces the consumption of sweet high-fat foods in obese and lean female binge eaters. Am J Clin Nutr. 1995;61:1206–12. doi: 10.1093/ajcn/61.6.1206. [DOI] [PubMed] [Google Scholar]
- 16.Fantino M, Hosotte J, Apfelbaum M. An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am J Physiol. 1986;251:R91–6. doi: 10.1152/ajpregu.1986.251.1.R91. [DOI] [PubMed] [Google Scholar]
- 17.Jonas JM, Gold MS. The use of opiate antagonists in treating bulimia: a study of low-dose versus high-dose naltrexone. Psychiatry Res. 1988;24:195–9. doi: 10.1016/0165-1781(88)90062-5. [DOI] [PubMed] [Google Scholar]
- 18.Chatoor I, Herman BH, Hartzler J. Effects of the opiate antagonist, naltrexone, on binging antecedents and plasma beta-endorphin concentrations. J Am Acad Child Adolesc Psychiatry. 1994;33:748–52. doi: 10.1097/00004583-199406000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Marrazzi MA, Bacon JP, Kinzie J, Luby ED. Naltrexone use in the treatment of anorexia nervosa and bulimia nervosa. Int Clin Psychopharmacol. 1995;10:163–72. doi: 10.1097/00004850-199510030-00005. [DOI] [PubMed] [Google Scholar]
- 20.Marrazzi MA, Wroblewski JM, Kinzie J, Luby ED. High-dose naltrexone and liver function safety. Am J Addict. 1997;6:21–9. doi: 10.3109/10550499708993159. [DOI] [PubMed] [Google Scholar]
- 21.Bencherif B, Guarda AS, Colantuoni C, Ravert HT, Dannals RF, Frost JJ. Regional mu-opioid receptor binding in insular cortex is decreased in bulimia nervosa and correlates inversely with fasting behavior. J Nucl Med. 2005;46:1349–51. [PubMed] [Google Scholar]
- 22.Waller DA, Kiser RS, Hardy BW, Fuchs I, Feigenbaum LP, Uauy R. Eating behavior and plasma beta-endorphin in bulimia. Am J Clin Nutr. 1986;44:20–3. doi: 10.1093/ajcn/44.1.20. [DOI] [PubMed] [Google Scholar]
- 23.Kales EF. Macronutrient analysis of binge eating in bulimia. Physiol Behav. 1990;48:837–40. doi: 10.1016/0031-9384(90)90236-w. [DOI] [PubMed] [Google Scholar]
- 24.Drewnowski A, Bellisle F, Aimez P, Remy B. Taste and bulimia. Physiol Behav. 1987;41:621–6. doi: 10.1016/0031-9384(87)90320-9. [DOI] [PubMed] [Google Scholar]
- 25.Raymond NC, Neumeyer B, Warren CS, Lee SS, Peterson CB. Energy intake patterns in obese women with binge eating disorder. Obes Res. 2003;11:869–79. doi: 10.1038/oby.2003.120. [DOI] [PubMed] [Google Scholar]
- 26.Avena NM. Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Exp Clin Psychopharmacol. 2007;15:481–91. doi: 10.1037/1064-1297.15.5.481. [DOI] [PubMed] [Google Scholar]
- 27.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–42. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Carr KD, Kutchukhidze N. Chronic food restriction increases fos-like immunoreactivity (FLI) induced in rat forebrain by intraventricular amphetamine. Brain Res. 2000;861:88–96. doi: 10.1016/s0006-8993(00)02018-7. [DOI] [PubMed] [Google Scholar]
- 29.Kim EM, Shi Q, Olszewski PK, Grace MK, O’Hare E, Billington CJ, et al. Identification of central sites involved in butorphanol-induced feeding in rats. Brain Res. 2001;907:125–9. doi: 10.1016/s0006-8993(01)02322-8. [DOI] [PubMed] [Google Scholar]
- 30.Ward HG, Nicklous DM, Aloyo VJ, Simansky KJ. Mu-opioid receptor cellular function in the nucleus accumbens is essential for hedonically driven eating. Eur J Neurosci. 2006;23:1605–13. doi: 10.1111/j.1460-9568.2006.04674.x. [DOI] [PubMed] [Google Scholar]
- 31.Ward HG, Simansky KJ. Chronic prevention of mu-opioid receptor (MOR) G-protein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology (Berl) 2006;187:435–46. doi: 10.1007/s00213-006-0463-7. [DOI] [PubMed] [Google Scholar]
- 32.Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ. An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1055–65. doi: 10.1152/ajpregu.00108.2003. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz GJ. Integrative capacity of the caudal brainstem in the control of food intake. Philos Trans R Soc Lond B Biol Sci. 2006;361:1275–80. doi: 10.1098/rstb.2006.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (Lond) 2009;33 (Suppl 1):S11–5. doi: 10.1038/ijo.2009.10. [DOI] [PubMed] [Google Scholar]
- 35.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–5. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 36.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–46. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 37.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 13:320–30. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav. 100:503–10. doi: 10.1016/j.physbeh.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150:2654–9. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emond M, Ladenheim EE, Schwartz GJ, Moran TH. Leptin amplifies the feeding inhibition and neural activation arising from a gastric nutrient preload. Physiol Behav. 2001;72:123–8. doi: 10.1016/s0031-9384(00)00393-0. [DOI] [PubMed] [Google Scholar]
- 41.Hellstrom PM, Geliebter A, Naslund E, Schmidt PT, Yahav EK, Hashim SA, et al. Peripheral and central signals in the control of eating in normal, obese and binge-eating human subjects. Br J Nutr. 2004;92 (Suppl 1):S47–57. doi: 10.1079/bjn20041142. [DOI] [PubMed] [Google Scholar]
- 42.Geliebter A, Yahav EK, Gluck ME, Hashim SA. Gastric capacity, test meal intake, and appetitive hormones in binge eating disorder. Physiol Behav. 2004;81:735–40. doi: 10.1016/j.physbeh.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Geliebter A, Hashim SA. Gastric capacity in normal, obese, and bulimic women. Physiol Behav. 2001;74:743–6. doi: 10.1016/s0031-9384(01)00619-9. [DOI] [PubMed] [Google Scholar]
- 44.Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Nomura S, Ding YQ, Kaneko T, Li JL, Mizuno N. Localization of mu-opioid receptor-like immunoreactivity in the central components of the vagus nerve: a light and electron microscope study in the rat. Neuroscience. 1996;73:277–86. doi: 10.1016/0306-4522(96)00027-9. [DOI] [PubMed] [Google Scholar]
- 46.Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ. mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain Res. 1994;643:245–65. doi: 10.1016/0006-8993(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 47.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, et al. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–38. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 48.Aicher SA, Goldberg A, Sharma S, Pickel VM. mu-opioid receptors are present in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius. J Comp Neurol. 2000;422:181–90. doi: 10.1002/(sici)1096-9861(20000626)422:2<181::aid-cne3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 49.Glass MJ, Briggs JE, Billington CJ, Kotz CM, Levine AS. Opioid receptor blockade in rat nucleus tractus solitarius alters amygdala dynorphin gene expression. Am J Physiol Regul Integr Comp Physiol. 2002;283:R161–7. doi: 10.1152/ajpregu.00480.2001. [DOI] [PubMed] [Google Scholar]
- 50.Kotz CM, Glass MJ, Levine AS, Billington CJ. Regional effect of naltrexone in the nucleus of the solitary tract in blockade of NPY-induced feeding. Am J Physiol Regul Integr Comp Physiol. 2000;278:R499–503. doi: 10.1152/ajpregu.2000.278.2.R499. [DOI] [PubMed] [Google Scholar]
- 51.Bello NT, Guarda AS, Terrillion CE, Redgrave GW, Coughlin JW, Moran TH. Repeated binge access to a palatable food alters feeding behavior, hormone profile, and hindbrain c-Fos responses to a test meal in adult male rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R622–31. doi: 10.1152/ajpregu.00087.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- 54.McGee HM, Amare B, Bennett AL, Duncan-Vaidya EA. Behavioral effects of withdrawal from sweetened vegetable shortening in rats. Brain Res. 1350:103–11. doi: 10.1016/j.brainres.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 55.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28:436–45. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 56.Berner LA, Avena NM, Hoebel BG. Bingeing, self-restriction, and increased body weight in rats with limited access to a sweet-fat diet. Obesity (Silver Spring) 2008;16:1998–2002. doi: 10.1038/oby.2008.328. [DOI] [PubMed] [Google Scholar]
- 57.Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008;33:524–35. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- 58.Blundell JE, Lawton CL, Cotton JR, Macdiarmid JI. Control of human appetite: implications for the intake of dietary fat. Annu Rev Nutr. 1996;16:285–319. doi: 10.1146/annurev.nu.16.070196.001441. [DOI] [PubMed] [Google Scholar]
- 59.Levine AS, Billington CJ. Opioids as agents of reward-related feeding: a consideration of the evidence. Physiol Behav. 2004;82:57–61. doi: 10.1016/j.physbeh.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 60.Kotz CM, Billington CJ, Levine AS. Opioids in the nucleus of the solitary tract are involved in feeding in the rat. Am J Physiol. 1997;272:R1028–32. doi: 10.1152/ajpregu.1997.272.4.R1028. [DOI] [PubMed] [Google Scholar]
- 61.Kotz CM, Grace MK, Briggs J, Levine AS, Billington CJ. Effects of opioid antagonists naloxone and naltrexone on neuropeptide Y-induced feeding and brown fat thermogenesis in the rat. Neural site of action. J Clin Invest. 1995;96:163–70. doi: 10.1172/JCI118017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Browning KN, Travagli RA. Short-term receptor trafficking in the dorsal vagal complex: an overview. Auton Neurosci. 2006;126–127:2–8. doi: 10.1016/j.autneu.2006.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Browning KN, Kalyuzhny AE, Travagli RA. Opioid peptides inhibit excitatory but not inhibitory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Neurosci. 2002;22:2998–3004. doi: 10.1523/JNEUROSCI.22-08-02998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herman MA, Alayan A, Sahibzada N, Bayer B, Verbalis J, Dretchen KL, et al. micro-Opioid receptor stimulation in the medial subnucleus of the tractus solitarius inhibits gastric tone and motility by reducing local GABA activity. Am J Physiol Gastrointest Liver Physiol. 299:G494–506. doi: 10.1152/ajpgi.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139:623–8. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]