Abstract
Reactive oxygen species enhance or impair autoregulation. Since superoxide is a vasoconstrictor, we tested the hypothesis that stretch generates superoxide that mediates myogenic responses. Increasing perfusion pressure of mouse isolated perfused renal afferent arterioles from 40 to 80 mmHg reduced their diameter by 13.3 ± 1.8 % (p < 0.001) and increased reactive oxygen species (ethidium: dihydroethidium fluorescence) by 9.8 ± 2.3 % (p < 0.05). Stretch-induced fluorescence was reduced significantly (p<0.05) by incubation with tempol (3.7 ± 0.8%), pegylated superoxide dismutase (3.2 ± 1.0%) or apocynin (3.5 ± 0.9%) but not by pegylated catalase, L-Nitroarginine methylester or Ca2+-free medium, relating it to Ca2+-independent vascular superoxide. Compared to vehicle, basal tone and myogenic contractions were reduced significantly (p<0.05) by pegylated superoxide dismutase (5.4±0.8), tempol (4.1 ± 1.0 %) apocynin (1.0 ± 1.3%;) and diphenyleneiodinium (3.9 ± 0.9%) but not by pegylated catalase (10.1 ± 1.6 %). L-Nitroarginine methylester enhanced basal tone but neither it (15.8 ± 3.3%), nor endolethlial nitric oxide synthase knockout (10.2 ± 1.8%) significantly changed myogenic contractions. Tempol had no further effect after superoxide dismutase but remained effective after catalase. H2O2 above 50μM caused contractions but at 25μM inhibited myogenic responses (7.4 ± 0.8%; p< 0.01). In conclusion, increasing the pressure within afferent arterioles led to Ca2+-independent increased vascular superoxide production from nicotinamide adenine dinucleotide phosephate oxidase which enhanced myogenic contractions largely independent of nitric oxide whereas H2O2 impaired pressure-induced contractions but was not implicated in the normal myogenic response.
Keywords: Oxidative stress, reactive oxygen species, hydrogen peroxide, renal autoregulation, hypertension
Introduction
Autoregulation maintains renal blood flow (RBF), glomerular filtration rate (GFR) and tubular fluid delivery during changes in perfusion pressure (PP)1. Defects in the buffering of arterial pressure by renal autoregulation have been implicated in renal barotrauma2. Renal autoregulation depends primarily on a rapid myogenic contraction of the afferent arteriole3 followed by a tubuloglomerular feedback (TGF) response1, 3.
Myogenic mechanisms in the afferent arteriole are incompletely understood1. Reactive oxygen species (ROS) have been implicated in the increased vascular reactivity of the renal afferent arterioles to angiotensin II (Ang II) in states of oxidative stress4–6. An increase in pressure in a large conduit artery increased vascular ROS generation by nicotine adenine dinucleotide phosphate (NADPH) oxidase, but conduit arteries have little myogenic reactivity7. Recently, ROS have been implicated in the enhanced myogenic contractions of renal afferent arterioles from spontaneously hypertensive rats (SHR)8 although the more modest myogenic contractions of normotensive rates were independent of ROS8. On the other hand, ROS may impair autoregulation. Thus, rat kidneys with oxidative stress caused by transforming growth factor beta (TGF-β)9 or by a high salt intake and Ang II infusion10 had impaired myogenic responses that were preserved by the redox-cycling nitroxide tempol11, while exposure of cerebral arterioles to ROS abolished autoregulation12. Therefore, it is presently unclear whether ROS contribute positively or negatively to myogenic responses13. This could indicate different effects of superoxide (O2∙ −) which was a potent stimulator of vascular reactivity4, 5 and hydrogen peroxide (H2O2) which had variable effects14, 15.
The mouse isolated perfused renal afferent arteriole displayed a linear increase in active wall tension above a perfusion pressure of approximately 40 mmHg which defined the myogenic response16. We used this preparation to test the hypothesis that stretch increased ROS and that O2∙ − and/or H2O2 were required for the myogenic contraction. We loaded vessels with dihydroethidium which is a ROS-sensitive fluorophore to determine release of ROS by increased perfusion pressure. Tempol was added to the bath to metabolize ROS. Since tempol can metabolize both O2∙ − and H2O2, the ROS responsible was assessed from the effects of bath addition of pegylated superoxide dismutase (PEG-SOD) or pegylated catalase (PEG-CAT) which are taken up into cells and metabolize O2∙ − or H2O2 respectively17. Although we found no effects of PEG-CAT on myogenic contractions in afferent arterioles from normal mice, we investigated the effects of bath addition of H2O2 on basal contractility and myogenic responses to determine its potential role in states of vascular oxidative stress. The source of ROS was assessed from the effects of bath addition of apocynin, diphenyleneidinium or L-nitroarginine methyl ester (L-NAME). Apocynin inhibited NADPH oxidase in renal afferent arterioles8 and L-NAME blocked ROS derived from uncoupled eNOS18. ROS have direct effects on vascular smooth muscle cell (VSMC) contractility11 or indirect effects via bioinactivation of nitric oxide (NO) by O2∙ −. NO blunted myogenic contractions in vivo in rat kidneys but this was ascribed to an indirect effect via tubuloglomerular feedback20 and blunted contractions in rabbit afferent arterioles but only when NO generation was stimulated by vascular flow21. Therefore, we assessed the effects of NO on myogenic responses by blockade of NOS with L-NAME and in mice with a knockout of endothelial nitric oxide synthase (eNOS −/−). Ca2+ is essential for myogenic responses, but its relationship to VSMC ROS is not established22. Therefore, we assessed myogenic responses and PP-induced ROS in Ca2+-free medium.
Methods and protocols
Male C57BL/6 mice, aged 3 to 5 months and weighing 25g to 28g (Jackson Laboratory, Bar Harbor, Maine) were fed a 0.4% NaCl (normal) control test diet (Harlan Teklad, CA) and allowed free access to tap water. Additional studies were undertaken in eNOS knockout mice from Jackson Labs, Maine. All procedures conformed to the Guide for Care and Use of Laboratory Animals prepared by The Institute for Laboratory Animal Research. Studies were approved by the Georgetown University Animal Care and Use Committee. Details of methods appear in supplement (please see http://hyper.ahajournals.org).
Animal preparation, dissection and mounting of afferent arterioles and surgery
Mice were anesthetized with 2% isoflurane and oxygen, the kidneys were removed and a single renal afferent arteriole prepared as described in detail16 and in the supplement (please see http://hyper.ahajournals.org).
Measurements of ROS and myogenic responses in afferent arterioles
These were as described previously16, 22–24 and detailed further in the supplement (please see http://hyper.ahajournals.org).
Pharmacological agents
The drugs used were: 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy (tempol), superoxide dismutase-polyethylene glycol, catalase-polyethylene glycol, apocynin, diphenyleneiodinium (DPI), L-nitroarginine methyl ester (L-NAME) and hydrogen peroxide (H2O2) from Sigma-Aldrich. Drugs were added to the bath 30 min prior to testing in the concentrations shown to be effective.
Statistics
Data were expressed as mean ± SEM. An analyses of variance (ANOVA) compared the effects of vehicle and drugs added to the bath. When appropriate, these calculations were followed by Bonferroni post hoc Student's t tests. Changes were analyzed using nonparametric statistics (GraphPad Prism, GraphPad Software). P < 0.05 was considered statistically significant.
Results
Data in arterioles from normal mice are shown in figure S1 in the supplement (please see http://hyper.ahajournals.org).
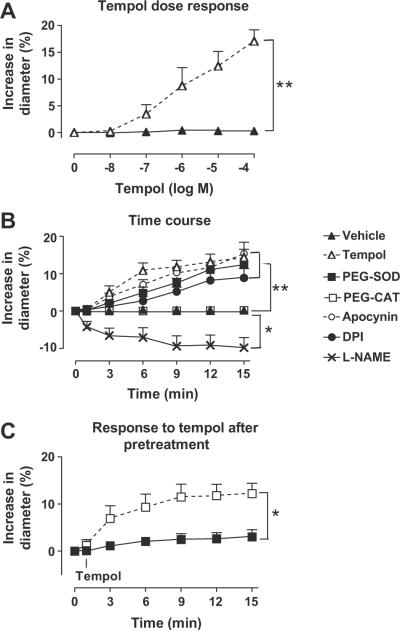
Increasing renal perfusion pressure from 40 to 80 mmHg increased the fluorescent signal for ROS, detected as the ratio of ethidium to dihydroethidium (E:DHE) by 9.8 ± 2.3% (Figure1). The ROS signal was significantly (p< 0.01) reduced by incubating with PEG-SOD (3.2 ± 1.0%), tempol (3.7 ± 0.8%) or apocynin (3.5 ± 0.9%) but it was not affected by PEG-CAT (10.2 ± 1.9%) or L-NAME (11.8 ± 2.8%) or removal of external Ca2+ with Ca-free bath and EGTA (11.7 ± 3.2%)16. The increase in vascular ROS with increased perfusion pressure detected with E:DHE was similar to that detected by tempo-9AC (n = 4) which was increased by 10.4 ± 1.6%.
Figure 1.
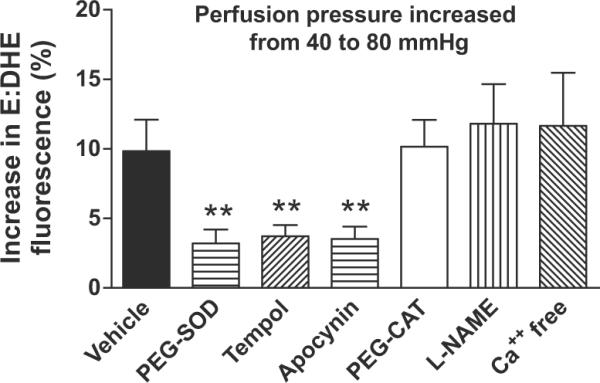
Mean ± SEM values (n=5–6) for change in ethidium to dihydroethidium fluorescence of afferent arterioles after increasing perfusion pressure from 40 to 80 mmHg with 30 min incubation in vehicle, 1000 μ·ml−1 pegylated catalase, 200 μ·ml−1 pegylated superoxide dismutase, 10−4M tempol, 10−5M apocynin, 10−4M L-NAME or in a calcium-free medium with EGTA. Compared to vehicle: **, p < 0.01
The effects of bath additional of tempol on the diameter of isolated renal afferent arterioles perfused at 60 mmHg are shown in Figure 2. Tempol caused graded increases in vascular diameter that were maximal at approximately 10−4 M (14.4 ± 2.2%; Figure 2A). The relaxation occurred over the first 6 minutes, and was stable by approximately 15 minutes (Figure 2B). Therefore, a dose of 10−4 M tempol and an incubation time of 30 minutes were selected for these studies. The basal diameter also was increased by incubation of arterioles with 200 u·ml−1 PEG-SOD (12.4 ± 0.6% p < 0.001), apocynin (15.4 ± 3.1%; p< 0.05) or DPI (8.9 ± 2.5%; p<0.05). Following incubation with 200 u·ml−1 PEG-SOD, the addition of 10−4 M tempol for 15 minutes did not further increase the basal diameter (3.0 ± 1.5%; p = ns). The basal diameter was not affected by incubation with 1000 u·ml−1 PEG-CAT (0.1 ± 0.1%; p = ns) but was reduced by L-NAME (−9.9 ± 2.7%; p<0.01). Following incubation with 1000 μ·ml−1 PEG-CAT, the addition of 10−4 M tempol for 15 minutes increased the diameter by 12.2 ± 2.2% (p < 0.05) which was similar to tempol alone.
Figure 2.
Mean ± SEM values (n=4 to 8) comparing afferent arterioles perfused at 60 mmHg and incubated with vehicle (solid triangles and continuous lines) or with graded concentrations of tempol (open triangles and interrupted lines) for change in diameter (A) or, for time after addition of vehicle, 10−4 M tempol, 200 μ·ml−1 PEG-SOD (solid squares and continuous lines), 1000 μ·ml−1 PEG-CAT (open squares and interrupted lines), 10−5M apocynin (open circles and interrupted lines), 10−5M DPI (solid circles and continuous lnes) or 10−4M L-NAME (crosses and continuous lines) (B), or for the effects of incubation with 10−4M tempol for the times shown after preincubation for 30 minutes with PEG-SOD (solid triangles and continuous lines) or PEG-CAT (open triangles and interrupted lines)(C). Comparing differences: *p<0.05; **, p < 0.01;***, p < 0.005.
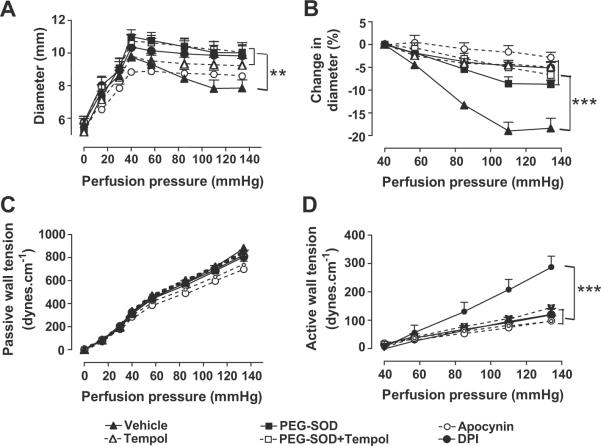
There were no significant differences in the basal luminal diameter measured at 40 mmHg between the groups (table 1). Compared to vehicle, incubation of the vessels with 10−4 M tempol, 200 u·ml−1 PEG-SOD, 10−5 M apocynin or 10−5M DPI all attenuated the pressure-induced reduction in diameter (Table 1 and Figure 3A and B) without affecting the passive wall tension (Figure 3C), resulting in significant reductions in active wall tension (Figure 3D; myogenic response, Table 1). Compared to vehicle, the myogenic response was reduced by 71% by tempol, by 58% by PEG-SOD, by 58% by apocynin and by 65% by DPI (table 1). After pre-incubation with PEG-SOD, the addition of tempol had no significant further effect.
Table 1.
Basal luminal diameter of afferent arterioles and characteristics of myogenic responses during increases in perfusion pressure from 40 to 135 mmHg after bath addition of vehicle or drugs
| Addition to bath | Strain | Number | Basal diameter (μm) | Δ diameter from 40 to 135 mmHg (%) | Myogenic response (dynes ˙ cm−1 /mmHg) |
|---|---|---|---|---|---|
| Vehicle | C57BL/6 | 8 | 10.1 ± 0.5 | −18.1 ± 2.5 | 3.1 ± 0.3 |
| Tempol | C57BL/6 | 8 | 9.8 ± 0.5 | −4.9 ± 1.2* | 0.9 ± 0.1* |
| PEG-SOD | C57BL/6 | 6 | 10.9 ± 0.5 | −8.7 ± 0.8* | 1.3 ± 0.2* |
| PEG-SOD then tempol | C57BL/6 | 5 | 10.7 ± 0.4 | −6.6 ± 0.9* | 1.4 ± 0.1* |
| Apocynin | C57BL/6 | 4 | 8.8 ± 0.2 | −2.8 ± 1.2* | 0.8 ± 0.2* |
| DPI | C57BL/6 | 5 | 10.3 ± 0.7 | −5.1 ± 1.1* | 1.1 ± 0.1* |
| PEG-CAT | C57BL/6 | 6 | 10.1 ± 0.6 | −18.2 ± 2.9 | 2.5 ± 0.2 |
| PEG-CAT then tempol | C57BL/6 | 6 | 10.2 ± 0.8 | −1.9 ± 2.6* | 1.3 ± 0.1* |
| H2O2 | C57BL/6 | 10 | 9.3 ± 0.3 | −10.4 ± 1.5* | 1.6 ± 0.1* |
| L-NAME | C57BL/6 | 6 | 8.9 ± 0.5 | −26.8 ± 3.4 | 3.1 ± 0.3 |
| Vehicle | eNOS −/− | 6 | 9.0 ± 0.8 | −16.8 ± 4.7 | 3.2 ± 0.3 |
Mean ± SEM. Basal diameter was measured at 40 mmHg perfusion pressure before drugs. Compared to vehicle;
, p<0.05.
Figure 3.
Mean ± SEM values (n = 5 to 8) for vessels incubated with a vehicle (solid triangles and continuous lines), 10−4 M tempol (open triangles and interrupted lines), 200 μ·ml−1 PEG-SOD (solid squares and continuous lines), 10−5M apocynin (open circles and interrupted lines), 10−5M DPI (solid circles and continuous lines) or PEG-SOD followed by tempol (open squares and broken lines). Data are shown for diameter (panel A), change in diameter (panel B), passive wall tension (panel C), and active wall tension (panel D). Comparing groups: **, p < 0.01;***, p < 0.005.
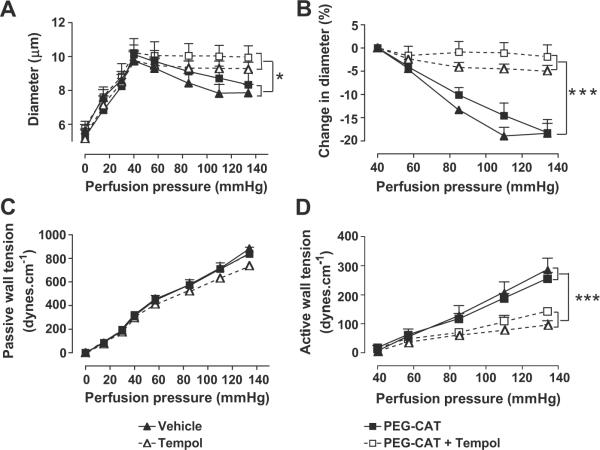
Incubation of vessels with 1000 u·ml−1 PEG-CAT had no significant effects on the reductions in vessel diameter with increasing perfusion pressure (Figure 4A and 4B) or the passive or active wall tensions (Figure 4C and 4D) and did not modify the response to 10−4 M tempol (table 1).
Figure 4.
Mean ± SEM values (n = 6 to 8) for vessels incubated with a vehicle (solid triangle and continuous lines), 10−4 M tempol, (open triangle and broken lines), 1000 μ·ml−1 PEG-catalase (solid square and continuous lines) and tempol following PEG-catalase (open square and broken lines). Comparing groups: *, p < 0.05; ***, p < 0.005.
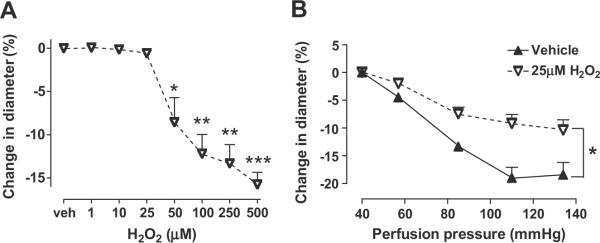
Afferent arterioles were perfused at 60 mmHg to provide some basal tone and incubated with graded concentration of H2O2 for 15 minutes. H2O2 significantly reduced the diameter at concentrations > 50μM (Fig 5A). A subthreshold concentration of H2O2 of 25μM blunted the reduction in luminal diameter with PP (Fig 5B) and the myogenic response (table 1).
Figure 5.
Mean ± SEM values (n=10) in panel A for vessels perfused at 60 mmHg and incubated by 30 mins with vehicle (solid triangles and continuous lines) or graded concentrations of hydrogen peroxide (open triangles and broken lines). In panel B, vessels were incubated for 30 mins with vehicle (solid triangles and continuous lines) or 25μmol·l−1 H2O2 (open triangles and broken line) and perfused at graded pressures. Comparing groups: *, p<0.05; **, p<0.01; ***, p <0.005.
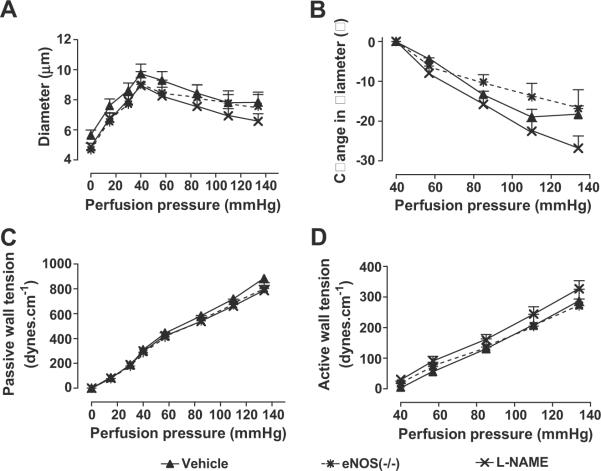
Bath addition of L-NAME or use of eNOS −/− mice had no significant effects on changes in vessel diameter with increasing perfusion pressure (Fig 6 A and B), passive or active wall tension (Fig 6 C and D) or myogenic responses (Table 1).
Figure 6.
Mean ± SEM values (n = 6) for vessels incubated with a vehicle (solid triangle and continuous lines) or 10−4 M L-NAME (crosses and continuous lines), or vessels from eNOS −/− mice (crosses and broken lines). Comparing groups: *, p < 0.05; **, p<0.01; ***, p < 0.005.
Discussion
The main new findings from this study of afferent arterioles from normal C57BL/6 mice were that increasing the perfusion pressure from 40 to 80 mmHg caused a myogenic contraction accompanied by an increase in ROS signal whether detected by dihydroethidium or tempo −9AC. The fluorescent ROS signal was predominately O2∙− since it was reduced by incubation with PEG-SOD or tempol but not with PEG-CAT and was upstream from Ca2+ since it persisted in Ca2+-free medium. Incubation of vessels with tempol, PEG-SOD, apocynin or DPI reduced basal and myogenic tone whereas PEG-CAT was not effective, indicating that the responses were enhanced by O2∙− generated from NADPH oxidase. The moderation of myogenic contractions by tempol was prevented by preincubation with PEG-SOD but was preserved by preincubation with PEG-CAT. H2O2 caused contractions at concentrations above 50μM but inhibited myogenic responses at 25μM. L-NAME increased basal tone but did not affect pressure-induced ROS generation. Neither L-NAME nor eNOS knockout affected myogenic contractions.
Tempol added to the bath prevented the enhanced Ang II contractions of perfused renal afferent arterioles of SOD-1 −/− mice6, moderated U-46,619-induced vasoconstriction25 and prevented the endothelium dependent contractions in rabbits with oxidative stress5. Tempol is an SOD mimetic and reduced tissue O2∙−11 but it might thereby increase tissue H2O211, 26–28. However, the increase in H2O2 in blood vessels was modest and lasted less than 2 minutes29. Moreover, tempol is a redox cycling nitroxide11 with catalase-like actions in tissues that prevented H2O2 accumulation30, 31. We found that the pressure-induced increase in fluorescence signal from the oxidation of DHE was reduced by tempol, similar to PEG-SOD, but was not reduced by PEG-CAT. This indicated that the ROS generated by vascular stretch was superoxide and that this was inhibited by tempol. Moreover, tempol had no further effect on the myogenic response in vessels preincubated with PEG-SOD, but retained its full efficacy after PEG-CAT. This related the inhibitory effects of tempol on the myogenic response to metabolism of O2∙− rather than to increased H2O2. Indeed, myogenic responses were blunted by PEG-SOD but not by PEG-CAT, indicating that the normal myogenic response was enhanced by O2∙− rather than by H2O2. However, although H2O2 was not implicated in normal myogenic responses, it may contribute if it accumulated sufficiently in the vessels since 25μM H2O2 inhibited myogenic contractions. H2O2 also inhibited angiotension-induced contractions and intracellular calcium in rat afferent arterioles22. Therefore, the catalase-like activity of tempol might explain its improvement in myogenic responses in models of severe or prolonged oxidative stress if H2O2 accumulated sufficiently to blunt myogenic contractions in these circumstances.
Apocynin inhibited the enhanced myogenic responses of afferent arterioles from SHR. Apocynin is not a specific inhibitor of NADPH oxidase32. However, similar results were obtained by inhibition of NADPH oxidase with gp9lds-tat8. The finding that apocynin and another NADPH oxidase inhibitor, DPI had similar effects as tempol or PEG-SOD in reducing basal and myogenic tone in mouse afferent arterioles in this study confirmed that the source of O2∙− was predominantly NADPH oxidase7. Since the perfusion-pressure-induced increase in contraction was abolished in Ca2+-free medium, yet the increase in ROS was unaffected, we concluded that the myogenic response was entirely dependent on Ca2+ and that changes in Ca2+ concentration or sensitivity were downstream from increased O2∙−.
We found comparable effects of the drugs that blocked O2∙− to reduce basal and active myogenic tone. This suggests that both depended on the generation of O2∙−, consistent with effects of NADPH oxidase to increase basal tone in SHR aortas33. Blockade of NOS by L-NAME increased the basal tone of the perfused afferent arteriole but did not change perfusion-pressure induced ROS or contractions. Thus, eNOS uncoupling did not contribute to O2∙− generation and NO did not modulate the myogenic response. Moreover, prolonged deletion of the eNOS gene also did not affect myogenic responses (fig 6 and table 1). This is consistent with the conclusions of Juncos et al21 that the stretch-induced contraction of rabbit isolated afferent arterioles was not dependent on NO although flow induced NO release affected basal tone.
The present conclusions differ from prior studies in normal rats where O2∙− contributed to the enhanced myogenic response of SHR afferent arterioles but had little influence under normal conditions8. Sharma et al9 reported that TGF-β blocked autoregulatory responses of the rat juxtamedullary nephron preparation by stimulating ROS. Saeed et al10 reported a reduced myogenic response in intact kidney of rats given a high salt intake and infused with angiotension II for 14 days that was preserved by tempol. Clearly, ROS may have opposite modulating effects on myogenic responses that may relate to experimental conditions (isolated arterioles vs intact kidneys), species (rat vs mouse) or ROS (O2∙− vs H2O2). The present study is the first to implicate O2∙− in myogenic responses of afferent arterioles from normal mice.
Perspective
Glomerular filtration requires a uniquely high capillary pressure that renders the glomerular capillaries susceptible to baratrauma if there is a breakdown of renal myogenic responses and a rise in blood pressure, as in some models of chronic kidney disease34. Thus, if afferent arteriolar ROS enhance myogenic responses, they might have a renal protective effect. However, the effects of metabolism of ROS by tempol vary widely from inhibitory effects on acute myogenic responses seen in mice arterioles in this study and in SHR8 to restorative effects in some others9, 10. Further study will be needed to determine if variable effects of tempol could underlie some discordant reports of its effects on the kidneys in models of chronic kidney disease that range from renal protection in the reduced renal mass model in rats35 or mice36 to no effect in models of diabetes mellitus28 or antiglomerular basement membrane nephritis27.
Supplementary Material
Acknowledgments
We thank Ms. Wing Kam (Emily) Chan and Glenda Baker for preparing and editing the manuscript.
Source of Funding This study was supported by grants to Christopher S. Wilcox and William J. Welch from the NIDDK (DK-036079 and DK-049870), William J. Welch (HL-089583) and from the NHLBI to Christopher S. Wilcox, William Welch and Anton Wellstein (HL-68686) and by funds from the George E. Schreiner Chair of Nephrology.
Footnotes
Conflict of Interest None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Just A. Mechanisms of renal blood flow autoregulation: dynamics and contributions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1–17. doi: 10.1152/ajpregu.00332.2006. [DOI] [PubMed] [Google Scholar]
- (2).Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertens. 2009;54:393–398. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Just A, Arendshorst WJ. A novel mechanism of renal blood flow autoregulation and the autoregulatory role of A1 adenosine receptors in mice. Am J Physiol Renal Physiol. 2007;293:F1489–F1500. doi: 10.1152/ajprenal.00256.2007. [DOI] [PubMed] [Google Scholar]
- (4).Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- (5).Wang D, Chabrashvili T, Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane-prostanoid receptors and endothelium. Circ Res. 2004;94:1436–1442. doi: 10.1161/01.RES.0000129578.76799.75. [DOI] [PubMed] [Google Scholar]
- (6).Carlstrom M, Lai EY, Ma Z, Steege A, Patzak A, Eriksson UJ, Lundberg JO, Wilcox CS, Persson AE. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin II via modulation of nitric oxide bioavailability. Hypertens. 2010;56:907–913. doi: 10.1161/HYPERTENSIONAHA.110.159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circ. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- (8).Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;298:H1769–H1775. doi: 10.1152/ajpheart.00537.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Sharma K, Cook A, Smith M, Valancius C, Inscho EW. TGF-beta impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol. 2005;288:F1069–F1077. doi: 10.1152/ajprenal.00345.2004. [DOI] [PubMed] [Google Scholar]
- (10).Saeed A, DiBona GF, Marcussen N, Guron G. High-NaCl intake impairs dynamic autoregulation of renal blood flow in ANG II-infused rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1142–R1149. doi: 10.1152/ajpregu.00326.2010. [DOI] [PubMed] [Google Scholar]
- (11).Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharm Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Zagorac D, Yamaura K, Zhang C, Roman RJ, Harder DR. The effect of superoxide anion on autoregulation of cerebral blood flow. Stroke. 2005;36:2589–2594. doi: 10.1161/01.STR.0000189997.84161.95. [DOI] [PubMed] [Google Scholar]
- (13).Just A, Arendshorst WJ. The role of reactive oxygen species in renal blood flow autoregulation. The FASEB Journal. 2008;22:761.19. Ref Type: Abstract. [Google Scholar]
- (14).Chen Y, Pearlman A, Luo Z, Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. Am J Physiol. 2007;293:H2085–H2092. doi: 10.1152/ajpheart.00968.2006. [DOI] [PubMed] [Google Scholar]
- (15).Iida Y, Katusic ZS. Mechanisms of cerebral arterial relaxations to hydrogen peroxide. Stroke. 2000;31:2224–2230. doi: 10.1161/01.str.31.9.2224. [DOI] [PubMed] [Google Scholar]
- (16).Lai E, Onozato ML, Solis G, Aslam S, Welch WJ, Wilcox CS. Myogenic responses of mouse isolated perfused renal afferent arterioles: effects of salt intake and reduced renal mass. Hypertens. 2010;55:983–989. doi: 10.1161/HYPERTENSIONAHA.109.149120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Beckman JS, Minor RL, Jr., White CW, Repine JE, Rosen GM, Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988;263:6884–6892. [PubMed] [Google Scholar]
- (18).Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wang D, Luo Z, Wang X, Jose PA, Falck JR, Welch WJ, Aslam S, Teerlink T, Wilcox CS. Impaired endothelial function and microvascular asymmetrical dimethylarginine in angiotensin II-infused rats: effects of tempol. Hypertens. 2010;56:950–955. doi: 10.1161/HYPERTENSIONAHA.110.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Just A, Arendshorst WJ. Nitric oxide blunts myogenic autoregulation in rat renal but not skeletal muscle circulation via tubuloglomerular feedback. J Physiol. 2005;569:959–974. doi: 10.1113/jphysiol.2005.094888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Juncos LA, Garvin J, Carretero OA, Ito S. Flow modulates myogenic responses in isolated microperfused rabbit afferent arterioles via endothelium-derived nitric oxide. J Clin Invest. 1995;95:2741–2748. doi: 10.1172/JCI117977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Fellner SK, Arendshorst WJ. Angiotensin II, reactive oxygen species, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol. 2005;289:F1012–F1019. doi: 10.1152/ajprenal.00144.2005. [DOI] [PubMed] [Google Scholar]
- (23).Wang D, Jose P, Wilcox CS. Beta1 receptors protect the renal afferent arteriole of angiotensin-infused rabbits from norepinephrine-induced oxidative stress. J Am Soc Nephrol. 2006;17:3347–3354. doi: 10.1681/ASN.2006030212. [DOI] [PubMed] [Google Scholar]
- (24).Harrison D, Gongora MC, Guzik TJ, Widder J. Oxidative stress and hypertension. Hypertens. 2007;1:30–44. doi: 10.1016/j.jash.2006.11.006. [DOI] [PubMed] [Google Scholar]
- (25).Schnackenberg CG, Welch WJ, Wilcox CS. TP receptor-mediated vasoconstriction in microperfused afferent arterioles: role O2− and NO. Am J Physiol. 2000;48:F302–F308. doi: 10.1152/ajprenal.2000.279.2.F302. [DOI] [PubMed] [Google Scholar]
- (26).Chen YF, Cowley AW, Jr., Zou AP. Increased H2O2 counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol. 2003;285:R827–R833. doi: 10.1152/ajpregu.00636.2002. [DOI] [PubMed] [Google Scholar]
- (27).Lu H, Zhen J, Wu T, Peng A, Ye T, Wang T, Yu X, Vaziri ND, Mohan C, Zhou XJ. Superoxide dismutase mimetic drug tempol aggravates anti-GBM antibody-induced glomerulonephritis in mice. Am J Physiol Renal Physiol. 2010;299:F445–F452. doi: 10.1152/ajprenal.00583.2009. [DOI] [PubMed] [Google Scholar]
- (28).Asaba K, Tojo A, Onozato ML, Goto A, Fujita T. Double-edged action of SOD mimetic in diabetic nephropathy. J Cardiovasc Pharmacol. 2007;49:13–19. doi: 10.1097/FJC.0b013e31802b6530. [DOI] [PubMed] [Google Scholar]
- (29).Chen X, Patel K, Connors S, Mendonca M, Welch WJ, Wilcox CS. Acute antihypertensive action of tempol in the spontaneously hypertensive rat. Am J Physiol. 2007;293:H3246–H3253. doi: 10.1152/ajpheart.00957.2007. [DOI] [PubMed] [Google Scholar]
- (30).Hahn SM, Mitchell JB, Shacter E. Tempol inhibits neutrophil and hydrogen peroxide-mediated DNA damage. Free Radic Biol Med. 1997;23:879–884. doi: 10.1016/s0891-5849(97)00079-8. [DOI] [PubMed] [Google Scholar]
- (31).Krishna MC, Samuni A, Taira J, Goldstein S, Mitchell JB, Russo A. Stimulation by nitroxides of catalase-like activity of hemeproteins. J Biol Chem. 1996;271:26018–26025. doi: 10.1074/jbc.271.42.26018. [DOI] [PubMed] [Google Scholar]
- (32).Schluter T, Steinbach AC, Steffen A, Rettig R, Grisk O. Apocynin-induced vasodilation involves Rho kinase inhibition but not NADPH oxidase inhibition. Cardiovasc Res. 2008;80:271–279. doi: 10.1093/cvr/cvn185. [DOI] [PubMed] [Google Scholar]
- (33).Lodi F, Cogolludo A, Duarte J, Moreno L, Coviello A, Peral De BM, Vera R, Galisteo M, Jimenez R, Tamargo J, Perez-Vizcaino F. Increased NADPH oxidase activity mediates spontaneous aortic tone in genetically hypertensive rats. Eur J Pharmacol. 2006;544:97–103. doi: 10.1016/j.ejphar.2006.06.028. [DOI] [PubMed] [Google Scholar]
- (34).Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- (35).Li P, Mendonca M, Welch WJ, Wilcox CS. Salt-sensitive hypertension in a model of chronic renal failure is ameliorated by tempol. J Am Soc Nephrol. 2007;18:846A. Ref Type: Abstract. [Google Scholar]
- (36).Hobo A, Yuzawa Y, Kosugi T, Kato N, Asai N, Sato W, Maruyama S, Ito Y, Kobori H, Ikematsu S, Nishiyama A, Matsuo S, Kadomatsu K. The growth factor midkine regulates the renin-angiotensin system in mice. J Clin Invest. 2009;119:1616–1625. doi: 10.1172/JCI37249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.