Abstract
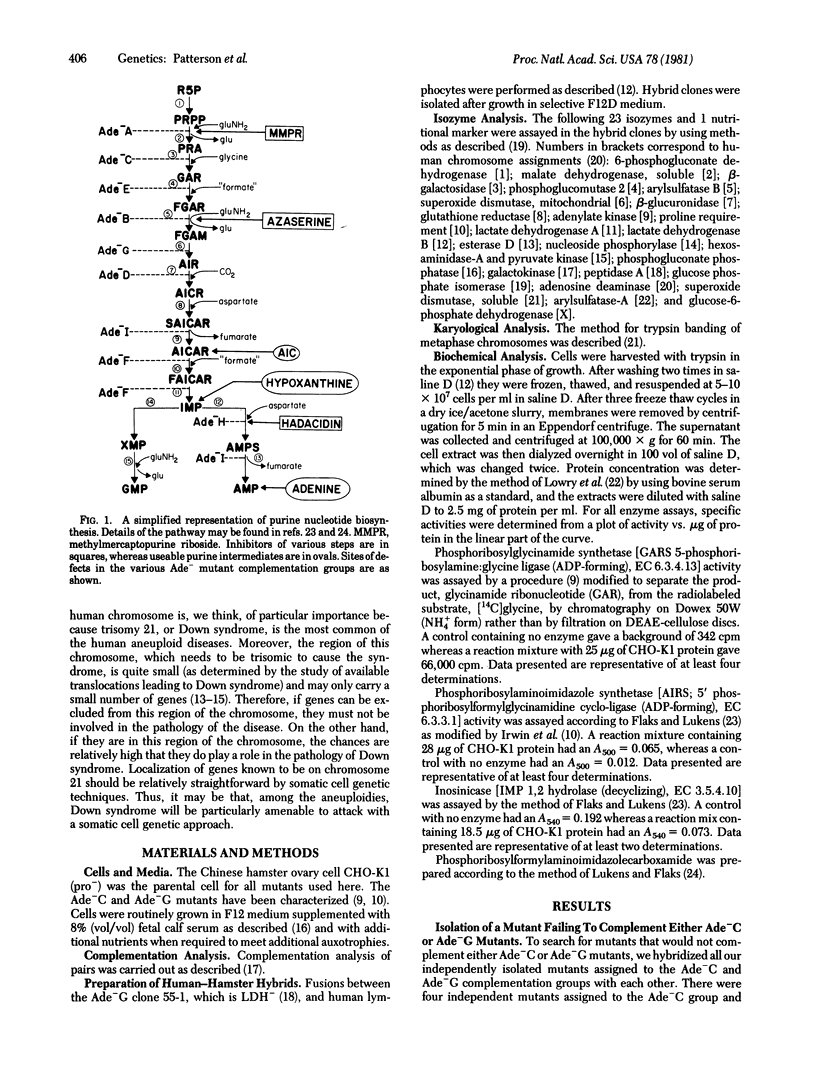
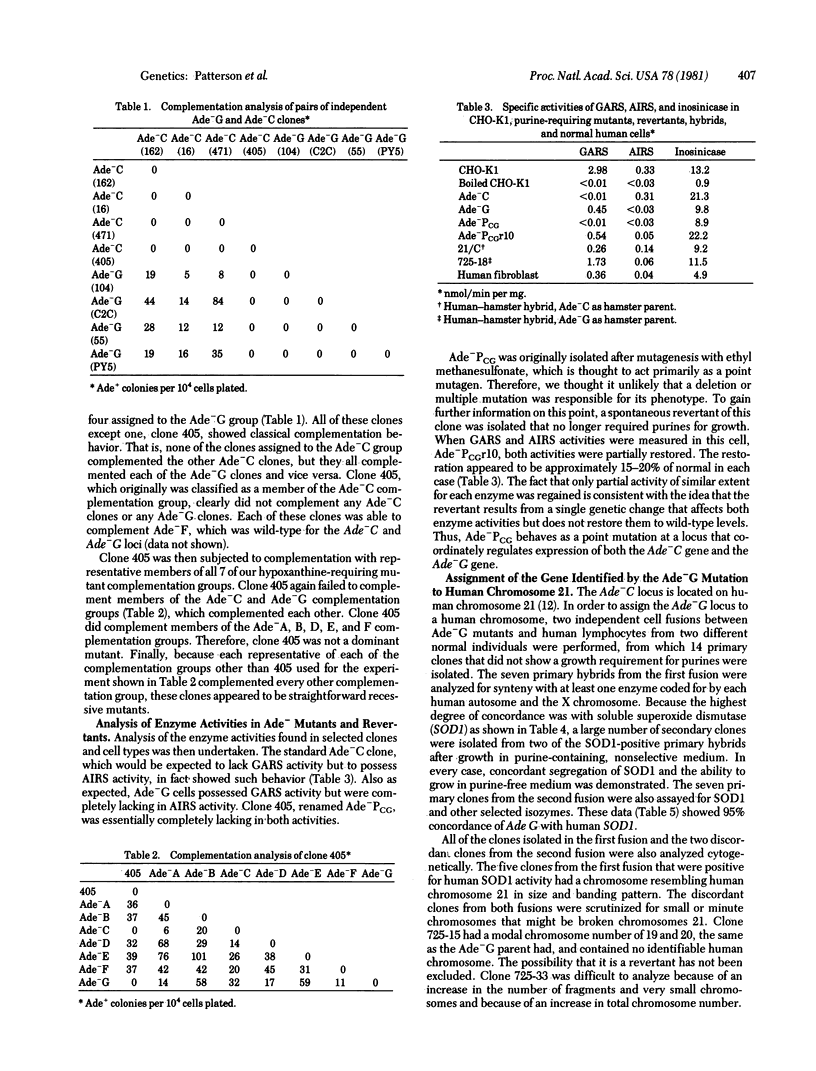
A method for determining coordinate genetic regulation is proposed for mammalian cells. The method involves (i) isolation of a set of mutants defective in the relevant pathway; (ii) complementation analysis of these mutants to determine dominance and to categorize the mutants into various different complementation groups; (iii) determination of the biochemical blocks in the mutants; (iv) identification of individual mutants that fail to complement the members of at least two distinct complementation groups that complement each other, such mutants being said to show coordinate regulation of the affected functions; (v) biochemical and reversion analysis of the relevant cell types to confirm the basis for the observed coordinate regulation; (vi) assignment of the individual genes to particular human chromosomes; (vii) mapping of the genes to determine contiguity on the genome; and (viii) examination of the structure of the relevant gene products. This method has allowed the demonstration of coordinate regulation between the gene coding for phosphoribosylglycineamide synthetase [5-phosphoribosylamine:glycine ligase (ADP-forming), EC 6.3.4.13], defective in our Ade-C mutants, and the gene coding for phoshoribosylaminoimidazole synthetase [5'-phosphoribosylformylglycinamidine cyclo-ligase (ADP-forming), EC 6.3.3.1], defective in our Ade-G mutants. Moreover, both genes can be assigned to human chromosome 21. Because at least two genes for purine biosynthesis have now been assigned to chromosome 21, and because patients with trisomy 21 (Down syndrome) show increased levels of serum purines, it may be that cells of these patients overproduce purines and that this overproduction may be relevant to the pathology of the syndrome.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartley J. A., Epstein C. J. Gene dosage effect for glycinamide ribonucleotide synthetase in human fibroblasts trisomic for chromosome 21. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1286–1289. doi: 10.1016/0006-291x(80)90629-4. [DOI] [PubMed] [Google Scholar]
- Coleman P. F., Suttle D. P., Stark G. R. Purification from hamster cells of the multifunctional protein that initiates de novo synthesis of pyrimidine nucleotides. J Biol Chem. 1977 Sep 25;252(18):6379–6385. [PubMed] [Google Scholar]
- Davidson J. N., Patterson D. Alteration in structure of multifunctional protein from Chinese hamster ovary cells defective in pyrimidine biosynthesis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1731–1735. doi: 10.1073/pnas.76.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULLER R. W., LUCE M. W., MERTZ E. T. Serum uric acid in mongolism. Science. 1962 Sep 14;137(3533):868–869. doi: 10.1126/science.137.3533.868. [DOI] [PubMed] [Google Scholar]
- Fluri R., Coddington A., Flury U. The product of the ade1: gene in Schizosaccharomyces pombe: a bifunctional enzyme catalysing two distinct steps in purine biosynthesis. Mol Gen Genet. 1976 Sep 23;147(3):271–282. doi: 10.1007/BF00582878. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Ammann A. J., Wara D. W., Sandman R., Diamond L. K. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975 May 3;1(7914):1010–1013. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Keys C., VarsanyiBreiner A., Kao F. T., Jones C., Puck T. T., Housman D. Isolation and localization of DNA segments from specific human chromosomes. Proc Natl Acad Sci U S A. 1980 May;77(5):2829–2833. doi: 10.1073/pnas.77.5.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeijer A., Smit E. M. Partial trisomy 21. Further evidence that trisomy of band 21q22 is essential for Down's phenotype. Hum Genet. 1977 Aug 31;38(1):15–23. doi: 10.1007/BF00295803. [DOI] [PubMed] [Google Scholar]
- Irwin M., Oates D. C., Patterson D. Biochemical genetics of Chinese hamster cell mutants with deviant purine metabolism: isolation and characterization of a mutant deficient in the activity of phosphoribosylaminoimidazole synthetase. Somatic Cell Genet. 1979 Mar;5(2):203–216. doi: 10.1007/BF01539161. [DOI] [PubMed] [Google Scholar]
- Jones C., Moore E. E., Lehman D. W. Genetic and biochemical analysis of the a1 cell-surface antigen associated with human chromosome 11. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6491–6495. doi: 10.1073/pnas.76.12.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROOTH R. S. PROPERTIES ODF DIPLOID CELL STRAINS DEVELOPED FROM PATIENTS WITH AN INHERITED ABNORMALITY OF URIDINE BIOSYNTHESIS. Cold Spring Harb Symp Quant Biol. 1964;29:189–212. doi: 10.1101/sqb.1964.029.01.024. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Jones C., Puck T. T. Genetics of somatic mammalian cells: genetic, immunologic, and biochemical analysis with Chinese hamster cell hybrids containing selected human chromosomes. Proc Natl Acad Sci U S A. 1976 Jan;73(1):193–197. doi: 10.1073/pnas.73.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krooth R. S., Hsiao W. L., Potvin B. W. Resistance to 5-fluoroorotic acid and pyrimidine auxotrophy: a new bidirectional selective system for mammalian cells. Somatic Cell Genet. 1979 Sep;5(5):551–569. doi: 10.1007/BF01542694. [DOI] [PubMed] [Google Scholar]
- Kurnit D. M. Down syndrome: gene dosage at the transcriptional level in skin fibroblasts. Proc Natl Acad Sci U S A. 1979 May;76(5):2372–2375. doi: 10.1073/pnas.76.5.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESCH M., NYHAN W. L. A FAMILIAL DISORDER OF URIC ACID METABOLISM AND CENTRAL NERVOUS SYSTEM FUNCTION. Am J Med. 1964 Apr;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levinson B. B., Ullman B., Martin D. W., Jr Pyrimidine pathway variants of cultured mouse lymphoma cells with altered levels of both orotate phosphoribosyltransferase and orotidylate decarboxylase. J Biol Chem. 1979 Jun 10;254(11):4396–4401. [PubMed] [Google Scholar]
- Moore E. E., Jones C., Kao F. T., Oates D. C. Synteny between glycinamide ribonucleotide synthetase and superoxide dismutase (soluble). Am J Hum Genet. 1977 Jul;29(4):389–396. [PMC free article] [PubMed] [Google Scholar]
- Oates D. C., Patterson D. Biochemical genetics of Chinese hamster cell mutants with deviant purine metabolism: characterization of Chinese hamster cell mutants defective in phosphoribosylpyrophosphate amidotransferase and phosphoribosylglycinamide synthetase and an examination of alternatives to the first step of purine biosynthesis. Somatic Cell Genet. 1977 Nov;3(6):561–577. doi: 10.1007/BF01539066. [DOI] [PubMed] [Google Scholar]
- Oates D. C., Vannais D., Patterson D. A mutant of CHO-K1 cells deficient in two nonsequential steps of de novo purine biosynthesis. Cell. 1980 Jul;20(3):797–805. doi: 10.1016/0092-8674(80)90326-8. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Coleman P. F., Stark G. R. N-(Phosphonacetyl)-L-aspartate-resistant hamster cells overaccumulate a single mRNA coding for the multifunctional protein that catalyzes the first steps of UMP synthesis. J Biol Chem. 1979 Feb 10;254(3):974–980. [PubMed] [Google Scholar]
- Patterson D. Isolation and characterization of 5-fluorouracil-resistant mutants of Chinese hamster ovary cells deficient in the activities of orotate phosphoribosyltransferase and orotidine 5'-monophosphate decarboxylase. Somatic Cell Genet. 1980 Jan;6(1):101–114. doi: 10.1007/BF01538699. [DOI] [PubMed] [Google Scholar]
- Patterson D., Kao F. T., Puck T. T. Genetics of somatic mammalian cells: biochemical genetics of Chinese hamster cell mutants with deviant purine metabolism. Proc Natl Acad Sci U S A. 1974 May;71(5):2057–2061. doi: 10.1073/pnas.71.5.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinet P. M., Couturier J., Dutrillaux B., Poissonnier M., Raoul O., Rethore M. O., Allard D., Lejeune J., Jerome H. Trisomie 21 et superoxyde dismutase-1 (IPO-A). Tentative de localisation sur la sous bande 21Q22.1. Exp Cell Res. 1976 Jan;97:47–55. doi: 10.1016/0014-4827(76)90653-4. [DOI] [PubMed] [Google Scholar]
- Stamato T. D., Jones C. Isolation of a lactic dehydrogenase-A-deficient CHO-K1 mutant by nylon cloth replica plating. Somatic Cell Genet. 1977 Nov;3(6):639–647. doi: 10.1007/BF01539071. [DOI] [PubMed] [Google Scholar]
- Suttle D. P., Stark G. R. Coordinate overproduction of orotate phosphoribosyltransferase and orotidine-5'-phosphate decarboxylase in hamster cells resistant to pyrazofurin and 6-azauridine. J Biol Chem. 1979 Jun 10;254(11):4602–4607. [PubMed] [Google Scholar]
- Watts R. W., Perera Y. S., Allsop J., Newton C., Platts-Mills T. A., Webster A. D. Immunological and purine enzyme studies on hyperuricaemic and normouricaemic patients with Down's syndrome. Clin Exp Immunol. 1979 Jun;36(3):355–363. [PMC free article] [PubMed] [Google Scholar]



