Abstract
The biosynthesis of nitric oxide (NO) and prostaglandin share many similarities. Two major forms of nitric oxide synthase (NOS) and cyclooxygenase (COX) have been identified: constitutive vs inducible. In general, the constitutive form functions in housekeeping and physiologic roles whereas the inducible form is up-regulated by mitogenic or inflammatory stimuli and is responsible for pathophysiological responses. The cross talk between the COX and NOS pathways was initially reported 1993 and since then, numerous studies have been undertaken to delineate the functional consequences of this interaction as well as the potential mechanism by which each pathway interacts. This review will focus in particular on recent advances in this field that extend our understanding of these two pathways under various systems.
Keywords: nitric oxide, nitric oxide synthase, cyclooxygenase, prostaglandin, s-nitrosylation, peroxynitrite
Generation of Nitric Oxide and its action
Redox Species of NO
NO is a highly reactive diatomic molecule that is produced within mammalian cells mostly by the enzyme NO synthase [1], which catalyzes the stepwise conversion of L-arginine to L-citrulline, releasing NO as a byproduct [2; 3]. In biological systems, NO exists as three redox-related species, and NO is used as a generic term to encompass all these species. Redox- related species of NO include NO•, which can modulate iron (Fe)-containing proteins by direct coordination to Fe centers (see below) and can have a cytotoxic effect by reacting with superoxide anion (O2•−) to produce peroxynitrite (ONOO−). Further oxidation of NO• species can generate nitrogen dioxide (NO2), dinitrogen trioxide (N2O3), dinitrogen tetroxide (N2O4), nitrite (NO2−), nitrate (NO3−) and others [4]. The second important species of NO, the nitrosonium ion (NO+), can nitrosylate thiol groups of proteins, a modification that may have important regulatory functions [5]. Nitrosonium ion has an extremely short half-life (≈ 10−10 s) in solution at physiological pH [6], and binds extremely rapidly to thiol groups. Following such binding, the resulting – SNO compounds maintains a “nitrosonium” characteristic and can transfer NO+ to other thiols, in a process called trans-nitrosylation [7–9]. The final redox-related species of NO is the nitronyl (NO−) anion, which was reported to have a similar reactivity to NO+ [10], leading to S-nitrosylation, however, the physiological relevance of this species is unclear.
Functions of NO
NO has two principal yet divergent functions in cells: homeostasis and cytotoxicity. For regulatory functions, NO is produced in small amounts under physiological conditions and mediates vasorelaxation, controls the adhesion and aggregation of platelets and neutrophils, and is involved in neurotransmission [1; 11]. Most of these actions are mediated through the binding of NO to Fe in the heme prosthetic group of soluble guanylate cyclase which catalyzes the conversion of GTP to cyclic GMP [12]. The importance of Fe in mediating the functions of NO is also apparent when examining the cytotoxic effects of the molecule, which are observed when NO produced in much larger quantities by macrophages, hepatocytes and other cells following their exposure to cytokines or microbial products. NO produced via such high-output systems inhibits proliferation of intracellular pathogens and tumor cells. These effects can be explained by the reactivity of NO• with Fe in the [Fe-S] centers of several important macromolecules, including aconitase, and Complex I and Complex II of the electron transport chain [13; 14]. The high affinity of NO• for iron probably results in both the removal of Fe from [Fe-S] centers and the formation of nitrosyl Fe species within [Fe-S] proteins. Thus NO• exerts its function through its interaction with the Fe of heme and non-heme Fe proteins.
S-nitrosylation
The reactivity of NO with iron containing moieties, such as heme or Fe-S clusters, adequately explains its immediate bioactivity but does not provide an explanation about the effects on cellular signaling pathways after post-translational modification of proteins by NO. In 1993, it was demonstrated that NO+, can interact with thiol groups of proteins to form S-nitrosothiols [7]. Importantly, S-nitrosylation of cysteine residues in proteins by the addition of NO+ results in the modification of their activities. S-nitrosylation can be achieved in a test tube by a reaction between the acidified nitrite and thiol groups (equations 1 and 2, in which RSH represents a thiol and RSNO an S-nitrosylated product).
| (1) |
| (2) |
It is highly unlikely that NO+ production can occur by this mechanism in biological systems because of the requirement for low pH. It is likely that biological production of NO+ requires iron-catalyzed oxidation of NO• which is generated by NOS [15; 16].
| (3) |
| (4) |
Support for this argument has been found in the detection of iron-nitrosyl complexes and an increase of S-nitrosothiols in LPS/IFN-γ treated macrophages, which produce a large quantity of NO [17].
As described above, simple S-nitrosylation is a result of a non-enzymatically catalyzed chemical reaction. Nevertheless, there appears to be some specificity in this reaction. First, not all proteins containing free cysteines are S-nitrosylated. Moreover, not every available cysteine in a given target is S-nitrosylated. For example, the ryanodine receptor contains 87 cysteines but only 13 of them are S-nitrosylated [18], suggesting a certain specificity of S-nitrosylation. Furthermore, it has been postulated that there is a primary consensus sequence for S-nitrosylation requiring the presence of acidic or basic residues adjacent to a target cysteines [19]. However, this acid-base consensus motif can also arise as a result of three-dimensional protein structure, as shown by analysis of the crystal structure of caspase-3 whose active cysteine is S-nitrosylated by NO in vivo [20]. Hence, the conditions for S-nitrosylation appear to be far more complex than what may be suggested by a primary sequence or availability of a target cysteine.
More than a hundred proteins have been identified as targets of S-nitrosylation which is associated with modification of their functions or structures [5; 8]. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is among the first proteins shown to be S-nitrosylated. It has been reported that NO production is correlated with inhibition of GAPDH in rat liver during chronic inflammation [21]; the S-nitrosylation of a cysteine residue in the active site of GAPDH causes reversible inactivation of the enzyme [22] as well as its translocation to nucleus leading to cell death [23]. Moreover, nitrosylated GAPDH functions as a carrier of NO and transnitrosylate nuclear proteins such as sirtuin-1 (SIRT1), histone deacetylase-2 (HDAC2) and DNA-activated protein kinase (DNA-PK) [9].
S-nitrosylation of hemoglobin was extensively studied and Cys93 has been shown to be a site for this modification. The binding of NO to hemoglobin is dependent on the allosteric state of hemoglobin [24]. When oxygenated, hemoglobin is S-nitrosylated and the NO group is released during arterial-venous transit [25]. This report concluded that NO released from hemoglobin plays an essential but previously unexpected role in the respiratory cycle by ensuring adequate blood flow to tissues.
S-nitrosylation of other proteins such as the N-methyl-D-aspartate (NMDA) receptor, p21ras, caspase-3, AMPA receptor and RASD1 affects their regulation or localization [26–29] and the list of S-nitrosylated proteins continues to grow [5]. Interestingly, p21ras can be S-nitrosylated and activated by NO [26], with the concentration of NO required to obtain maximal activation of this protein in vitro being 1000 fold higher than that required for activation using intact cells, suggesting that the intracellular environment is more favorable for nitrosothiol formation [30].
Peroxynitrite
Superoxide is generated by various sources under physiological conditions and its production is tightly regulated by superoxide dismutases (SODs) [31]. However, during acute and chronic inflammation, superoxide production exceeds the capacity of endogenous SODs to remove it [32; 33]. Under the same conditions, NO production is also enhanced. Excess superoxide scavenges NO, generating peroxynitrite, thus destroying the biological activity of NO [34]. Peroxynitrite is highly reactive and exerts its action via various mechanisms, however, it is the interaction with tyrosine residues in proteins forming nitrotyrosine that has received the most attention. So far, numerous proteins have been identified as targets for nitrotyrosine modification but the specificity is not well understood. Nevertheless, nitrotyrosine formation within proteins leads to either gain or loss of function [35–37]. MnSOD is a good example of an enzyme whose activity was lost after tyrosine nitration in vivo [31; 38]. The functional consequence of nitrotyrosine formation of MnSOD is quite severe as loss of activity enhances the production of superoxide, leading to an increase in peroxynitriate, followed by additional modifications on other proteins such as aconitase, ATPase, and cytochrome c, further disrupting mitochondria respiratory function and consequently resulting in apoptosis [36; 39; 40]. This nitrotyrosine modification has been detected in various pathophysiological conditions such as ischemia and reperfusion injury, Alzheimer’s disease, and organ transplant [41–43]. Thus, peroxynitrite functions as a potent cytotoxic and anti-inflammatory molecule [37; 42; 44]. Nitrotyrosine formation is similar to S-nitrosyaltion in that the NO moiety modifies a specific residue in the target proteins but many differences also exist [5; 37]. Unlike the labile S-nitrosylation modification, once a tyrosine residue is modified by peroxynitrite, it appears to be long-lasting. One way to remove the nitrotyrosine is by protein degradation [37; 42; 44]. A yet unidentified protein, termed “denitrase”, appears to be able to remove the nitro group without protein degradation and has been observed in tissues [45].
Cycolooxygenases and the role of prostaglandin in biology
COX1 and 2
Cyclooxygenase (COX) was first purified in 1976 from sheep seminal vesicles a homodimer of molecular mass of 71kDa containing both cyclooxygenase and hydroperoxidase activities [46; 47]. Subsequently, several laboratories speculated an existence of COX isoenzymes as certain stimuli could induce the production of prostaglandin [48; 49]. Later Needleman’s group reported production of prostaglandin generated from de novo synthesis of new COX protein in mouse peritoneal macrophage in vivo [50]. The following year, cyclooxygenase was shown indeed to have two isoforms initially called prostaglandin H synthase-1 and -2 and now commonly referred to as COX1 and COX2 [51; 52]. These are encoded by two different genes; the human COX2 gene of 8.3kb is a small immediate early gene on chromosome 1 while COX1 is a much longer 22kb gene on chromosome 9. Human COX1 and COX2 are homodimers sharing 60% identity in sequence [53; 54]. Both isoforms are composed of 3 domains; the epidermal growth factor domain, the membrane bound domain and the catalytic domain containing cyclooxygenase and peroxygenase active sites on the either side of heme prosthetic group which is important for both activities. Peroxidase active site, on the opposite side of membrane binding domain, consists of heme prosthetic group [54; 55]. Interestingly, sequences of sidechain between the isoforms near peroxidase site are less conserved than any other catalytic domain area and yet hemes from both isoforms have comparable affinities for cyanide as the sixth axial ligand [56]. The cyclooxygenase active site is located on the top of a long hydrophobic channel originating from the membrane binding domain near the heme. The binding site for fatty acids as well as competitive COX inhibitors resides above this channel, and is the most strongly conserved region in COX isoforms [54; 57]. When arachidonic acid binds to the cyclooxygenase site, it sits at the end of channel and carbon-13 of arachidonic acid is placed near Tyr-385, which is a crucial amino acid for cyclooxygenase reaction. However, mutation of the Tyr-385 does not impact the midpoint potential of heme in COX2 further suggesting that peroxidase site is independent of cyclooxygenase site. Moreover, crystallographic as well as biochemical data showed that two active sites are physically separated as fatty acids do not interfere peroxidase catalysis [57].
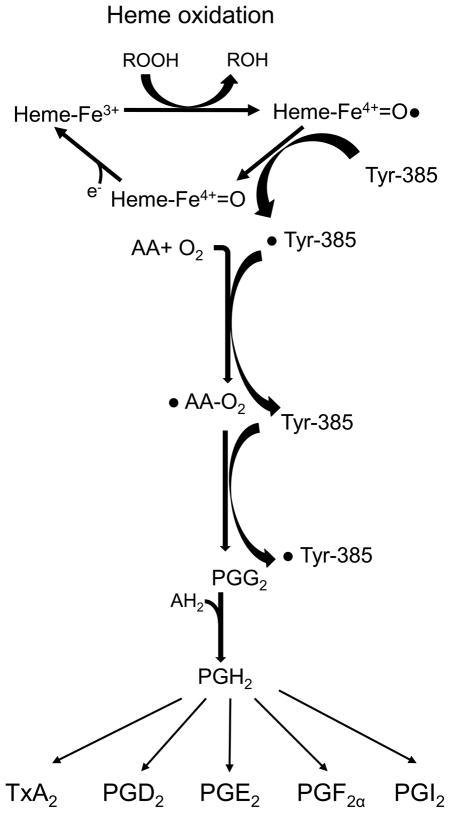
The conversion of arachidonic acid to the hydroperoxyendoperxoide, PGG2 is initiated by abstraction of the pro-S hydrogen atom from carbon-13 and then dioxygen traps the pentadienyl radical at carbon 11. This reaction is followed by a series of radical reaction which is similar to polyunsaturated fatty acid autoxidation producing prostaglandinG2 (PGG2). The enzyme activation, which is dependent of peroxidase activity, is required for this cyclooxygenase reaction. In this process, an electron is transferred from heme to Tyr-385 generating tyrosl radical in the cylooxygenase active site and initiating a reaction. Moreover, tyrosl radical is regenerated by reducing perxyl radical to the hydroperoxide to from PGG2. After cyclooxygenase reaction, peroxidasereduces the 15-hyroperoxy of pGG2 to the corresponding alcohol of PGH2 (Fig. 1) [54; 57–59]. Even though the two isoforms appear to follow the same basic mechanism and their 3 dimensional structures can be almost superimposed [54], there is a minor difference within the substrate binding site in catalytic domain which explains the specificity of non-steroid anti-inflammatory drugs (NSAIDs) [55].
Figure 1. The scheme of COXs enzyme reaction mechanism.
Cox enzymes produce PGH2 by two step chemical reactions; cyclooxygenase and peroxidase. PGH2 is further converted to other prostanoid by tissue specific enzymes.
Despite the structural similarity of the COX isoforms, their expression patterns and localization are very distinct. COX1 is widely distributed and constitutively expressed in most tissues while COX2 is activated by various inflammatory and proliferative stimuli [54]. Due to this obvious difference in expression patterns, it had been speculated that COX1 produces prostaglandins which are required for homeostatic functions such as gastric protection and hemostasis whereas COX2 participates in pathophysiological process including inflammation and tumorigenesis. This idea drove pharmaceutical companies to invest massive resources to develop COX2 specific inhibitors as anti-inflammatory drugs to avoid common side effects of traditional NSAIDs such as gastrointestinal bleeding [60; 61]. However, continued research has revealed that this path may not be ideal. Firstly, deletion of COX1 in mice did not show an increased susceptibility of gastric ulcer [62], while COX2 inhibitors have been shown to attenuate the healing process [63]. Secondly, COX1 is also expressed in inflammatory cells [64] and COX2 is constitutively expressed in certain tissues including brain, and kidney [62; 65–67]. Interestingly, it has been showed that COX1 knockout rather than COX2 deletion reduced the LPS-mediated inflammatory response in the brain [68]. Moreover, COX2 knockout mice showed unexpected results: disruption of postnatal kidney development, infertility in female mice due to failure to ovulate and embryo implantation [66; 67; 69; 70]. Hence, to ascribe such a dichotomy in function between the COX isoforms may be considered too general, though, there is clearly some differential functions between them. An elegant study performed by Funk’s group showed that replacement of COX2 with COX1 cannot compensate for a loss of function in COX2 [71] strongly suggesting that there are specific roles assigned to each isoform.
Function of prostaglandin signaling in biology
Virtually all cells except for red blood cells can produce prostaglandin from arachindonic acid which is prevalent at high concentrations in phospholipids of the cell membrane. Prostaglandins are members of a lipid family which are enzymatically derived from polyunsaturated fatty acids with 20-carbon chains and 4 cis-double bonds (20:4) via the cyclooxygenase pathway. Prostaglandins are also known as “prostanoids,” which includes classical prostaglandin as well as specialized species such as thromboxane and prostacyclin [72; 73].
The first report of the existence of prostaglandins was made in 1930 showing that fresh semen led to rhythmic contraction of human myometrium in vitro [74]. A few years later, a couple of groups independently confirmed that presence of an active substance responsible for muscle contraction and named it “prostaglandin,” as an homage to the origin being the prostate gland [73; 75; 76]. Continued research has revealed that prostaglandins are a mixture of biologically active substances such as prostaglandin E or prostaglandin F, but that ironically, the actual origin of prostaglandins is the seminal vesicle and not the prostate gland [77]. One of the most important discoveries in the field was when Vane showed that anti-inflammatory activity of aspirin and other NSAIDs was due to an inhibition of cyclooxygenase [78], proposing that major pharmacological effects of NSAIDs were attributable to an inhibition of this enzyme.
Nervous System
The presence of prostaglandin in the brain was first reported in 1964 [79]. It has been postulated that prostaglandin production from COX2 plays a prominent role in fever, pain or any pathological conditions [80]. Moreover, COX2 is up-regulated in the brains of patients with Alzheimer’s disease (AD) suggesting abnormal modulation of the COX pathway [81]. Several clinical trials were performed to evaluate the effects of NSAIDs or COX2 specific inhibitors but the outcomes have been inconclusive [82; 83].
Dramatic increases in COX2 mRNA and protein levels have been observed under excitotoxic conditions, defined as a process by which high levels of glutamate or its analogs excite neurons and induce neuronal cell death [84]. Glutamate enhances calcium influx, which can initiate multiple cascades of events including an increase in NO production by neuronal NOS (nNOS) and activation of enzymes involved in generation of arachidonic acids and its metabolism, such as phospholipase A2, COX2 and lipoxygenase [85; 86]. Therefore, it is not surprising that glutamate-mediated excitotoxic conditions enhance prostaglandin production. Indeed, our laboratory has shown that N-methyl D-aspartate (NMDA)-mediated activation of COX2 requires nNOS and plays a crucial role in NMDA-excitotoxicity which can be attenuated by COX2 specific inhibitors [87].
Ischemic insults also influence synthesis or relocation of prostaglandins and other arachidonic acid metabolites [88]. Interestingly, both COX1 and COX2 are increased in brain ischemia. Furthermore, either selective inhibition of COX1 or COX2 significantly reduces neuronal cell death, suggesting that both COX isoforms participate in the progression of neuronal damage following global cerebral ischemia and identifies a potential therapeutic use of COX inhibitors in cerebral ischemia [89].
One interesting aspect of neuronal COX2 is that unlike in other tissues, COX2 is constitutively expressed [65], thus, we can speculate that COX2 may play a role in not only pathological progression as described above but also in physiological neuronal function such as synaptic plasticity. Worley’s group has shown that the basal expression of COX2 is regulated by NMDA receptor-dependent synaptic activity and especially up-regulated by long term potentiation (LTP) suggesting that the expression and activity of COX2 may be associated with long-term synaptic plasticity [65; 90]. This idea was further confirmed by showing that COX2 specific inhibitors or suppression of COX2 gene reduced high-frequency stimulation (HFS) induced LTP implying that constitutively expressed COX2 plays a crucial role in synaptic plasticity [65; 84]. However, hippocampal slices prepared from COX2-deleted mice did not show any significant difference on HFS-induced LTP [91], implying that COX1 may be able to compensate for the loss of COX2 and its own role in synaptic plasticity [92].
Cardiovascular
Prostaglandin E series are generally potent vasodilators of the vascular bed [93]. They usually affect small vessels including arterioles and pre/postcapillary venules [94]. Thromboxane and prostacyclin are more specialized prostaglandins that exert major impacts on the cardiovascular system [94; 95]. Thromboxane is normally produced by platelets, acting as a very potent vasoconstrictor, and plays an important role on platelet aggregation and hemostasis. Its effect is counterbalanced by prostacyclin, which is a powerful vasodilator and inhibitor of platelet aggregation. Prostacyclin is the main product in the vascular system [96] and is produced in endothelial cells [97; 98]. As prostaglandin production in the endothelial cell is dependent on COX2 induction [99], the usage of COX2 specific inhibitors under chronic inflammatory conditions has raised concerns [100; 101], leading to some COX2 specific inhibitors being withdrawn from the market due to an increased cardiovascular risk.
Cancer
The potential link between prostaglandin and tumorigenesis was initially proposed several decades ago [102; 103]. Further studies have shown COX2 elevation particularly in colon cancers, while epidemiological studies have indicated that NSAIDs indeed reduce the incidence of colon cancer [104; 105]. The effects of prostaglandins on tumors is not limited to colon cancer and extends to cancers in other tissues such as breast [106], pancreas [107], esophagus [108], lung [109] and gastric [110]. Hence, COX2 expression is dysregulated in many types of cancer and COX2-derived prostaglandin E2 (PGE2) has been shown to elicit multiple oncogenic signals to promote carcinogenesis [111; 112]. Significant research efforts are currently being undertaken to identify more selective targets and elucidate the molecular pathway of prostaglandin-mediated oncogenesis. As the rate-limiting enzyme for this pathway, COX2 is garnering considerable attention in hopes that COX2 specific inhibitors may be used as anti-cancer drugs or for cancer prevention, however, further studies are necessary.
Metabolism
While prostaglandins are produced in virtually every system in the human body and play a role in cellular homeostasis, recent studies have uncovered an unexpected role for COX2-produced prostaglandins. Kristiensan’s group first reported that uncoupling protein 1 (UCP1), a hallmark protein in brown adipocytes crucial for cold- or diet-induced thermogenesis, is regulated by COX2 [113]. Moreover, Herzig’s group demonstrated that overexpression of COX2 in white adipose tissue, where excess fat is stored, can convert these cells to become brown adipose tissue, which burns fat to produce heat [114]. This brown fat is usually prominent in infants who require more energy to maintain their body temperature and continuously regress with age. A considerable amount of this brown fat still remains in adulthood and is correlated to leanness [115; 116]. Hence, increasing brown fat content in humans has become an attractive target for anti-obesity drugs and manipulation of COX2 activity may be a new therapeutic strategy, though a strong note of caution is warranted, as the triggering of the inflammatory response is a potential side-effect of enhanced prostaglandin production.
Crosstalk between the NO and Prostaglandin pathways
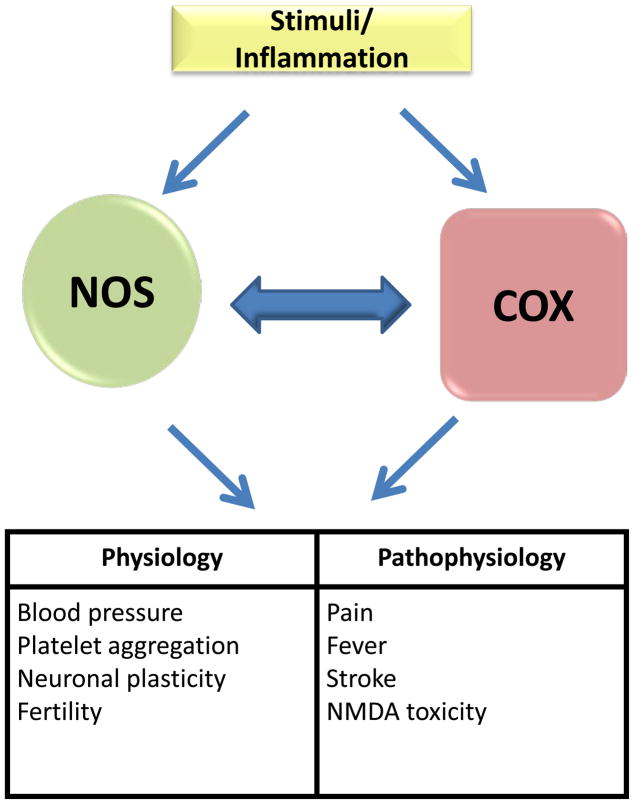
NO and prostaglandin pathways share numerous similarities and the two molecules can be produced simultaneously in the same tissues, as described in a number of models of inflammation such as endotoxin induced septic shock, carrageenan-induced pouch or paw inflammation (Fig. 2) [117]. Hence numerous studies have explored the potential crosstalk between NO and prostaglandin pathways and increasing evidence supports this idea. However, the detailed mechanisms by which NO regulates prostaglandin production or vice versa is still controversial, partly because the interaction between these two pathways occurs at multiple levels along with the complexity of NO redox chemistry [117; 118]. An initial report detailing the cross talk between NO and prostaglandin was made by Needleman’s group showing that NO can activate cyclooxygenase [119]. In this study, NOS and COX2 activity were induced in a macrophage cell line, RAW264.7, treated with LPS to produce both NO and prostaglandin. The production of both was attenuated by NOS inhibitors. As the NOS inhibitors used in this study do not have any NSAID characteristics, the inhibition of prostaglandin production was likely due to the decrease in NO production and not as a direct effect on COX2 [119]. This study was further corroborated by in vitro studies showing that even exogenous NO gas or chemical donors can induce COX1 activity [120; 121]. Moreover, NO-mediated activation of COX does not seem to be limited to its enzyme activity. Lie et al showed that NO can increase COX2 mRNA levels via the β-catenin/TCF pathway leading to activation of the polyoma enhancer activator 3 (PEA3) transcription factor [122]. Furthermore, NO also interacts with various other pathways which can influence COX expression such as the cAMP/PKA/CRE and JNK/Jun/ATF2 signaling cascades [122; 123].
Figure 2. The role of Nitric oxide Synthase (NOS) and Cyclooxygenase.
Both enzymes share the similar pathway for activation and their physiological as well as pathophysiological implication.
In addition to this body of evidence showing an up-regulation of COX activity by NO, a large number of reports supporting the idea that NO can inactivate COX under certain conditions also exists. Levi’s group showed that LPS-induced COX2 expression and production of prostaglandin in microglia cells are augmented by NOS inhibitors [124]. Indeed, similar findings were reported in various other cells types including a J774 macrophage cell line and the rat Kupper cells [125; 126]. Interestingly, the interaction between NO and prostaglandin is not uni-directional, as it has been reported that NSAIDs such as aspirin and indomethacin can significantly reduced NOS activity [127].
The mechanism by which the NOS and COX pathways interact is complicated by conflicting reports in the field. For instance, while most of the actions by NO are mediated by its interaction with Fe in heme prosthetic group of soluble guanylate cyclase (sGC), Salvemini et al has shown that inhibition of sGC with the sGC-specific inhibitor, methylene blue, does not attenuate NO-mediated activation of prostaglandin biosynthesis [119] and has proposed that NO may directly activate COX enzyme. This theory is further supported by numerous reports demonstrating that exogenous NO donors can activate recombinant COX. However, Tsai et al reported that it is unlikely that NO directly binds to heme group in COX to activate under anaerobic condition [128]. One reason for this disagreement may be due to the complexity of NO chemistry with the result that various NO species are generated, as described earlier.
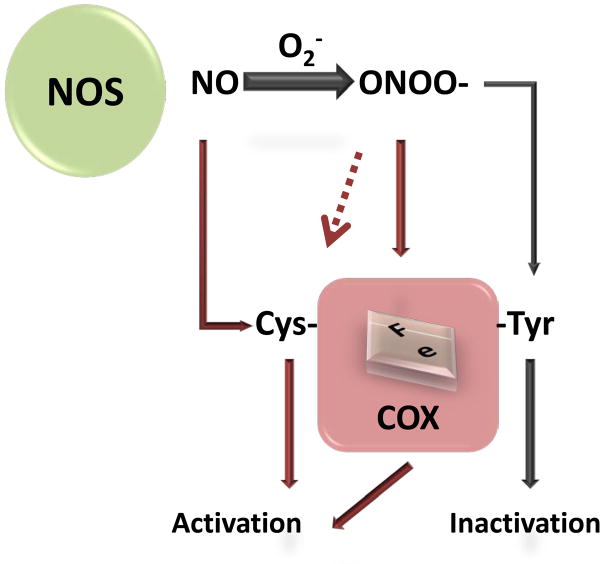
Currently, there are several proposed mechanisms by which NO mediates prostaglandin synthesis (Fig. 3). One is that NO and superoxide can be produced simultaneously and can react together to form peroxynitrite, which can feedback to modify COX. Another possible mechanism is the direct S-nitrosylation of COX enzymes. These two post-translational modifications can alter the function of these enzymes and are further discussed below.
Figure 3. Proposed mechanisms of NO-mediated regulation of prostaglandin production.
Nitric oxide produced by NOS can directly modify cysteine(s) in the COXs and activate it. Moreover, superoxide can be simultaneously generated under the same environment which can enhance NO production. Superoxide rapidly interacts with NO producing peroxynitrite which can interact with either heme or tyrosine residue(s) in COX2.
Peroxynitrite-mediated regulation of prostaglandin synthesis
Superoxide and nitric oxide are readily produced in the cells under both physiologic and pathological conditions [37]. Upon production these two compounds, peroxynitrite can be easily formed. Many laboratories have reported that peroxynitrite can interact with COX enzymes but the consequence of the interaction differs depending on where this interaction occurs. So far, two peroxynitrite binding sites in COX enzymes have been identified: within the endogenous heme moiety and at tyrosine residues on the protein.
Peroxynitrite has been shown to activate both COX1 and COX2 by several groups. In particular, treatment of cells with SIN-1, an exogenous peroxynitrite generator, enhances prostaglandin synthesis and this activation is inhibited by oxygen radical scavengers, strongly implicating peroxynitrite as an activator of COX [129]. The mechanism by which peroxynitrite activates COX activity is still unclear, however, it has been speculated that peroxynitrite may be interacting with Fe in the heme group of COX and forms a radical intermediate product which can accelerate the enzyme reaction [130–132]. This hypothesis is supported by the fact that peroxynitrite is known to interact with the heme group of certain proteins such as myeloperoxidase and horseradish peroxidase to generate an intermediate product [133].
Most of the studies about peroxynitrite and COX enzymes have been focused on tyrosine residue modification in the enzyme and the formation of a nitrotyrosine residue. It appears that this modification, especially at Tyr385, leads to an inactivation of enzyme [134–137], as demonstrated by inhibition of COX1 enzyme activity when either purified enzyme COX1 or smooth muscle cells are treated with NO donors and arachidonic acid simultaneously [138]. It is interesting to note that pure NO donors such as NOC-7 does not lead to formation of nitrotyrosine in the absence of arachidonic acid, while peroxynitrite, SIN-1 or tetranitromethane (TMN) can readily carry out this reaction.
It appears that peroxynitrite has dual actions on COX depending on where it interacts in the enzyme. Even though nitrotyrosine modification of COXs is detected in many pathological conditions and clearly inactivates its enzyme activity in vitro, the exact mechanism of nitration remains elusive. The levels of peroxynitrite markedly increase under pathological conditions, as inflammation induces NO production from iNOS and superoxide production from by NAD(P)H and xanthine oxidase and correlates to formation of nitrotyrosine in COXs. However, further investigation is required to identify different conditions that determine the target selectivity of peroxynitrite: to choose between interacting with tyrosine to inactivate an enzyme versus interaction with heme in COXs to activate it. Interestingly, Deeb et al showed that holoCOX1 (heme-containing) can be nitrated at Tyr 385 by peroxynitrite, even in the absence of arachidonic acid, while apoCOX1 (heme-deficient) is modified at the different site, thus indicating a heme requirement for peroxynitrite-mediated Tyr385 nitration [139]. The identity of the peroxynitrated tyrosine residue in apoCOX1 and its downstream biological function are yet to be determined.
It has been proposed that peroxynitrite may interact with Fe in the heme moiety of COX to generate a short-lived intermediate which can facilitate the formation of nitrotyrosine [140]. While heme is essential for this reaction within COX, the role of this co-factor is unclear, as there are other proteins which can be nitrated without heme moiety in the protein [141]. It is possible that Fe binding in the heme molecule in COX may expose the Tyr385 residue, which resides just below the heme moiety, making it more readily accessible to peroxynitrite. It will be interesting to examine whether recombinant COX with non-Fe protoporphyrin such as Zn-protoporphyrin or Sn-protoporphyrin can be nitrated by peroxynitrite. This theory, however, conflicts in part with the aforementioned evidence that peroxynitrite binding to the heme moiety in COX leads to activation of the enzyme while nitration of Tyr385 leads to enzyme inactivation. This may be partially explained by differing concentrations of arachidonic acid, which can be oxidized by peroxynitrite, with this oxidized substrate having different impacts on COX activities. Deeb et al has indeed shown that higher arachidonic acid reduces formation nitrotyrosine in COX1 [142].
Most importantly, this peroxynitration modification of COX enzymes has been detected in many pathological or chronic inflammatory conditions such as atherosclerosis, Parkinson’s disease or Alzheimer’s disease. However, it is not clear whether an inactivation of this enzyme by nitrotyrosine formation contributes to the pathology of these diseases or simply an indication of nitrossative stress.
Finally, COXs are not the only target which can be modified by Peroxynitrite. Prostacyclin synthase (PGCI) is selectively inhibited by peroxynitrite at low concentration (IC50=50 nM) while thromboxane A2 (TxA2)-synthase is activated [143; 144]. In particular, PGCI contains one Cys at position 469 which is involved in heme binding and spectrophotometry study showed that this thiolate ligand at the fifth coordination position of the heme iron is not affected by peroxynitrite. It appears that nitration of tyrosine residue 430 in PGCI interferes the metal center of the active site and inhibits its activity [144; 145].
S-nitrosylation of COX
The recent development of a simple method to detect S-nitrosylation flourished identification of new targets and its functional consequence since this modification was first reported with albumin by Stamler et al. COX has also been identified as a target of this modification [5; 6; 146]. It was first proposed by Hajjar et al showing that S-nitrosothiol may be responsible for NO-mediated activation of COX as it occurs in an heme-independent manner [147]. On the other hand, Marnett’s group investigated the role of free cysteines in COX1 and showed that Cys313 and Cys540 mutation into serine alone inhibited its enzyme activity implying that NO-mediated Cys modification may interfere with COX1 activity [148]. Interestingly, Marnett’s and Hajjar’s groups independently reported that SNAP, an NO chemical donor, cannot activate COX1 [138; 149], thus confusion exists over whether S-nitrosylation of COX1 leads to its activation.
Our group has shown that iNOS can specifically bind to COX2, leading to direct S-nitrosylation of cysteine residues and activate COX2 [150]. This interaction and modification is specific for COX2 as iNOS does not interact with COX1. In particular, we observed more than one S-nitrosylated cysteine residues in LPS/IFN-γ activated RAW264.7 cells and individually mutated each cysteine to serine. We have identified Cys523, located near the catalytic domain, as the only cysteine which does not affect base level enzyme activity and when mutated, NO can no longer activate COX2. This cysteine is located in near the catalytic site of COX2 and we speculated that this modification leads to a slight conformational change in COX2 to affect its enzyme kinetics. Indeed, we performed viscosity studies examining enzyme activity in various concentrations of sucrose and showed that release of product is accelerated in the presence of NO donor. However, it is not possible to exclude a potential involvement of any other cysteines for NO-mediated activation of COX2 as mutation of other cysteines alone affected the basal level enzyme activity of COX2. Given that NO has a high affinity for heme, it is unclear how NO can specifically S-nitrosylate cysteines of COX2 rather than interacting with heme moiety. This may be partially explained by the fact that S-nitrosylation of COX2 by iNOS requires physical interaction between the two proteins, specifically between the subunit binding site of iNOS and catalytic domain of COX2. Hence, we speculated that NO generated from iNOS is simply in direct proximity of the S-nitrosylation target residue in COX2 for immediate delivery and modification. Indeed, disruption of iNOS/COX2 binding diminishes S-nitrosylation and activation of COX2 in LPS-induced RAW264.7 cells. Interestingly, Goodwin et al demonstrated that removal of superoxide in LPS-activated RAW264.7 cells inhibited prostaglandin formation to the same extent as NOS inhibitors [137]. While the immediate speculation will be peroxynitrite-mediated nitrotyrosine formation of COX2, Cross’ group showed that peroxynitrite can also generate S-nitrosothiol [151] and therefore, further studies are necessary to identify the form of NO-mediated modification of COX2 in activated macrophages.
iNOS-mediated regulation of prostaglandin production is not limited to COX enzymes. Xu et al showed that iNOS activates cytolosic phospholipase A2a (cPLA2α) by S-nitrosylation [152]. In this case, cPLA2α does not directly interact with iNOS, but rather forms ternary complex with COX2 and iNOS, and hence induction of COX2 is a crucial step for S-nitroyslation and activation of cPLA2α [152].
S-nitrosylation of COX2 does not seem to be limited to macrophages. Unlike other tissues, COX2 in the brain is constitutively expressed. We showed that COX2 is S-nitrosylated in neuronal cells by nNOS [87]. To our surprise, nNOS binding is mediated by its PDZ domain, differing from the domain near the catalytic region that mediates iNOS binding to COX-2. We also showed that a physical interaction between nNOS and COX2 is required for S-nitrosylation of COX2 and that disruption of binding attenuated NMDA-mediated excitotoxicity, as did a selective COX2 inhibitor.
Perspective/Conclusion
The prostaglandin pathway has been the center of attention as a main target to treat numerous pathological conditions such as pain, neuroexcitotoxicity, ischemia, neurodegeneration, cancer, and metabolic deficiencies. There is no doubt that NO signaling is tightly coupled to prostaglandin pathway and new evidence also supports that NO-conjugated COX inhibitors (e.g. nitroaspirin) may have a great potential for the treatment of both inflammatory as well as non-inflammatory conditions. Yet we do not have clear understanding of the molecular mechanism by which the pathways interact. Efforts from many outstanding laboratories have proposed multiple potential mechanisms but some of them are quite contradictory. It may be partially due to a lack in sophisticated enough tools to examine the exact identity of NO, both spatially and temporally in vivo, as it appears that the total amount and ratio of NO and superoxide produced in the cells may play a key role in explaining the discrepancies among many reports. Moreover, there is a considerable difference depending on which cell type or tissue is examined and the surrounding microenvironment may also be a crucial factor. Undoubtedly, further studies are necessary to understand the molecular basis of interaction between NO and prostaglandin and that these will unveil a crucial target for intervention in numerous pathological conditions.
Research highlight.
Nitric oxide regulates prostaglandin synthesis.
Nitric oxide activates cyclooxygenase2 activity via S-nitrosylation.
Peroxinitrite inactivates cyclooxygenase1/2 via Tyrosine nitration.
Interaction of Peroxinitrite and heme in cyclooxygenase can activate their activity.
Acknowledgments
The author thanks J Cheah for reading a manuscript and providing insightful input. This work was supposed by NIH grant MH079614 and DK084336.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASE J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 2.Stuehr DJ, Kwon NS, Nathan CF, Griffith OW, Feldman PL, Wiseman J. N omega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J Biol Chem. 1991;266:6259–6263. [PubMed] [Google Scholar]
- 3.Abu-Soud HM, Presta A, Mayer B, Stuehr DJ. Analysis of neuronal NO synthase under single-turnover conditions: conversion of Nomega-hydroxyarginine to nitric oxide and citrulline. Biochemistry. 1997;36:10811–10816. doi: 10.1021/bi971414g. [DOI] [PubMed] [Google Scholar]
- 4.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 5.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 8.Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 9.Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JV, Snowman AM, Law L, Hester LD, Snyder SH. GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol. 2010;12:1094–1100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim WK, Choi YB, Rayudu PV, Das P, Asaad W, Arnelle DR, Stamler JS, Lipton SA. Attenuation of NMDA receptor activity and neurotoxicity by nitroxyl anion, NO−. Neuron. 1999;24:461–469. doi: 10.1016/s0896-6273(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 11.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 12.Ignarro LJ. Physiology and pathophysiology of nitric oxide. Kidney Int Suppl. 1996;55:S2–S5. [PubMed] [Google Scholar]
- 13.Drapier JC, Hibbs JB., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988;140:2829–2838. [PubMed] [Google Scholar]
- 14.Henry Y, Lepoivre M, Drapier JC, Ducrocq C, Boucher JL, Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASE J. 1993;7:1124–1134. doi: 10.1096/fasebj.7.12.8397130. [DOI] [PubMed] [Google Scholar]
- 15.Akaike T, Inoue K, Okamoto T, Nishino H, Otagiri M, Fujii S, Maeda H. Nanomolar quantification and identification of various nitrosothiols by high performance liquid chromatography coupled with flow reactors of metals and Griess reagent. J Biochem. 1997;122:459–466. doi: 10.1093/oxfordjournals.jbchem.a021774. [DOI] [PubMed] [Google Scholar]
- 16.Inoue K, Akaike T, Miyamoto Y, Okamoto T, Sawa T, Otagiri M, Suzuki S, Yoshimura T, Maeda H. Nitrosothiol formation catalyzed by ceruloplasmin. Implication for cytoprotective mechanism in vivo. J Biol Chem. 1999;274:27069–27075. doi: 10.1074/jbc.274.38.27069. [DOI] [PubMed] [Google Scholar]
- 17.Lancaster JR, Jr, Hibbs JB., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990;87:1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 19.Stamler JS, Toone EJ, Lipton SA, Sucher NJ. (S)NO signals: translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 20.Hess DT, Matsumoto A, Nudelman R, Stamler JS. S-nitrosylation: spectrum and specificity. Nat Cell Biol. 2001;3:E46–E49. doi: 10.1038/35055152. [DOI] [PubMed] [Google Scholar]
- 21.Vedia L, McDonald B, Reep B, Brune B, Di SM, Billiar TR, Lapetina EG. Nitric oxide-induced S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. J Biol Chem. 1992;267:24929–24932. [PubMed] [Google Scholar]
- 22.Mohr S, Hallak H, de BA, Lapetina EG, Brune B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1999;274:9427–9430. doi: 10.1074/jbc.274.14.9427. [DOI] [PubMed] [Google Scholar]
- 23.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 24.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 25.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 26.Lander HM, Ogiste JS, Teng KK, Novogrodsky A. p21ras as a common signaling target of reactive free radicals and cellular redox stress. J Biol Chem. 1995;270:21195–21198. doi: 10.1074/jbc.270.36.21195. [DOI] [PubMed] [Google Scholar]
- 27.Mannick JB, Hausladen A, Liu L, Hess DT, Zeng M, Miao QX, Kane LS, Gow AJ, Stamler JS. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Man HY, Sekine-Aizawa Y, Han Y, Juluri K, Luo H, Cheah J, Lowenstein C, Huganir RL, Snyder SH. S-nitrosylation of N-ethylmaleimide sensitive factor mediates surface expression of AMPA receptors. Neuron. 2005;46:533–540. doi: 10.1016/j.neuron.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 30.Lander HM, Hajjar DP, Hempstead BL, Mirza UA, Chait BT, Campbell S, Quilliam LA. A molecular redox switch on p21(ras). Structural basis for the nitric oxide-p21(ras) interaction. J Biol Chem. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 31.McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969;244:6056–6063. [PubMed] [Google Scholar]
- 32.Fridovich I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann N Y Acad Sci. 1999;893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 33.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 35.Nowak P, Wachowicz B. Peroxynitrite-mediated modification of fibrinogen affects platelet aggregation and adhesion. Platelets. 2002;13:293–299. doi: 10.1080/0953770021000007230. [DOI] [PubMed] [Google Scholar]
- 36.Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 37.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 38.Yamakura F, Kawasaki H. Post-translational modifications of superoxide dismutase. Biochim Biophys Acta. 2010;1804:318–325. doi: 10.1016/j.bbapap.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 40.Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F. Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J Biol Chem. 2003;278:33972–33977. doi: 10.1074/jbc.M303734200. [DOI] [PubMed] [Google Scholar]
- 41.Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 42.Ischiropoulos H. Protein tyrosine nitration--an update. Arch Biochem Biophys. 2009;484:117–121. doi: 10.1016/j.abb.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 43.Mollace V, Nottet HS, Clayette P, Turco MC, Muscoli C, Salvemini D, Perno CF. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 2001;24:411–416. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- 44.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irie Y, Saeki M, Kamisaki Y, Martin E, Murad F. Histone H1.2 is a substrate for denitrase, an activity that reduces nitrotyrosine immunoreactivity in proteins. Proc Natl Acad Sci U S A. 2003;100:5634–5639. doi: 10.1073/pnas.1131756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hemler M, Lands WE. Purification of the cyclooxygenase that forms prostaglandins. Demonstration of two forms of iron in the holoenzyme. J Biol Chem. 1976;251:5575–5579. [PubMed] [Google Scholar]
- 47.Miyamoto T, Ogino N, Yamamoto S, Hayaishi O. Purification of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1976;251:2629–2636. [PubMed] [Google Scholar]
- 48.Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the anti-pyretic activity of paracetamol (4-acetamidophenol) Nature. 1972;240:410–411. doi: 10.1038/240410a0. [DOI] [PubMed] [Google Scholar]
- 49.Lin AH, Bienkowski MJ, Gorman RR. Regulation of prostaglandin H synthase mRNA levels and prostaglandin biosynthesis by platelet-derived growth factor. J Biol Chem. 1989;264:17379–17383. [PubMed] [Google Scholar]
- 50.Morrison AR, Moritz H, Needleman P. Mechanism of enhanced renal prostaglandin biosynthesis in ureter obstruction. Role of de novo protein synthesis. J Biol Chem. 1978;253:8210–8212. [PubMed] [Google Scholar]
- 51.Bailey JM, Muza B, Hla T, Salata K. Restoration of prostacyclin synthase in vascular smooth muscle cells after aspirin treatment: regulation by epidermal growth factor. J Lipid Res. 1985;26:54–61. [PubMed] [Google Scholar]
- 52.Sano H, Hla T, Maier JA, Crofford LJ, Case JP, Maciag T, Wilder RL. In vivo cyclooxygenase expression in synovial tissues of patients with rheumatoid arthritis and osteoarthritis and rats with adjuvant and streptococcal cell wall arthritis. J Clin Invest. 1992;89:97–108. doi: 10.1172/JCI115591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Otto JC, Smith WL. Prostaglandin endoperoxide synthases-1 and –2. J Lipid Mediat Cell Signal. 1995;12:139–156. doi: 10.1016/0929-7855(95)00015-i. [DOI] [PubMed] [Google Scholar]
- 54.Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2009;50(Suppl):S29–S34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garavito RM, Mulichak AM. The structure of mammalian cyclooxygenases. Annu Rev Biophys Biomol Struct. 2003;32:183–206. doi: 10.1146/annurev.biophys.32.110601.141906. [DOI] [PubMed] [Google Scholar]
- 56.Goodwin DC, Rowlinson SW, Marnett LJ. Substitution of tyrosine for the proximal histidine ligand to the heme of prostaglandin endoperoxide synthase 2: implications for the mechanism of cyclooxygenase activation and catalysis. Biochemistry. 2000;39:5422–5432. doi: 10.1021/bi992752f. [DOI] [PubMed] [Google Scholar]
- 57.Kulmacz RJ, van der Donk WA, Tsai AL. Comparison of the properties of prostaglandin H synthase-1 and –2. Prog Lipid Res. 2003;42:377–404. doi: 10.1016/s0163-7827(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 58.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 59.Tsai AL, Kulmacz RJ. Prostaglandin H synthase: resolved and unresolved mechanistic issues. Arch Biochem Biophys. 2010;493:103–124. doi: 10.1016/j.abb.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DuBois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 61.Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, Isakson PC, Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci U S A. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Davis BJ, Lee CA. Disruption of the mouse cyclooxygenase 1 gene. Characteristics of the mutant and areas of future study. Adv Exp Med Biol. 1997;407:87–92. [PubMed] [Google Scholar]
- 63.Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706–714. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- 64.Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASE J. 2008;22:1491–1501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 66.Lipsky PE, Brooks P, Crofford LJ, DuBois R, Graham D, Simon LS, Van De Putte LB, Abramson SB. Unresolved issues in the role of cyclooxygenase-2 in normal physiologic processes and disease. Arch Intern Med. 2000;160:913–920. doi: 10.1001/archinte.160.7.913. [DOI] [PubMed] [Google Scholar]
- 67.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 68.Aid S, Langenbach R, Bosetti F. Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. J Neuroinflammation. 2008;5:17. doi: 10.1186/1742-2094-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McAdam BF, Mardini IA, Habib A, Burke A, Lawson JA, Kapoor S, FitzGerald GA. Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation. J Clin Invest. 2000;105:1473–1482. doi: 10.1172/JCI9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Langenbach R, Loftin C, Lee C, Tiano H. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol. 1999;58:1237–1246. doi: 10.1016/s0006-2952(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 71.Yu Y, Fan J, Hui Y, Rouzer CA, Marnett LJ, Klein-Szanto AJ, FitzGerald GA, Funk CD. Targeted cyclooxygenase gene (ptgs) exchange reveals discriminant isoform functionality. J Biol Chem. 2007;282:1498–1506. doi: 10.1074/jbc.M609930200. [DOI] [PubMed] [Google Scholar]
- 72.Smith WL, Marnett LJ, DeWitt DL. Prostaglandin and thromboxane biosynthesis. Pharmacol Ther. 1991;49:153–179. doi: 10.1016/0163-7258(91)90054-p. [DOI] [PubMed] [Google Scholar]
- 73.Miller SB. Prostaglandins in health and disease: an overview. Semin Arthritis Rheum. 2006;36:37–49. doi: 10.1016/j.semarthrit.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 74.KURZROK R, Lieb C. Biochemical studies of human semen. II. Proc Soc Exp BiolMed. 1930;28:268–272. [Google Scholar]
- 75.Goldblatt MW. Properties of human seminal plasma. J Physiol. 1935;84:208–218. doi: 10.1113/jphysiol.1935.sp003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.von Euler US. On the specific vaso-dilating and plain muscle stimulating substances from accessory genital glands in man and certain animals (prostaglandin and vesiglandin) J Physiol. 1936;88:213–234. doi: 10.1113/jphysiol.1936.sp003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bergstrom S, Sjovall J. The isolation of prostaglandin. ActaChem Scand. 11:1086–1957. Ref Type: Journal (Full) [Google Scholar]
- 78.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 79.Samuelsson B. IDENTIFICATION OF A SMOOTH MUSCLE-STIMULATING FACTOR IN BOVINE BRAIN. PROSTAGLANDINS AND RELATED FACTORS 25. Biochim Biophys Acta. 1964;84:218–219. [PubMed] [Google Scholar]
- 80.Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009;30:174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pasinetti GM, Aisen PS. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience. 1998;87:319–324. doi: 10.1016/s0306-4522(98)00218-8. [DOI] [PubMed] [Google Scholar]
- 82.Launer L. Nonsteroidal anti-inflammatory drug use and the risk for Alzheimer’s disease: dissecting the epidemiological evidence. Drugs. 2003;63:731–739. doi: 10.2165/00003495-200363080-00001. [DOI] [PubMed] [Google Scholar]
- 83.Townsend KP, Pratico D. Novel therapeutic opportunities for Alzheimer’s disease: focus on nonsteroidal anti-inflammatory drugs. FASE J. 2005;19:1592–1601. doi: 10.1096/fj.04-3620rev. [DOI] [PubMed] [Google Scholar]
- 84.Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002;87:2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- 85.Bosetti F, Langenbach R, Weerasinghe GR. Prostaglandin E2 and microsomal prostaglandin E synthase-2 expression are decreased in the cyclooxygenase-2-deficient mouse brain despite compensatory induction of cyclooxygenase-1 and Ca2+-dependent phospholipase A2. J Neurochem. 2004;91:1389–1397. doi: 10.1111/j.1471-4159.2004.02829.x. [DOI] [PubMed] [Google Scholar]
- 86.Piomelli D, Wang JK, Sihra TS, Nairn AC, Czernik AJ, Greengard P. Inhibition of Ca2+/calmodulin-dependent protein kinase II by arachidonic acid and its metabolites. Proc Natl Acad Sci U S A. 1989;86:8550–8554. doi: 10.1073/pnas.86.21.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian J, Kim SF, Hester L, Snyder SH. S-nitrosylation/activation of COX-2 mediates NMDA neurotoxicity. Proc Natl Acad Sci U S A. 2008;105:10537–10540. doi: 10.1073/pnas.0804852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phillis JW, O’Regan MH. The role of phospholipases, cyclooxygenases, and lipoxygenases in cerebral ischemic/traumatic injuries. Crit Rev Neurobiol. 2003;15:61–90. doi: 10.1615/critrevneurobiol.v15.i1.30. [DOI] [PubMed] [Google Scholar]
- 89.Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Alvarez D, Al-Dalain S, Martinez G, Leon OS, Springer JE. Assessment of the relative contribution of COX-1 and COX-2 isoforms to ischemia-induced oxidative damage and neurodegeneration following transient global cerebral ischemia. J Neurochem. 2003;86:545–555. doi: 10.1046/j.1471-4159.2003.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ballou LR, Botting RM, Goorha S, Zhang J, Vane JR. Nociception in cyclooxygenase isozyme-deficient mice. Proc Natl Acad Sci U S A. 2000;97:10272–10276. doi: 10.1073/pnas.180319297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen C, Bazan NG. Lipid signaling: sleep, synaptic plasticity, and neuroprotection. Prostaglandins Other Lipid Mediat. 2005;77:65–76. doi: 10.1016/j.prostaglandins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 93.Bergstrom S, Carlson LA, Weeks JR. The prostaglandins: a family of biologically active lipids. Pharmacol Rev. 1968;20:1–48. [PubMed] [Google Scholar]
- 94.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50(Suppl):S423–S428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhagwat SS, Hamann PR, Still WC, Bunting S, Fitzpatrick FA. Synthesis and structure of the platelet aggregation factor thromboxane A2. Nature. 1985;315:511–513. doi: 10.1038/315511a0. [DOI] [PubMed] [Google Scholar]
- 96.Bunting S, Gryglewski R, Moncada S, Vane JR. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976;12:897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- 97.Weksler BB, Marcus AJ, Jaffe EA. Synthesis of prostaglandin 12 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977;74:3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maclntyre DE, Pearson JD, Gordon JL. Localisation and stimulation of prostacyclin production in vascular cells. Nature. 1978;271:549–551. doi: 10.1038/271549a0. [DOI] [PubMed] [Google Scholar]
- 99.Schmedtje JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272:601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 100.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 101.Solomon DH, Schneeweiss S, Glynn RJ, Kiyota Y, Levin R, Mogun H, Avorn J. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation. 2004;109:2068–2073. doi: 10.1161/01.CIR.0000127578.21885.3E. [DOI] [PubMed] [Google Scholar]
- 102.Williams ED, Karim SM, Sandler M. Prostaglandin secretion by medullary carcinoma of the thyroid. A possible cause of the associated idarrhoea. Lancet. 1968;1:22–23. doi: 10.1016/s0140-6736(68)90010-x. [DOI] [PubMed] [Google Scholar]
- 103.Sykes JA, Moddox IS. Prostaglandin production by experimental tumours and effects of anti-inflammatory compounds. Nat New Biol. 1972;237:59–61. doi: 10.1038/newbio237059a0. [DOI] [PubMed] [Google Scholar]
- 104.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 105.Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, Speizer FE. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 106.Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 107.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ., III Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 108.Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 109.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- 110.Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, Shiozaki H, Hori M. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999;94:451–455. doi: 10.1111/j.1572-0241.1999.876_e.x. [DOI] [PubMed] [Google Scholar]
- 111.Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007;26:525–534. doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- 112.Tian M, Schiemann WP. PGE2 receptor EP2 mediates the antagonistic effect of COX-2 on TGF-beta signaling during mammary tumorigenesis. FASEB J. 2010;24:1105–1116. doi: 10.1096/fj.09-141341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Madsen L, Pedersen LM, Lillefosse HH, Fjaere E, Bronstad I, Hao Q, Petersen RK, Hallenborg P, Ma T, De MR, Araujo P, Mercader J, Bonet ML, Hansen JB, Cannon B, Nedergaard J, Wang J, Cinti S, Voshol P, Doskeland SO, Kristiansen K. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PLoS One. 2010;5:e11391. doi: 10.1371/journal.pone.0011391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel DM, Rozman J, Hrabe de AM, Nusing RM, Meyer CW, Wahli W, Klingenspor M, Herzig S. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 115.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 116.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cuzzocrea S, Salvemini D. Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney Int. 2007;71:290–297. doi: 10.1038/sj.ki.5002058. [DOI] [PubMed] [Google Scholar]
- 118.Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–252. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- 119.Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salvemini D, Seibert K, Masferrer JL, Misko TP, Currie MG, Needleman P. Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation. J Clin Invest. 1994;93:1940–1947. doi: 10.1172/JCI117185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salvemini D, Masferrer JL. Interactions of nitric oxide with cyclooxygenase: in vitro, ex vivo, and in vivo studies. Methods Enzymol. 1996;269:12–25. doi: 10.1016/s0076-6879(96)69005-3. [DOI] [PubMed] [Google Scholar]
- 122.Liu Y, Borchert GL, Phang JM. Polyoma enhancer activator 3, an ets transcription factor, mediates the induction of cyclooxygenase-2 by nitric oxide in colorectal cancer cells. J Biol Chem. 2004;279:18694–18700. doi: 10.1074/jbc.M308136200. [DOI] [PubMed] [Google Scholar]
- 123.Park SW, Sung MW, Heo DS, Inoue H, Shim SH, Kim KH. Nitric oxide upregulates the cyclooxygenase-2 expression through the cAMP-response element in its promoter in several cancer cell lines. Oncogene. 2005;24:6689–6698. doi: 10.1038/sj.onc.1208816. [DOI] [PubMed] [Google Scholar]
- 124.Minghetti L, Polazzi E, Nicolini A, Creminon C, Levi G. Interferon-gamma and nitric oxide down-regulate lipopolysaccharide-induced prostanoid production in cultured rat microglial cells by inhibiting cyclooxygenase-2 expression. J Neurochem. 1996;66:1963–1970. doi: 10.1046/j.1471-4159.1996.66051963.x. [DOI] [PubMed] [Google Scholar]
- 125.Stadler J, Harbrecht BG, Di SM, Curran RD, Jordan ML, Simmons RL, Billiar TR. Endogenous nitric oxide inhibits the synthesis of cyclooxygenase products and interleukin-6 by rat Kupffer cells. J Leukoc Biol. 1993;53:165–172. doi: 10.1002/jlb.53.2.165. [DOI] [PubMed] [Google Scholar]
- 126.Swierkosz TA, Mitchell JA, Warner TD, Botting RM, Vane JR. Co-induction of nitric oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostanoids. Br J Pharmacol. 1995;114:1335–1342. doi: 10.1111/j.1476-5381.1995.tb13353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen KD, Chen LY, Huang HL, Lieu CH, Chang YN, Chang MD, Lai YK. Involvement of p38 mitogen-activated protein kinase signaling pathway in the rapid induction of the 78-kDa glucose-regulated protein in 9L rat brain tumor cells. J Biol Chem. 1998;273:749–755. doi: 10.1074/jbc.273.2.749. [DOI] [PubMed] [Google Scholar]
- 128.Tsai AL, Wei C, Kulmacz RJ. Interaction between nitric oxide and prostaglandin H synthase. Arch Biochem Biophys. 1994;313:367–372. doi: 10.1006/abbi.1994.1400. [DOI] [PubMed] [Google Scholar]
- 129.Boulos C, Jiang H, Balazy M. Diffusion of peroxynitrite into the human platelet inhibits cyclooxygenase via nitration of tyrosine residues. J Pharmacol Exp Ther. 2000;293:222–229. [PubMed] [Google Scholar]
- 130.Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J Biol Chem. 1999;274:22903–22906. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]
- 131.Tsai AL, Palmer G, Kulmacz RJ. Prostaglandin H synthase. Kinetics of tyrosyl radical formation and of cyclooxygenase catalysis. J Biol Chem. 1992;267:17753–17759. [PubMed] [Google Scholar]
- 132.Karthein R, Dietz R, Nastainczyk W, Ruf HH. Higher oxidation states of prostaglandin H synthase. EPR study of a transient tyrosyl radical in the enzyme during the peroxidase reaction. Eur J Biochem. 1988;171:313–320. doi: 10.1111/j.1432-1033.1988.tb13792.x. [DOI] [PubMed] [Google Scholar]
- 133.Floris R, Piersma SR, Yang G, Jones P, Wever R. Interaction of myeloperoxidase with peroxynitrite. A comparison with lactoperoxidase, horseradish peroxidase and catalase. Eur J Biochem. 1993;215:767–775. doi: 10.1111/j.1432-1033.1993.tb18091.x. [DOI] [PubMed] [Google Scholar]
- 134.Boulos C, Jiang H, Balazy M. Diffusion of peroxynitrite into the human platelet inhibits cyclooxygenase via nitration of tyrosine residues. J Pharmacol Exp Ther. 2000;293:222–229. [PubMed] [Google Scholar]
- 135.Shi W, Hoganson CW, Espe M, Bender CJ, Babcock GT, Palmer G, Kulmacz RJ, Tsai A. Electron paramagnetic resonance and electron nuclear double resonance spectroscopic identification and characterization of the tyrosyl radicals in prostaglandin H synthase 1. Biochemistry. 2000;39:4112–4121. doi: 10.1021/bi992561c. [DOI] [PubMed] [Google Scholar]
- 136.Deeb RS, Resnick MJ, Mittar D, McCaffrey T, Hajjar DP, Upmacis RK. Tyrosine nitration in prostaglandin H(2) synthase. J Lipid Res. 2002;43:1718–1726. doi: 10.1194/jlr.m200199-jlr200. [DOI] [PubMed] [Google Scholar]
- 137.Goodwin DC, Gunther MR, Hsi LC, Crews BC, Eling TE, Mason RP, Marnett LJ. Nitric oxide trapping of tyrosyl radicals generated during prostaglandin endoperoxide synthase turnover. Detection of the radical derivative of tyrosine 385. J Biol Chem. 1998;273:8903–8909. doi: 10.1074/jbc.273.15.8903. [DOI] [PubMed] [Google Scholar]
- 138.Upmacis RK, Deeb RS, Hajjar DP. Regulation of prostaglandin H2 synthase activity by nitrogen oxides. Biochemistry. 1999;38:12505–12513. doi: 10.1021/bi983049e. [DOI] [PubMed] [Google Scholar]
- 139.Deeb RS, Hao G, Gross SS, Laine M, Qiu JH, Resnick B, Barbar EJ, Hajjar DP, Upmacis RK. Heme catalyzes tyrosine 385 nitration and inactivation of prostaglandin H2 synthase-1 by peroxynitrite. J Lipid Res. 2006;47:898–911. doi: 10.1194/jlr.M500384-JLR200. [DOI] [PubMed] [Google Scholar]
- 140.Upmacis RK, Deeb RS, Hajjar DP. Oxidative alterations of cyclooxygenase during atherogenesis. Prostaglandins Other Lipid Mediat. 2006;80:1–14. doi: 10.1016/j.prostaglandins.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 141.Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am J Physiol Heart Circ Physiol. 2005;288:H371–H381. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- 142.Deeb RS, Cheung C, Nuriel T, Lamon BD, Upmacis RK, Gross SS, Hajjar DP. Physical evidence for substrate binding in preventing cyclooxygenase inactivation under nitrative stress. J Am Chem Soc. 2010;132:3914–3922. doi: 10.1021/ja910578y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zou M, Martin C, Ullrich V. Tyrosine nitration as a mechanism of selective inactivation of prostacyclin synthase by peroxynitrite. Biol Chem. 1997;378:707–713. doi: 10.1515/bchm.1997.378.7.707. [DOI] [PubMed] [Google Scholar]
- 144.Zou MH, Ullrich V. Peroxynitrite formed by simultaneous generation of nitric oxide and superoxide selectively inhibits bovine aortic prostacyclin synthase. FEBS Lett. 1996;382:101–104. doi: 10.1016/0014-5793(96)00160-3. [DOI] [PubMed] [Google Scholar]
- 145.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 147.Hajjar D, Lander H, Pearce S, Upmacis R, Pomerantz KB. Nitric oxide enhances prostaglandin-H synthase-1 activity by a hemeindependent mechanism: evidence implicating nitrosothiols. J Am Chem Soc. 1995;117:3340–3346. Ref Type: Journal (Full) [Google Scholar]
- 148.Kennedy TA, Smith CJ, Marnett LJ. Investigation of the role of cysteines in catalysis by prostaglandin endoperoxide synthase. J Biol Chem. 1994;269:27357–27364. [PubMed] [Google Scholar]
- 149.Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 1996;93:15069–15074. doi: 10.1073/pnas.93.26.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–1970. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 151.van dV, Hoen PA, Wong PS, Bast A, Cross CE. Formation of S-nitrosothiols via direct nucleophilic nitrosation of thiols by peroxynitrite with elimination of hydrogen peroxide. J Biol Chem. 1998;273:30255–30262. doi: 10.1074/jbc.273.46.30255. [DOI] [PubMed] [Google Scholar]
- 152.Xu L, Han C, Lim K, Wu T. Activation of cytosolic phospholipase A2alpha through nitric oxide-induced S-nitrosylation. Involvement of inducible nitric-oxide synthase and cyclooxygenase-2. J Biol Chem. 2008;283:3077–3087. doi: 10.1074/jbc.M705709200. [DOI] [PubMed] [Google Scholar]