Abstract
Percutaneous osseointegrated prostheses are being investigated as an alternative strategy to attach prosthetic limbs to patients. Although the use of these implants has shown to be promising in clinical trials; the ability to maintain a skin seal around an osseointegrated implant interface is a major challenge to prevent superficial and deep periprosthetic infections.
The specific aim of this study was to establish a translational load-bearing ovine model to assess postoperative limb compensation and gait symmetry following a percutaneous integrated implant. We tested the following hypotheses: (1) the animals would return to pre-amputation limb loads within 12-months; (2) the animals would return to a symmetrical gait pattern (stride length and time in stance) within 12-months.
The results demonstrated that one month following surgery, the sheep loaded their amputated limb to a mean value of nearly 80% of their pre-amputation loading condition; by 12-months, this mean had dropped to approximately 74%. There were no statistical differences between the symmetry of the amputated forelimb and the contralateral forelimb at any time point for the animals stride length or the time spent in the stance phase of their gait cycle. Thus, the data showed that while the animals maintained symmetric gait patterns, they did not return to full weight-bearing after 12-months. The results of this study showed that a large animal load-bearing model had a symmetric gait and was weight bearing for up to 12 months. While the current investigation utilizes an ovine model, there data show that osseointegrated implant technology with postoperative follow-up can help our human patients return to symmetric gait and maintain an active lifestyle, leading to an improvement in their quality of life following amputation.
Keywords: Percutaneous, osseointegrated, gait, amputation, ovine
Introduction
Limb amputation is a devastating outcome for those who experience severe traumas, survive certain cancers, or have certain congenital abnormalities. Furthermore, the number of patients afflicted with diabetes continues to rise, leading to a concomitant increase in the number of amputations related to diabetes (1998; 2001; Cowie et al. 2009; Cowie et al. 2010; 2011). Finally, as the number and severity of political conflicts increases worldwide, so does the number of combat-related amputations (Fischer 2009). With the increasing number of amputations, it is important to continue to explore potential improvements in the advancement of patient care with advanced prosthetic technology.
Socket-type attachment of an exoprosthesis is the standard of care for these patients, but is not always suitable for all patients. The technology fails when applied to limbs of limited length, especially in patients with multiple short residual limbs. Proper socket fit can be difficult to achieve and poor socket fit directly affects the activity level and satisfaction of the patient. These on-going limitations of the residual limb require repeated socket adjustments due to scarring, neuromas and fluctuations in the volume and/or length of the residual limb.
Even patients that are fitted with socket-type attachments can be dissatisfied since they frequently experience pain from persistent skin deterioration of the soft tissues resulting from poor socket fit (DesGroseilliers et al. 1978; Ehde et al. 2000; Dillingham et al. 2001; Hagberg and Branemark 2001; Mak et al. 2001; Marshall et al. 2002).
Finally, heterotopic bone formation can be induced by surgery or by trauma and can grow exuberantly into the soft tissues of the residual limb (Henrot et al. 2000; Potter et al. 2006; Potter et al. 2007) causing significant disability and limited range of motion. When load is applied through the socket to this underlying heterotopic bony mass, pressure damage occurs from necrosis as the skin and soft tissues are trapped between these hard surfaces (Henrot et al. 2000).
Percutaneous osseointegrated prostheses (POP) used as a docking system, is an alternant technology to sockets. These POP devices, also known in the literature as osseointegrated (Albrektsson et al. 1981; Branemark 1983; Hagert et al. 1986; Branemark 2003; Brånemark et al. 2005; Webster et al. 2009; Chou et al. 2010; Isaacson et al. 2010; Jeyapalina et al. 2011; Shelton et al. 2011) and endo-exoprosthesis (Aschoff 2009; Aschoff et al. 2009), represent a relatively new technology that is presently being investigated worldwide in an attempt to obviate the multiple shortcomings of socket technology by providing direct attachment in order to secure the exoprosthetic limb. Currently, there are three primary groups working with selected human amputee volunteers with this technology – Branemark and co-workers in Sweden, Aschoff and colleagues in Germany, and Blunn and Pendergrass in England). Their patients have reported significant improvements in their functionality as evidenced by improved range of motion (Moller et al. 1999; Sullivan et al. 2003), the lack of skin or residual limb problems associated with socket prosthetic attachment (Hagberg and Branemark 2009), greater overall satisfaction with their prosthetic device function (Moller et al. 1999; Henrot et al. 2000; Sullivan et al. 2003) and the previously unrecognized benefit of “osseoperception”, the central sensory feedback from the environment through the implanted bone (Jacobs et al. 2000; Sullivan et al. 2003; Klineberg 2005; Jacobs and Van Steenberghe 2006; Hagberg and Branemark 2009). Although there are multiple significant advantages seen in these clinical populations, the patients in these trials also show problems with high infection rates at the skin-implant interface, periprosthetic osteomyelitis, implant loosening, and long rehabilitation times associated with some of the existing procedures (Sullivan et al. 2003; Hagberg and Branemark 2009). The high infection rates are challenges that could be significantly reduced or eliminated by assuring that the natural antibacterial skin barrier were maintained at the skin-implant interface. Currently, these devices are not approved for clinical use in the United States and some other parts of the world due to the reported 18-50% infection rates (Hagberg and Branemark 2001; Hagberg and Branemark 2009).
To facilitate the global approval and clinical use of these osseointegrated percutaneous implants, we believe that a weight-bearing, large animal model needs to be established to study the efficacy of decreasing the infection rates at the skin implant interface. Previously, the ovine model has shown promise for the translational testing of bone endoprosthetic implants (Willie et al. 2004; Bloebaum et al. 2007; Pearce et al. 2007). Sheep have a body weight similar to that of most humans, have a bone morphology, structure and size appropriate for the testing of human implants (Newman et al. 1995; Willie et al. 2004) and have a bone remodeling rate similar to that of humans (Willie et al. 2004).
Gait analysis on amputees has also been performed as an important tool for assessing ambulation and weight bearing to confirm rehabilitation milestones and clinical endpoints (Kulkarni et al. 2005; Gard 2006). In quadrupeds, the analyses are complex in that animals have been reported to offload their injured limb and apply more loads to their three other limbs (Duda et al. 1998; Seebeck et al. 2005; Bockstahler et al. 2009). However, most of the quadruped gait literature deals with fracture healing events. Healing around a percutaneous, osseointegrated implant for amputees would most appropriately be compared with appositional bone formation into porous coated total joint replacements that has been well documented as appositional bone attachment (Hofmann et al. 1991; Bloebaum et al. 1992; Bloebaum et al. 1992; Hofmann et al. 1993; Bloebaum et al. 1994; Bloebaum et al. 1997; Bloebaum et al. 1998; Bloebaum et al. 2007) taking up to one year to achieve.
The specific aim of this study was to establish a load-bearing amputation animal model that could be used to evaluate percutaneous osseointegrated implant technology and to monitor the animal’s changes in gait patterns over time. The continued ambulation of the animal postoperatively would allow us to assess the effectiveness of the device, as reflected by weight bearing and gait analyses. Thus, we tested two hypotheses: (1) The animals would return to pre-amputation limb loads within 12-months; (2) The animals would return to a symmetrical gait pattern within 12-months compared to this contralateral limb.
Methods
Model Selection
Various animal models were investigated prior to this study, but sheep were chosen for this investigation because it was a large animal model with both body weights and bone remodeling rates comparable to an adult human (Willie et al. 2004; Peters et al. 2006; Bloebaum et al. 2007). Previous studies such as (Duda et al. 1998) show the efficacy of the forelimb application because the anatomical orientation of the forelimb was more vertical than the hindlimb, making it a better model for the implant design and postoperative observations and care.
Implant Design and Fabrication
The metacarpal III bones from an initial 20 mature crossbred sheep carcasses were imaged by using a clinically based CT scanner (LightSpeed VCT XT™, GE Healthcare Worldwide, United Kingdom) at a tube voltage of 100 kVp with an automatically calibrated variable current and stored digitally as DICOM files. These digital CT images were reconstructed using a commercially available software package (MIMICS, Materialise Corp., Plymouth, MI, USA), then ported to a custom analysis program for analyses (MatLab, MathWorks Corp., Natick, MA, USA) to provide not only the anteroposterior and mediolateral dimensions, but also the three-dimensional morphology of the medullary canal at 1 mm increments throughout the length of each bone. From these data, 3 implant sizes and surgical broaches, corresponding to the 25th, 50th, and 75th percentiles, were designed (Intelligent Implant Systems, LLC, Charlotte, NC, USA) and fabricated (IMDS Co-Innovative, Logan, UT, USA) from the medical grade Ti6Al4V titanium alloy.
The intramedullary portion of each implant was textured by grit blasting to facilitate immediate bone attachment and to achieve bone/implant integration. The subdermal barrier and the most distal portion of the stem were coated with a 500-750 micron thick commercially pure titanium porous coating (P2 type, Thortex Corp., Portland, OR, USA) with a porosity of 52 ± 12%. All of the implants were passivated following ASTM standard B 600 - 09, then sterilized using high pressure saturated steam at 121 C for around 20 minutes using an autoclave.
Surgery
Following an institutionally approved protocol, the right Metacarpal III bone of nine skeletally mature female sheep of mixed breeds (Rambouillet, Targhee, Suffolk, Crossbred, Polypay), aged 2-4 years, and weighing 80-95 kg were fitted with percutaneous osseointegrated implants. Each animal underwent a screening process prior to surgery to ensure they were healthy and the metacarpal was sized for implant selection radiographically. Next, the animals were anesthetized and prepared for surgery. An anteriorly based skin flap was made on the distal end of the right forelimb and the dewclaws were excised posteriorly. Care was taken to preserve the blood supply to the skin. The distal portion of the third metacarpal was transected at the metaphyseal flare and the medullary canal was reamed and a porous-coated (on the distal end), titanium implant was pressed and malleted into place. The fit and fill of the implant within the medullary canal was verified using intraoperative radiography. A central longitudinal “stab” wound was made in the skin flap and the flap was pulled over the Morse taper extending from the end of the implant. A Delrin® /polyurethane exoprosthetic limb, designed to weigh approximately the same as the amputated limb, was attached using a titanium adapter attached to the implant via the Morse taper protruding through the skin (Figure 1). Subjects were given 100-mcg fentanyl patch 12 hours prior to surgery and ketoprofen and buprenorphine immediately before surgery. Ketoprofen was administered for 7 days post-op and fentanyl patches were replaced every three days for a total of nine days (including the day prior to surgery).
Figure 1.
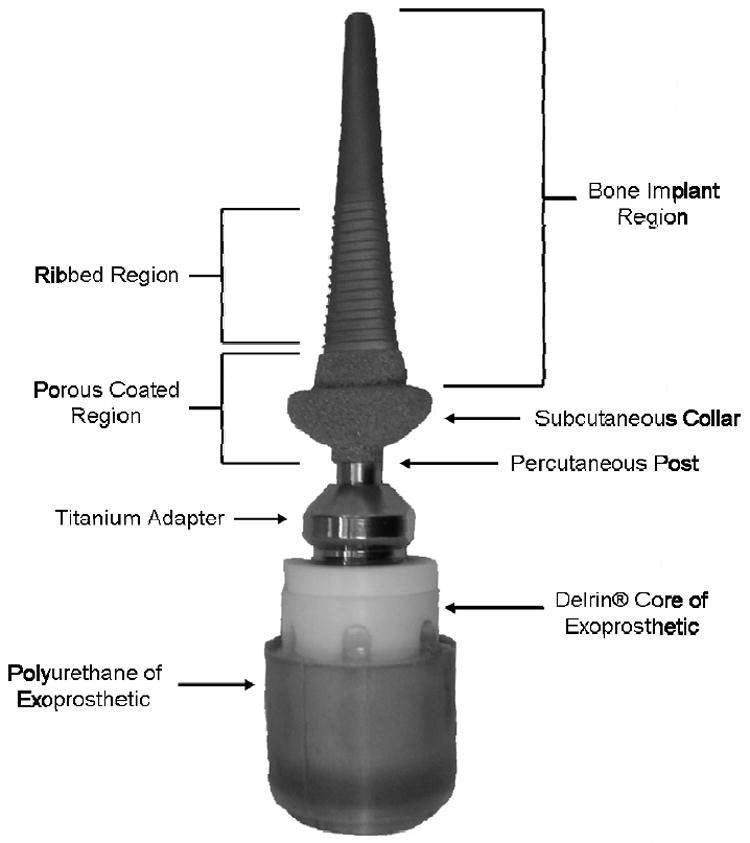
Photograph of the percutaneous, osseointegrated implant used in the study showing the bone implant region, ribbed region, porous coated region, titanium adapter, and exoprosthesis.
Gait Analysis
A commercially available pressure mat (Tekscan HR Mat, Tekscan, Inc., South Boston, MA, USA) was used as it allowed the sheep to ambulate freely across the transducer while peak vertical forces (PVFs) were recorded from each limb as the animal moved through the gait cycle (Figure 2 right). The mat (width=0.45m, length=1.5m) was placed in the straight edge of an enclosed metal elliptical walkway (Figure 2 left). The sheep maintained a walking speed throughout the testing by the presence of a caretaker following them around the walkway. The walkway was approximately 0.6 m in width while the enclosure was 10 m in length, 4 m in total width, and 1 m high. A 3 mm thick outdoor carpet was placed over the sensor as a shim to prevent the animals from slipping and to protect the sensors and to avoid shying of the sheep as has been done in previous sheep studies (Seebeck et al. 2005). Force calibration was performed with the shim in place before each testing session.
Figure 2.

(Left) Photograph of a sheep standing on the pressure mat within the enclosure. (Right) Output of the pressure sensing mat showing the Right Hindlimb (RH), Left Hindlimb (LH), Right Forelimb (RF), and Left Forelimb (LF).
The PVFs, the stride lengths, and the stance phases as a percent of the gait cycle were recorded for each animal before amputation (Time 0) and at 1-, 2-, 3-, 6-, 9-, and 12-months following amputation. During each recording session the animals walked across the sensor at least five times while data were recorded from each of the four limbs and synched with digital video images at a sampling rate of 30 Hz. This frequency was selected as it was the sampling rate of the digital images, ensuring that one frame from the pressure mat corresponded to one frame from the video recordings, allowing each limb strike to be analyzed correctly. The rationale for the frequent time periods chosen in this investigation was based on the endosteal bone response to endoprosthesis (Bloebaum et al. 1994; Hofmann et al. 1997; Willie et al. 2004). The fracture healing model time periods previously used (Duda et al. 1998) did not allow for the longer term 12-month follow up used in this study.
Once PVFs were obtained from each limb, the PVF weight distribution for each limb was obtained, as has been done with previous ovine studies (Kim and Breur 2008) using the following equation:
where the superscript PVFRF, PVFLF, PVFRH, and PVFLH indicate the peak vertical forces for the Right Forelimb, Left Forelimb, Right Hindlimb, and Left Hindlimb respectively. The advantage of using the PVF Distribution over other measures is that it minimizes the influence of both the weight and the velocity of the animal. The postoperative PVF distribution for each limb was compared to the preoperative PVF distribution for each limb using a Two-Tailed Paired t-Test using commercially available software (Microsoft Excel, Microsoft Corporation, Redmond, WA, USA), and then adjusted for multiple comparisons using the Hochberg multiple comparison procedure.
Stride length was calculated as the distance from hoof strike to hoof strike, from the same limb, while stance phase as a percentage of the gait cycle, was taken as the time the animal was in stance phase divided by the time it took to complete one gait cycle. Temporospatial parameters, such as these, can be used to measure lameness in quadrupeds (Breur and Kim 2008; Sanchez-Bustinduy et al. 2010). The stride length and stance phase as percentages of the gait cycle from the amputated right forelimb were compared to the contralateral left forelimb at each time point to determine if the animals had a symmetrical gait. Statistical significance was determined using a Two-Tailed Paired t-Test (Microsoft Excel, Microsoft Corporation, Redmond, WA, USA) with the p-values adjusted for multiple comparisons using the Hochberg multiple comparison procedure. Data are displayed as means and 95% confidence intervals.
Results
Peak Vertical Forces (PVFs)
Preoperatively, the forelimbs of the sheep were loaded with approximately 33% more weight than the hindlimbs. This back-to-front load distribution was similar in trend with work previously reported in other ovine studies (Kim and Breur 2008). One month following surgery, animals loaded their amputated right forelimb with 80.4 ± 2.5% of the preoperative load (p<0.05) (Figure 3). The PVF distribution remained relatively constant for the next two months. However, between months 3 and 6, the value dropped significantly (p=0.011) to 78.6 ± 4.8% of the preoperative PVF distribution. By the end of the 12-month study, the PVF distribution had continued to decrease to 74.4 ± 4.0% of the preoperative value (p<0.05).
Figure 3.
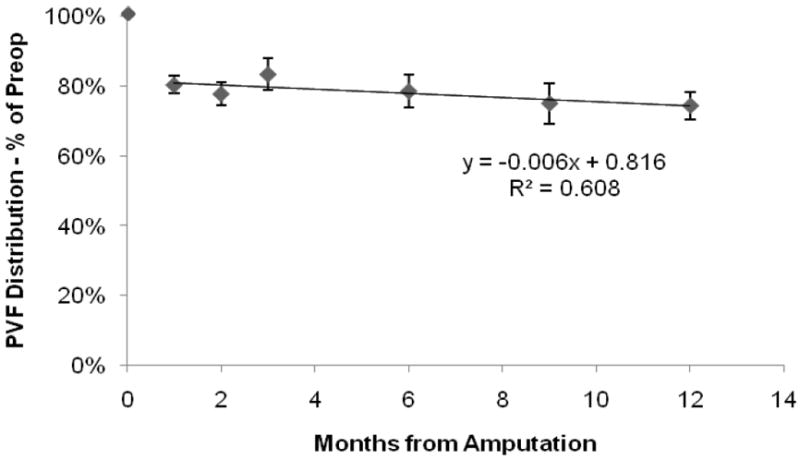
Percent of preoperative PVF Distribution for the right (amputated) forelimb showing the amputated limb was loaded less after surgery for all time points, obtaining a value nearly 74% of the preamputation value.
At 12-months, the animals were applying greater loads to the non-amputated left forelimb yielding a PVF distribution 15.3 ± 5.7% greater than the pre-amputation condition (p<0.05) while the left and right hindlimbs respectively had a PVF distribution of 8.2 ± 7.4% (p<0.05) and 10.1 ± 7.1% (p<0.05) greater than their pre-amputation condition (Figure 4). This shows that the sheep were applying more loads to the other three limbs and that less load was applied to the amputated right forelimb.
Figure 4.
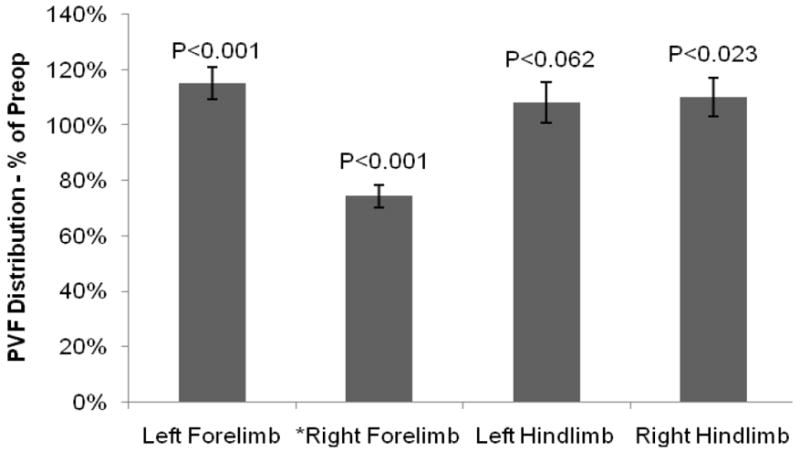
Percent of preoperative PVF Distribution for the left forelimb, left hindlimb, and right hindlimb at 12-months postop, demonstrating that the three untreated limbs had a higher PVF Distribution following surgery to compensate for the decreased load on the right forelimb.
Stride Length
The stride length was approximately 1 m for all four limbs preoperatively and there was no statistical difference measurable in stride length between the forelimbs or the hindlimbs (p>0.05). One month following amputation, and throughout the remainder of this 12-month study, the amputated right limb and non-amputated left limbs were consistent with a stride length around 1 m. There was no statistical difference (p>0.05) measurable between the right and left forelimbs at any postoperative time point, indicating a symmetrical stride length between the amputated right forelimb and contralateral left forelimb.
Stance Phase as a Percent of Gait Cycle
Before surgery, the stance phase for each limb was between 54-60% of the total gait cycle. Preoperatively, there was no statistical difference measurable between the forelimbs or the hindlimbs (p>0.05). Over the course of the 12-month study, the animals spent approximately 60% of their gait cycle in stance phase. There was no statistical difference measurable (p>0.05) between the right forelimb and left forelimb at any postoperative time point. This shows that the sheep spent an equal amount of time, on each forelimb, at each time point throughout the study and supports the observation that there was no measureable gait asymmetry.
Discussion
This investigation confirmed that an amputation model was load bearing and would allow the assessment of percutaneous osseointegrated implant function and the ability to test the efficacy of the device. One month postoperative, the animals loaded their prosthetic limb less than they had preoperatively and their PVF distribution was never restored to pre-amputation loads disproving Hypothesis 1. The sheep had a physiologically symmetrical stride length and uniform time in stance phase, over the 12-month evaluation period, supporting Hypothesis 2.
Although the sheep never returned to their preoperative load status, the results of this study are promising as they conclude that an adequate skin seal was maintained preventing infection and allowing them to resume symmetrical gait patterns. For the first three months following surgery, the PVF distribution was approximately 80% of the preoperative value. Despite being load with less weight, these load amounts indicate a good initial fit showing implant stability and maintained skin seal. One very possible explanation for altered loading of the implant one month postoperatively could be the changed proprioception with the artificial implant. The Delrin/polyurethane exoprosthetic limb could have altered the feeling of balance and grip with the surface. However, one would expect that over time, the animals would have acclimated to the shortcomings of the exoprosthetic limb. Instead, over the 12-month period, the applied loads decreased to nearly 74% of the preoperative value. We believe that the decrease in loading over time could be due to stress shielding of the bone by the implants with resorption of the stress shielded bone and a subsequent decrease in cortical bone strength (Chou and Bloebaum 2008). Finite element models have shown that percutaneous, osseointegrated endoprosthetics can significantly change the stress and strain energy density levels in bones due to both the implant and altered loading conditions, providing another explanation for the decrease over time (Tomaszewski et al. 2010). Based upon the radiographic findings, all of the animals exhibited at least some distal resorption of the implanted third metacarpal, and this loss of bone may explain the change that occurred in the forelimb loading conditions over the 12-month study. This bone loss could be diminished by an implant design that would provide more uniform distal stress distribution along the bone-implant interface (Van Rietbergen et al. 1993; Aamodt et al. 2001; Swider et al. 2006; Xu and Robinson 2008). Given our present system design, it is unknown what would occur to the prosthetic limb loads after 12-months. A longer term study would be needed to determine if loads would continue to decrease, remain the same, or improve over time, as the bone continues to remodel around the implant. To compensate for the decreased load on the amputated limb, the animals applied a greater load to the other three limbs, with the greatest shift to the contralateral left forelimb.
Although animals loaded their prosthetic limb less throughout the 12-months study, they exhibited a symmetrical gait with regards to both stride length and time in stance phase throughout the study. These findings are supportive of the observation that lameness, caused by pain or discomfort (Breur and Kim 2008; Sanchez-Bustinduy et al. 2010) was absent, also reflecting the maintenance of skin seal in this investigation. Studies on unilateral above-knee amputees’ show a 20% offload between the amputated and non-amputated limbs (Summers et al. 1987; Gauthier-Gagnon et al. 2000). However, these patients display an asymmetrical gait, especially with regards to time spent in stance phase (Jaegers et al. 1995; Nolan et al. 2003).
One possible limitation of this study was the potential saturation of individual elements on the pressure mat (sensels) with the exoprosthesis. The exoprosthesis had a smaller surface area than the hoof so there was the risk of some sensels becoming saturated during a limb strike; however, the number of saturated sensels was small and would not account for the 20-26% difference in postoperative PVF distribution from preoperative conditions. The accuracy might be improved by designing an exoprosthetic foot with a surface area similar to the hoof size of a sheep and by using a different shim material; although, only 2-3% error was noted suggesting good precision in the measurement system.
In conclusion, the ovine amputation model seems to be an effective load-bearing model at the 12 month time period which would allow continued investigations of developing a permanent skin seal for the prevention of high infection rates currently being promoted in clinical studies (Aschoff et al. 2009; Hagberg and Branemark 2009). Although our sheep did not return to preamputation loading conditions within 12-months, they were viable and active within the first month following amputation surgery and appeared to reach load bearing levels of 80% similar to patients with above human knee amputations. This model may be used with future amputation studies as a means to assess various implant designs that could lead to a better understanding of the stress needed to encourage bone remodeling and maintenance. A valuable application of this knowledge could be in the prevention and/or reversal of osteoporosis and osteopenia seen in the residual limbs of patients with amputations; conditions that persist with conventional socket technology (Rush et al. 1994; Kulkarni et al. 1998).
Acknowledgments
This research project is sponsored by the U.S. Department of the Army Award #W81XWH-06-1-0574. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. The content of this research does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred. Research was conducted in compliance with the Animal Welfare Act Regulations and other Federal statutes relating to animals and experiments involving animals and adheres to the principles set forth in the Guide for Care and Use of Laboratory Animals, National Research Council, 1996. This work is also supported in part by the Office of Rehabilitation R&D Service, DVA SLC HCS, Salt Lake City, Utah, by the NIH/NICHD Grant R01HD061014 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, and by the Department of Orthopaedics, University of Utah School of Medicine, Salt Lake City, Utah. We would also like to acknowledge George and Lisa Ethridge and James and Maria Hess, the Albert and Margaret Hofmann Chair, Bruce MacWilliams, Ph.D. for his input on gait analysis, and Greg Stoddard for his contributions with the statistical analyses.
Footnotes
Conflict of Interest Statement The authors have no conflict of interest associated with this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- M M a M W R. Vol. 47. CDC US Department of Health and Human Services, MMWR; Atlanta, GA: 1998. Diabetes-related amputations of lower extremities in the Medicare population - Minnesota 1993-1995; pp. 649–664. [PubMed] [Google Scholar]
- M M a M W R. Vol. 50. CDC US Department of Health and Human Services, MMWR; Atlanta, GA: 2001. Hospital discharge rates for nontraumatic lower extremity amputation by Diabetes status - United States, 1997; pp. 954–958. [PubMed] [Google Scholar]
- D o D T. CDC National Center for Chronic Disease Prevention and Health Promotion, CDC; Atlanta, GA: 2011. National Diabetes fact sheet, 2011; pp. 1–12. [Google Scholar]
- Aamodt A, Lund-Larsen J, Eine J, Andersen E, Benum P, Husby OS. Changes in proximal femoral strain after insertion of uncemented standard and customised femoral stems. An experimental study in human femora. J Bone Joint Surg [Br] 2001;83-B(6):921–929. doi: 10.1302/0301-620x.83b6.9726. [DOI] [PubMed] [Google Scholar]
- Albrektsson T, Branemark I-I, Hansson H-A, Lindstrom J. Osseointegrated titanium implants. Acta Orthop Scand. 1981;52(2):155–170. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- Aschoff HH. The endo - exo - femoral prosthesis 2009 [Google Scholar]
- Aschoff HH, Clausen A, Hoffmeister T. The endo-exo femur prosthesis--a new concept of bone-guided, prosthetic rehabilitation following above-knee amputation. Z Orthop Unfall. 2009;147(5):610–615. doi: 10.1055/s-0029-1185893. [DOI] [PubMed] [Google Scholar]
- Bloebaum RD, Bachus KN, Hofmann AA. Progression of human bone ingrowth into porous coated devices. Am Acad Orthop Surgeons. 1992;59(7):45. doi: 10.3109/17453679709004000. [DOI] [PubMed] [Google Scholar]
- Bloebaum RD, Bachus KN, Jensen JW, Hofmann AA. Postmortem analysis of consecutively retrieved asymetric porous-coated tibial components. J Arthroplasty. 1997;12(8):920–929. doi: 10.1016/s0883-5403(97)90162-5. [DOI] [PubMed] [Google Scholar]
- Bloebaum RD, Bachus KN, Jensen JW, Scott DF, Hofmann AA. Porous-coated metal-backed patellar components in total knee replacement. J Bone Joint Surg [Am] 1998;80-A(4):518–528. doi: 10.2106/00004623-199804000-00008. [DOI] [PubMed] [Google Scholar]
- Bloebaum RD, Bachus KN, Momberger NG, Hofmann AA. Mineral apposition rates of human cancellous bone at the interface of porous coated implants. J Biomed Mater Res. 1994;28(5):537–544. doi: 10.1002/jbm.820280503. [DOI] [PubMed] [Google Scholar]
- Bloebaum RD, Rubman MH, Hofmann AA. Bone ingrowth into porous-coated tibial components implanted with autograft bone chips: Analysis of ten consecutively retrieved implants. J Arthroplasty. 1992;7(4):483–493. doi: 10.1016/s0883-5403(06)80069-0. [DOI] [PubMed] [Google Scholar]
- Bloebaum RD, Willie BM, Mitchell BS, Hofmann AA. Relationship between bone ingrowth, mineral apposition rate, and osteoblast activity. J Biomed Mater Res A. 2007;81A(2):505–514. doi: 10.1002/jbm.a.31087. [DOI] [PubMed] [Google Scholar]
- Bockstahler BA, Vobornik A, Muller M, Peham C. Compensatory load redistribution in naturally occurring osteoarthritis of the elbow joint and induced weight-bearing lameness of the forelimbs compared with clinically sound dogs. Vet J. 2009;180(2):202–212. doi: 10.1016/j.tvjl.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Brånemark P-I, Chien S, Gröndahl H-G, Robinson K, editors. The Osseointegration Book. From Calvarium to Calcaneus. Berlin, Germany: Quintessenz Verlags-GmbH; 2005. [Google Scholar]
- Branemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50(3):399–410. doi: 10.1016/s0022-3913(83)80101-2. [DOI] [PubMed] [Google Scholar]
- Branemark R. W R A M C a D o V A A H P Workshop. Sahlgren University Hospital; Goteborg SWEDEN: 2003. Osseointegration. [Google Scholar]
- Breur GJ, Kim J. Should gait analysis be a part of clinical orthopaedic reports? J Small Anim Pract. 2008;49(3):113–114. doi: 10.1111/j.1748-5827.2008.00560.x. [DOI] [PubMed] [Google Scholar]
- Chou TG, Bloebaum RD. Examining Pexiganan effects on pin track infection in a transcutaneous implant model. 54th Annual Meeting of the Orthopaedic Research Society; San Francisco, CA. Trans Orthopaed Res Soc; 2008. [Google Scholar]
- Chou TG, Petti CA, Szakacs J, Bloebaum RD. Evaluating antimicrobials and implant materials for infection prevention around transcutaneous osseointegrated implants in a rabbit model. J Biomed Mater Res A. 2010;92(3):942–952. doi: 10.1002/jbm.a.32413. [DOI] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, Bainbridge KE, Fradkin JE. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010;33(3):562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseilliers JP, DesJardins JP, Germain JP, Krol AL. Dermatologic problems in amputees. Can Med Assoc J. 1978;118(5):535–537. [PMC free article] [PubMed] [Google Scholar]
- Dillingham TR, Pezzin LE, MacKenzie EJ, Burgess AR. Use and satisfaction with prosthetic devices among persons with trauma-related amputations: A long-term outcome study. Am J Phys Med Rehabil. 2001;80(8):563–571. doi: 10.1097/00002060-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Duda GN, Eckert-Hubner K, Sokiranski R, Kreutner A, Miller R, Claes L. Analysis of inter-fragmentary movement as a function of musculoskeletal loading conditions in sheep. J Biomech. 1998;31(3):201–210. doi: 10.1016/s0021-9290(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Ehde DM, Czerniecki JM, Smith DG, Campbell KM, Edwards WT, Jensen MP, Robinson LR. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000;81(8):1039–1044. doi: 10.1053/apmr.2000.7583. [DOI] [PubMed] [Google Scholar]
- Fischer H. United States military casualty statistics: Operation Iraqi freedom and operation enduring freedom. CRS Report for Congress, Congressional Research Service: CRS1-5 2009 [Google Scholar]
- Gard SA. Use of Quantitative Gait Analysis for the Evaluation of Prosthetic Walking Performance. Journal of Prosthetics & Orthotics. 2006;18(6):93–104. [Google Scholar]
- Gauthier-Gagnon C, Gravel D, St-Amand H, Murie C, Goyette M. Changes in ground reaction forces during prosthetic training of people with transfemoral amputations: A pilot study. Journal of Prosthetics & Orthotics. 2000;12(3):72–76. [Google Scholar]
- Hagberg K, Branemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25(3):186–194. doi: 10.1080/03093640108726601. [DOI] [PubMed] [Google Scholar]
- Hagberg K, Branemark R. One hundred patients treated with osseointegrated transfemoral amputation prostheses--rehabilitation perspective. J Rehabil Res Dev. 2009;46(3):331–344. [PubMed] [Google Scholar]
- Hagert C-G, Branemark P-I, Albrektsson T, Strid K-G, Irstam L. Metacarpophalangeal joint replacement with osseointegrated endoprostheses. Scand J Plast Reconstr Surg. 1986;20:207–218. doi: 10.3109/02844318609006321. [DOI] [PubMed] [Google Scholar]
- Henrot P, Stines J, Walter F, Martinet N, Paysant J, Blum A. Imaging of the painful lower limb stump. Radiographics. 2000;20 Spec No:S219–235. doi: 10.1148/radiographics.20.suppl_1.g00oc14s219. [DOI] [PubMed] [Google Scholar]
- Hofmann AA, Bachus KN, Bloebaum RD. Comparative study of human cancellous bone remodeling to titanium and hydroxyapatite coated implants. J Arthroplasty. 1993;8(2):157–166. doi: 10.1016/s0883-5403(06)80056-2. [DOI] [PubMed] [Google Scholar]
- Hofmann AA, Bloebaum RD, Bachus KN. Progression of human bone ingrowth into porous-coated implants. Acta Orthop Scand. 1997;68(2):161–166. doi: 10.3109/17453679709004000. [DOI] [PubMed] [Google Scholar]
- Hofmann AA, Rubman MH, Bloebaum RD, Bachus KN. Effect of autograft bone chips applied at the bone/implant interface of porous coated devices in human cancellous bone. Am Acad Orthop Surgeons. 1991;58(61):82. [Google Scholar]
- Isaacson BM, Stinstra JG, MacLeod RS, Pasquina PF, Bloebaum RD. Developing a quantitative measurement system for assessing heterotopic ossification and monitoring the bioelectric metrics from electrically induced osseointegration in the residual limb of service members. Ann Biomed Eng. 2010;38(9):2968–2978. doi: 10.1007/s10439-010-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R, Branemark R, Olmarker K, Rydevik B, Van Steenberghe D, Branemark PI. Evaluation of the psychophysical detection threshold level for vibrotactile and pressure stimulation of prosthetic limbs using bone anchorage or soft tissue support. Prosthet Orthot Int. 2000;24(2):133–142. doi: 10.1080/03093640008726536. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Van Steenberghe D. From osseoperception to implant-mediated sensory-motor interactions and related clinical implications. J Oral Rehabil. 2006;33(4):282–292. doi: 10.1111/j.1365-2842.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- Jaegers SM, Arendzen JH, de Jongh HJ. Prosthetic gait of unilateral transfemoral amputees: a kinematic study. Arch Phys Med Rehabil. 1995;76(8):736–743. doi: 10.1016/s0003-9993(95)80528-1. [DOI] [PubMed] [Google Scholar]
- Jeyapalina S, Beck JP, Bachus KN, Bloebaum RD. Periprosthetic bone remodeling and adaptation around load-bearing percutaneous osseointegrated implants. Orthopaedic Research Society 57th Annual Meeting; Long Beach, CA. Trans Orthopaed Res Soc; 2011. [Google Scholar]
- Kim J, Breur GJ. Temporospatial and kinetic characteristics of sheep walking on a pressure sensing walkway. Can J Vet Res. 2008;72(1):50–55. [PMC free article] [PubMed] [Google Scholar]
- Klineberg I. Introduction: from osseointegration to osseoperception. The functional translation. Clin Exp Pharmacol Physiol. 2005;32(1-2):97–99. doi: 10.1111/j.1440-1681.2005.04135.x. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348–353. doi: 10.1191/026921598672393611. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Gaine WJ, Buckley JG, Rankine JJ, Adams J. Chronic low back pain in traumatic lower limb amputees. Clin Rehabil. 2005;19(1):81–86. doi: 10.1191/0269215505cr819oa. [DOI] [PubMed] [Google Scholar]
- Mak AF, Zhang M, Boone DA. State-of-the-art research in lower-limb prosthetic biomechanics-socket interface: a review. J Rehabil Res Dev. 2001;38(2):161–174. [PubMed] [Google Scholar]
- Marshall HM, Jensen MP, Ehde DM, Campbell KM. Pain site and impairment in individuals with amputation pain. Arch Phys Med Rehabil. 2002;83(8):1116–1119. doi: 10.1053/apmr.2002.33121. [DOI] [PubMed] [Google Scholar]
- Moller K, Sollerman C, Geijer M, Branemark PI. Early results with osseointegrated proximal interphalangeal joint prostheses. J Hand Surg Am. 1999;24(2):267–274. doi: 10.1053/jhsu.1999.0267. [DOI] [PubMed] [Google Scholar]
- Newman E, Turner AS, Wark JD. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone. 1995;16(4 Suppl):277S–284S. doi: 10.1016/8756-3282(95)00026-a. [DOI] [PubMed] [Google Scholar]
- Nolan L, Wit A, Dudzinski K, Lees A, Lake M, Wychowanski M. Adjustments in gait symmetry with walking speed in trans-femoral and trans-tibial amputees. Gait Posture. 2003;17(2):142–151. doi: 10.1016/s0966-6362(02)00066-8. [DOI] [PubMed] [Google Scholar]
- Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 2007;13:1–10. doi: 10.22203/ecm.v013a01. [DOI] [PubMed] [Google Scholar]
- Peters CL, Hines JL, Bachus KN, Craig MA, Bloebaum RD. Biological effects of calcium sulfate as a bone graft substitute in ovine metaphyseal defects. J Biomed Mater Res (Part A) 2006;76A(3):456–462. doi: 10.1002/jbm.a.30569. [DOI] [PubMed] [Google Scholar]
- Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski D. Heterotopic ossification in the residual limbs of traumatic and combat-related amputees. J Am Acad Orthop Surg. 2006;14(10 Suppl):S191–197. doi: 10.5435/00124635-200600001-00042. [DOI] [PubMed] [Google Scholar]
- Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89(3):476–486. doi: 10.2106/JBJS.F.00412. [DOI] [PubMed] [Google Scholar]
- Rush PJ, Wong JS, Kirsh J, Devlin M. Osteopenia in patients with above knee amputation. Arch Phys Med Rehabil. 1994;75:112–115. [PubMed] [Google Scholar]
- Sanchez-Bustinduy M, de Medeiros MA, Radke H, Langley-Hobbs S, McKinley T, Jeffery N. Comparison of kinematic variables in defining lameness caused by naturally occurring rupture of the cranial cruciate ligament in dogs. Vet Surg. 2010;39(4):523–530. doi: 10.1111/j.1532-950X.2010.00672.x. [DOI] [PubMed] [Google Scholar]
- Seebeck P, Thompson MS, Parwani A, Taylor WR, Schell H, Duda GN. Gait evaluation: A tool to monitor bone healing? Clin Biomech. 2005;20(9):883–891. doi: 10.1016/j.clinbiomech.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Shelton TJ, Bloebaum RD, Beck JP, Bachus KN. Percutaneous, osseointegrated implants: Attachment strength in a 12 month amputation ovine model. Orthopaedic Research Society 57th Annual Meeting; Long Beach, CA. Trans Orthopaed Res Soc; 2011. [Google Scholar]
- Sullivan J, Uden M, Robinson KP, Sooriakumaran S. Rehabilitation of the trans-femoral amputee with an osseointegrated prosthesis: the United Kingdom experience. Prosthet Orthot Int. 2003;27(2):114–120. doi: 10.1080/03093640308726667. [DOI] [PubMed] [Google Scholar]
- Summers GD, Morrison JD, Cochrane GM. Foot loading characteristics of amputees and normal subjects. Prosthet Orthot Int. 1987;11(1):33–39. doi: 10.3109/03093648709079378. [DOI] [PubMed] [Google Scholar]
- Swider P, Pedrono A, Mouzin O, Soballe K, Bechtold JE. Biomechanical analysis of the shear behaviour adjacent to an axially loaded implant. J Biomech. 2006;39(10):1873–1882. doi: 10.1016/j.jbiomech.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Tomaszewski PK, Verdonschot N, Bulstra SK, Verkerke GJ. A comparative finite-element analysis of bone failure and load transfer of osseointegrated prostheses fixations. Ann Biomed Eng. 2010;38(7):2418–2427. doi: 10.1007/s10439-010-9966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rietbergen B, Huiskes R, Weinans H, Sumner DR, Turner TM, Galante JO. ESB Research Award 1992. The mechanism of bone remodeling and resorption around press-fitted THA stems. J Biomech. 1993;26(4-5):369–382. doi: 10.1016/0021-9290(93)90001-u. [DOI] [PubMed] [Google Scholar]
- Webster JB, Chou T, Kenly M, English M, Roberts TL, Bloebaum RD. Perceptions and acceptance of osseointegration among individuals with lower limb amputations: A prospective survey study. J Prosthet Orthot. 2009;21(4):215–222. [Google Scholar]
- Willie B, Bloebaum R, Bireley W, Bachus K, Hofmann A. Determining relevance of a weight-bearing ovine model for bone ingrowth assessment. J Biomed Mater Res Part A. 2004;69A(3):567–576. doi: 10.1002/jbm.a.30038. [DOI] [PubMed] [Google Scholar]
- Xu W, Robinson K. X-ray image review of the bone remodeling around an osseointegrated trans-femoral implant and a finite element simulation case study. Ann Biomed Eng. 2008;36(3):435–443. doi: 10.1007/s10439-007-9430-7. [DOI] [PubMed] [Google Scholar]


