Abstract
Background
The membrane bound bile acid (BA) receptor, TGR5, is located on myenteric, cholinergic and nitrergic neurons in colon and proximal small intestine. Our aim was to assess the association of genetic variation in TGR5 and small bowel (SBT) and colonic transit.
Methods
In 230 healthy controls and 414 patients with lower functional GI disorders (FGID: irritable bowel syndrome [IBS]-alternators [Alt] 84, IBS-constipation [IBS-C] 157, IBS-diarrhea [IBS-D] 173), we tested the association between TGR5 SNP rs11554825 (minor allele frequency 41%) with symptom phenotype (total cohort) and intermediate phenotype (SBT or colonic transit by radioscintigraphy) which was available in 213 people in this cohort. The association with symptom phenotype was assessed using logistic regression, while the association with colonic filling at 6h [CF6], and colonic transit (geometric center [GC] at 24h) was assessed using ANCOVA, in each instance assuming a dominant genetic model.
Key Results
There was no significant association with symptom phenotype. We observed a potential association of SNP rs11554825 with overall transit: CF6 (p=0.061) and GC24 (p=0.083). The association of the SNP with CF6 in the IBS-D subgroup (p=0.017) indicated the TC/CC subgroup had an average 50% faster SBT compared to the TT subgroup. In IBS-D patients, GC24 was not significantly associated with rs11554825 (TC/CC vs TT).
Conclusions and Inferences
Variation in TGR5 may contribute to altered SBT and colonic transit in lower FGID. Further studies are required to characterize the potential role of BA receptor TGR5 in the mechanism and treatment of bowel dysfunction in lower FGID.
Keywords: GpBAR1, diarrhea, intestine, colon
Bile acids (BAs) have significant effects on diverse gastroenterological, hepatic, endocrine and metabolic functions; BAs regulate cells by activating nuclear and membrane-bound receptors that regulate the expression of biosynthetic enzymes and binding proteins for BAs. However, BAs also rapidly activate signaling pathways and cellular functions without the requirement of transcription; these pathways include adenylate cyclase-cAMP generation and calcium mobilization and generation of a series of kinases, which are involved in some of the secretory and motor effects of BAs in the digestive tract. At the level of the intestine, the ileal bile acid transporter or IBAT (also called the apical sodium-dependent bile acid transporter, ASBT) which is responsible for the active absorption of BAs in the ileum and the maintenance of BA homeostasis. After BAs are absorbed, they bind to the nuclear farnesoid-X receptors in the enterocytes, and this controls the synthesis of proteins such as ileal bile acid binding protein and FGF-19 (1,2), the latter providing negative feedback to the hepatic synthesis of bile acids.
There is a second membrane-bound BA receptor, TGR5 (also known as GpBAR1), which is ubiquitous (3,4) and is activated by primary and secondary BAs to generate intracellular cAMP (4). TGR5 has been associated with several metabolic functions including the control of lipids and obesity (5), and the potential effects of bile acids on glycemic control (6) through the BA activation of TGR5 receptors on the L cells of the intestine that produce peptides such as the incretin glucagon-like peptide-1 (GLP-1) which may influence glycemic control, peptide YY (PYY) and oxyntomodulin which affect gut motility (7).
Poole et al. recently reported that TGR5 is expressed by myenteric, cholinergic and particularly nitrergic neurons in the colon as well as in the proximal small intestine; this observation suggested that BAs may alter intestinal and colonic motility (8). Indeed, the same authors showed that the secondary BA, deoxycholic acid, which is a GpBAR1 agonist, caused a rapid and sustained inhibition of spontaneous phasic activity of isolated segments of ileum and colon by a neurogenic, cholinergic and nitrergic mechanism, and delayed gastric and intestinal transit (8).
Given these observations in animal and in vitro studies, we wished to test the hypothesis that genetic variation in TGR5 is associated with altered small bowel and colonic transit. Specifically, given the inhibition of transit when BA activates TGR5, we considered that an abnormally functioning TGR5 receptor (caused by a functional genetic variation in TGR5 gene) would reduce the inhibitory effect of endogenous BAs on transit and would, therefore, be associated with acceleration of transit relative to the wild type variant. Our aim was to assess the association of genetic variation in TGR5 and colonic and small bowel transit in humans who were either healthy volunteers or patients with lower functional gastrointestinal diseases (FGID).
METHODS
Design
In a cohort of 230 healthy controls and 414 patients with lower FGID, we tested the association between TGR5 SNP rs11554825 (located in exon 1, minor allele frequency 41%) with symptom phenotype and intermediate phenotype (colonic transit) in health and FGID based on Rome II criteria [irritable bowel syndrome (IBS)-alternators (Alt) 84, IBS-constipation (IBS-C) or functional constipation (FC) 157, IBS-diarrhea (IBS-D) or functional diarrhea (FD) 173]. The designation of symptom subgroups was based on a standard bowel disease questionnaire (9) with specific questions a priori identified in the instrument to characterize the FGID sub-phenotype. Small bowel or colonic transit (by radioscintigraphy) was available in 213 people in this cohort.
Subjects
Genomic DNA and phenotype data were collected with written informed consent in previous studies from 2000 to 2010 (10–16). The Mayo Clinic Institutional Review Board had reviewed and approved use of the collected data for this study. All participants had authorized use of medical records and genomic DNA for research.
Gastrointestinal and Colonic Transit
An established, validated scintigraphic method was used to measure gastrointestinal and colonic transit as previously described. Colonic transit by scintigraphy is a valid biomarker of colonic dysmotility. The conduct and performance characteristics of this test are summarized elsewhere (17–20).
During transit studies, participants continued estrogen replacement, birth control pills or depot injection, stable doses of thyroid replacement, low-dose aspirin, and selective serotonergic antidepressants. Exclusion criteria included organic diseases that might explain the patients’ symptoms and use of medication for IBS or bowel dysfunction within 7 days before or during the study.
The primary endpoint of colonic transit is the geometric center at 24 hours (GC24). Secondary endpoints were the colonic GC at 8 and 48 hours, gastric emptying and small bowel transit. The GC is the weighted average of count percentages in the different colonic regions: ascending (AC), transverse (TC), descending (DC), rectosigmoid (RS), and stool; the weighting factors are 1 to 5 respectively.
A change in colonic GC24 of 1 unit is associated with a discernible (~0.65 point) change in stool consistency on a validated 7-point stool form scale.
Genotyping
Genomic DNA was isolated from whole blood shortly after blood draw using standard methods and stored at −80°C until genotyping. Genotyping for TGR5 SNP rs11554825 (located in chromosome 2 position 218,834,054, exon 1, with minor allele frequency in our patient cohort of 41%) was performed using TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, CA) per the manufacturer’s instructions. Following PCR amplification, end reactions were analyzed using ABI 7300FAST Real-Time PCR System by Sequence Detection Software (Applied Biosystems). According to this genotyping, T is the major allele and C the minor allele.
Statistical Analysis
Hardy-Weinberg equilibrium (HWE) for the genotype distribution of the TGR5 SNP was assessed using Weir’s exact test (21). The association with symptom phenotype (healthy volunteers vs. FGID overall, and separately, vs. FGID subtypes) was assessed using logistic regression models incorporating gender as a covariate, the later model employed a generalized logit link with healthy volunteers as the reference category. The association with colonic filling at 6 hours (CF6), and colonic transit [geometric center (GC) at 24 hours] was assessed using analysis of covariance (ANCOVA) models incorporating symptom phenotype (HV, IBS-C, IBS-D, and IBS-A) as a covariate. In particular, ANCOVA models which included a genotype by symptom phenotype interaction term were also examined to assess potential differential genotype associations by symptom phenotype. A dominant genetic model coding (TC/CC vs. TT) was used in both the logistic and ANCOVA analyses. The reported p-values are unadjusted for examining the two endpoints of interest nor for these specific assessments within the 4 symptom phenotype subgroups (IBS-Alt, IBS-C, IBS-D, and healthy subjects).
RESULTS
Hardy-Weinberg Equilibrium (HWE) for Candidate SNP
TGR5 SNP rs11554825 in our entire cohort was in HWE relative to published data (22).
Demographics and Genotypes of Symptom and Transit Cohorts
Demographic characteristics and genotyping information of the full cohort of 644 participants (414 patients, 230 healthy volunteers) and the subset of 213 with colonic transit are shown in Tables I and II, respectively. There was a significant association of having a transit study with age and gender, but not the TGR5 genotype, with increasing odds for a transit study in females (relative to males) and a decreasing odds with older age. However, incorporating symptom phenotype in the model “explained” the association with gender, although not surprisingly, the odds for having a transit study were significantly increased in each subtype (relative to healthy controls).
Table I.
Demographics and Genotypes of All Participants
| Group | Healthy | IBS-Alt | IBS-C | IBS-D | ||||
|---|---|---|---|---|---|---|---|---|
| Genotype | TC/CC | TT | TC/CC | TT | TC/CC | TT | TC/CC | TT |
| N | 154 | 76 | 55 | 29 | 108 | 49 | 112 | 61 |
| Gender n (%F) | 108(70) | 54(71) | 51(93) | 26(90) | 106(98) | 44(89) | 90(80) | 52(85) |
| Mean(±SEM) Age | 37 ± 1 | 36 ± 2 | 41 ± 2 | 40 ± 2 | 46± 1 | 44 ± 2 | 45 ± 1 | 46 ± 2 |
Table II.
Demographics and Genotypes of Participants with Transit Measurements
| Group | Healthy | IBS-Alt | IBS-C | IBS-D | ||||
|---|---|---|---|---|---|---|---|---|
| Genotype | TC/CC | TT | TC/CC | TT | TC/CC | TT | TC/CC | TT |
| N | 32 | 6 | 18 | 7 | 46 | 24 | 50 | 30 |
| Gender n (%F) | 26(81) | 6(100) | 18(100) | 7(100) | 46(100) | 23(96) | 39(78) | 23(77) |
| Mean(±SEM)Age | 34.0±1.7 | 33.3±6.1 | 38.3±2.8 | 34.1±4.3 | 41.2±1.4 | 34.8±2.2 | 42.8±2.0 | 41.1±3.0 |
| Mean(±SEM)BMI | 26.4±1.0 | 24.4±1.1 | 27.9±1.1 | 29.1±1.4 | 25.6±0.7 | 25.6±0.6 | 27.2±0.8 | 28.0±1.0 |
Association in Overall Cohort of TGR5 SNP with Symptom Phenotypes and Transit Measurements
There was no significant association with symptom phenotype (Table III).
Table III.
Prevalence of Symptom Phenotype in the Lower FGID Cohort
| IBS-Alt | FC and IBS-C | FD and IBS-D | Healthy Controls |
|||||
|---|---|---|---|---|---|---|---|---|
| Genotype | TC/CC | TT | TC/CC | TT | TC/CC | TT | TC/CC | TT |
| N (% of specific phenotype) | 55 (65.5) | 29 (34.5) | 108 (68.8) | 49 (31.2) | 112 (64.7) | 61 (35.3) | 154 (67.1) | 76 (32.9) |
| % of total genotype with specified phenotype | 12.8 | 13.5 | 25.2 | 22.8 | 26.1 | 28.4 | 35.9 | 35.4 |
FC= functional constipation; FD= functional diarrhea
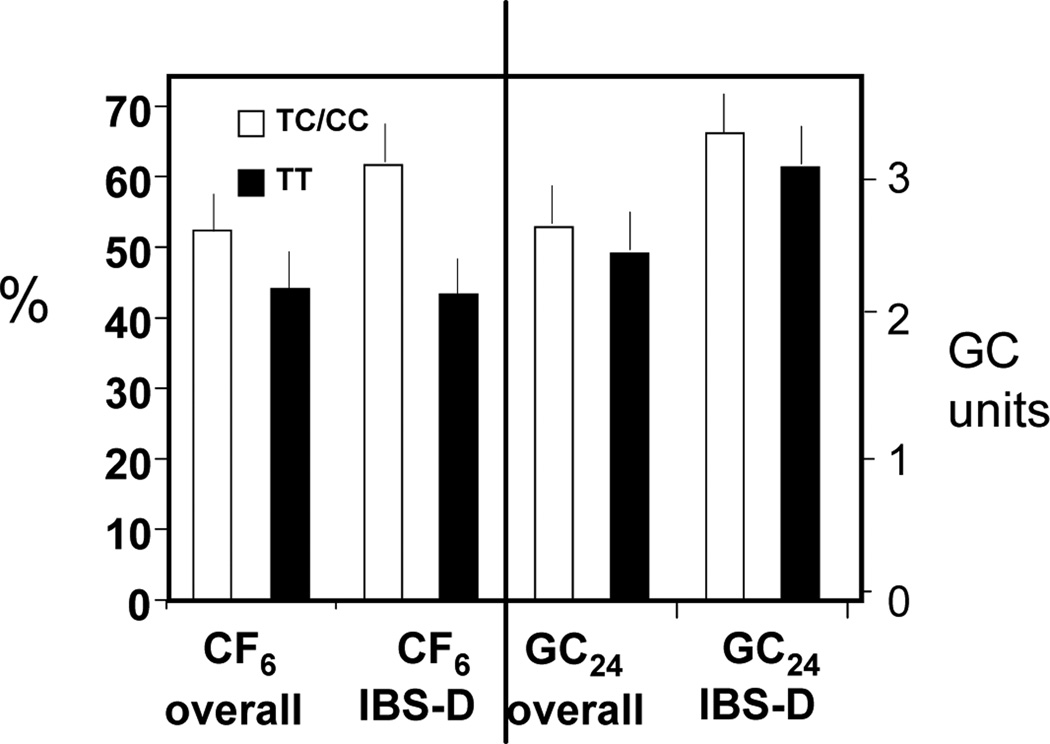
We observed a modest overall association of SNP rs11554825 with transit in the entire cohort of participants who underwent transit: CF6 (p=0.061) and colonic GC24 (p=0.083) (Figure 1). The association with CF6 was most pronounced in the group of patients with IBS-D (p=0.017) with the TC/CC subgroup having an average 50% faster small bowel transit time compared to CC subgroup. In contrast, colonic transit in IBS-D patients was not significantly different in TC/CC compared to TT subgroups (p=0.35).
Figure 1.
Association of TGR5 SNP rs11554825 with small bowel and colonic transit in the overall cohort of patients and healthy controls who underwent transit measurement and, specifically, in patients with IBS-D. A modest overall association is noted between SNP rs11554825 and transit in the entire IBS cohort for Colonic filling at 6 h (CF6, p=0.061) and colonic transit (GC24, p=0.083). The association with CF6 was most pronounced in IBS-D (p=0.017) with the TC/CC subgroup having an average 50% faster small bowel transit time compared to the TT subgroup. In contrast to the numerical difference in CF6 in the TC/CC subgroup compared to TT subgroup in IBS-D, colonic transit in IBS-D patients was not significantly different in the 2 subgroups.
DISCUSSION
Our study shows that there is an association between the TGR5 genotype variations (rs11554825) and small intestinal transit in IBS-D patients, and possibly with both small intestinal and colonic transit in a cohort of patients with lower FGID and healthy controls.
These findings are consistent with recent studies showing that TGR5 receptors are located on nitrergic neurons whose activation results in inhibition of ileal and colonic motility and inhibition of gastric and small intestinal transit (8). The TT genotype of rs11554825 (T being the major allele) is associated with numerically slower small bowel and colonic transit compared to the TC/CC subgroup, though neither reached statistical significance in the entire IBS group or in the IBS-D group if adjustment for multiple endpoints and 4 subgroups was applied. Our data are consistent with the hypothesis that the TC/CC subgroup alters the function of the TGR5 gene, reduces its expression, and hence reduces the nitrergic inhibition of intestinal motility and transit, which manifests as faster transit in the TC/CC subgroup. The functional significance of SNP rs11554825 is unclear, even though Hov et al. (22) showed it was significantly associated with ulcerative colitis (p= 8.5*10−7). In fact, the coding part of TGR5 is entirely in exon 2, and using confocal microscopy, flow cytometry and a cAMP-sensitive luciferase assay, five of the non-synonymous mutations (W83R, V178M, A217P, S272G and Q296X) in exon 2 were found to reduce or abolish TGR5 function (22).
Our participant cohort is representative of adults in the Unites States as there was no deviation from the Hardy-Weinberg equilibrium. There was a significant association of having a transit study with age and gender, but not with the TGR5 genotype. There were increased odds for undergoing transit measurement in females (relative to males); however, incorporating symptom phenotype in the model accounted for the association with gender and this is attributable to the increased prevalence of IBS in females. The decreased odds of undergoing transit measurements with older age are consistent with the higher prevalence of lower FGIDs in younger adults. It was not surprising that the odds for having a transit study were significantly increased in each subtype of lower FGID relative to healthy controls.
In experimental animal studies, activation of TGR5 was associated with delayed gastric and small intestinal transit (8). The mechanism of this effect is not fully understood (23); however, it is conceivable that this results from activation of TGR5 receptors on enteroendocrine cells, such as L cells, leading to the release of glucagon-like peptide-1 (GLP-1) with subsequent inhibition of proximal gut transit. Alternatively, activation of neuronal mechanisms such as intrinsic primary afferent neurons and nitrergic neurons ultimately inhibit intestinal transit.
In contrast, bile acids, such as chenodeoxycholic acid, induce acceleration of colonic transit as shown in health and IBS-D patients (24,25), and induce high amplitude propagated contractions (26), presumably by a different mechanism that may be activated by a higher dose of the intraluminal bile acids than the dose that stimulates the neural or hormonal mechanisms that retard gastric and small intestinal transit. It is interesting to note that administrations of the bile acid, chenodeoxycholate, and the bile acid sequestrant, colesevelam, in humans were associated with retardation of gastric emptying (24,25), suggesting that both pathways that result in retardation of gastric emptying and acceleration or delay of colonic transit in IBS-C and IBS-D respectively may occur concurrently. There is evidence that another bile acid sequestrant, colestimide, increased secretion of GLP-1 in patients with type 2 diabetes mellitus, and this effect is mediated through the nuclear receptor farnesoid X receptor (FXR) and the membrane receptor, TGR5. These actions may enhance glycemic control either through the effects of GLP-1 on gastric emptying or FXR-mediated regulation of energy substrate mobilization and storage (27–29).
Our study should be regarded as hypothesis-generating. Thus our hypothesis is that TGR5 impacts small bowel and colonic transit in humans. The functional significance of rs11554825 is still not reported and, although it is in strong linkage disequilibrium with SNPs in the 5’ untranslated region of the TGR5 gene, it has not been demonstrated to be in linkage disequilibrium with the functional SNPs in exon 2 which is the section of the gene that leads to the transcribed protein (22). It is also possible that SNP rs11554825 is in linkage disequilibrium with the causative genetic variation in TGR5 that is responsible for the alteration of gastrointestinal motility and that genetic variant has yet to be identified.
The strengths of our study include the use of the standard, validated measurement of small bowel and colonic transit as an intermediate phenotype with defined coefficient of variation, and the relatively large number of participants included in the genetic association analysis. The weaknesses are: first, that SNP rs11554825 is not yet proven to have functional consequences and therefore it may serve only as a marker of a genetic association; second, the symptom phenotype information is based on a bowel disease questionnaire with insufficient information to more accurately characterize bowel frequency and consistency; and third, that the associations with colonic filling and colonic transit in the IBS group as a whole are not statistically significant, and the subgroup (IBS-D) analysis (unadjusted for 4 groups) is not statistically significant. Our data are therefore hypothesis-generating.
In summary, variation in TGR5 may contribute to altered small bowel and colonic transit in patients with lower FGID, particularly IBS-D patients. Further studies are indicated to characterize the potential role of the bile acid receptor, TGR5, in the mechanism and treatment of bowel dysfunction in lower FGID.
Acknowledgements
This work was funded by grant RO1-DK079866 from National Institutes of Health to Dr. M. Camilleri and by Mayo Clinic CTSA grant (RR24150). We thank Cindy Stanislav for excellent secretarial assistance.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
Authors’ contributions: M. Camilleri: study conceptualization, writing protocol and paper; M.I. Vazquez-Roque: writing protocol and paper; P. Carlson: technologist genotyping; D. Burton: technologist transit measurements; B.S. Wong: writing paper; A.R. Zinsmeister: database management and statistical analysis.
Contributor Information
Michael Camilleri, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic College of Medicine, Rochester, Minnesota
Maria I. Vazquez-Roque, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic College of Medicine, Rochester, Minnesota
Paula Carlson, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic College of Medicine, Rochester, Minnesota
Duane Burton, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic College of Medicine, Rochester, Minnesota.
Banny S. Wong, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic College of Medicine, Rochester, Minnesota
Alan R. Zinsmeister, Division of Biomedical Statistics and Informatics, Department of Health Sciences Research, Mayo Clinic College of Medicine, Rochester, Minnesota
REFERENCES
- 1.Claudel T, Staels B, Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25:2020–2030. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 3.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 4.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 5.Kuipers F, Stroeve JH, Caron S, Staels B. Bile acids, farnesoid X receptor, atherosclerosis and metabolic control. Curr Opin Lipidol. 2007;18:289–297. doi: 10.1097/MOL.0b013e3281338d08. [DOI] [PubMed] [Google Scholar]
- 6.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 7.Bajor A, Gillberg PG, Abrahamsson H. Bile acids: short and long term effects in the intestine. Scand J Gastroenterol. 2010;45:645–664. doi: 10.3109/00365521003702734. [DOI] [PubMed] [Google Scholar]
- 8.Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22:814–825. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talley NJ, Phillips SF, Wiltgen CM, et al. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–1479. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 10.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2009;8:159–165. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andresen V, Camilleri M, Kim HJ, et al. Is there an association between GNbeta3-C825T genotype and lower functional gastrointestinal disorders? Gastroenterology. 2006;130:1985–1994. doi: 10.1053/j.gastro.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri CE, Carlson PJ, Camilleri M, et al. A study of candidate genotypes associated with dyspepsia in a U.S. community. Am J Gastroenterol. 2006;101:581–592. doi: 10.1111/j.1572-0241.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M, Carlson P, Zinsmeister AR, et al. Mitochondrial DNA and gastrointestinal motor and sensory functions in health and functional gastrointestinal disorders. Am J Physiol. 2009;296:G510–G516. doi: 10.1152/ajpgi.90650.2008. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Camilleri M, Carlson PJ, et al. Association of distinct alpha(2) adrenoceptor and serotonin transporter polymorphisms with constipation and somatic symptoms in functional gastrointestinal disorders. Gut. 2004;53:829–837. doi: 10.1136/gut.2003.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manabe N, Wong BS, Camilleri M, Burton D, McKinzie S, Zinsmeister AR. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22 doi: 10.1111/j.1365-2982.2009.01442.x. 293-e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao AS, Wong BS, Camilleri M, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549–1558. doi: 10.1053/j.gastro.2010.07.052. and Supplementary Material at www.gastrojournal.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton DD, Camilleri M, Mullan BP, Forstrom LA, Hung JC. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med. 1997;38:1807–1810. [PubMed] [Google Scholar]
- 18.Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 19.Deiteren A, Camilleri M, Bharucha AE, Burton D, McKinzie S, Rao AS, Zinsmeister AR. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–423. e95. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camilleri M. Scintigraphic biomarkers for colonic dysmotility. Clin Pharmacol Ther. 2010;87:748–753. doi: 10.1038/clpt.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weir BS. Hardy-Weinberg Disequilibrium. In: Weir BS, editor. Genetic Data Analysis II: Methods for Discrete Population Genetic Data. Sunderland, MA: Sinauer Associates Publishers; 1996. pp. 98–101. [Google Scholar]
- 22.Hov JR, Keitel V, Laerdahl JK, et al. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS One. 2010;5:e12403. doi: 10.1371/journal.pone.0012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keely SJ. Missing link identified: GpBAR1 is a neuronal bile acid receptor. Neurogastroenterol Motil. 2010;22:711–717. doi: 10.1111/j.1365-2982.2010.01528.x. [DOI] [PubMed] [Google Scholar]
- 24.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159–165. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao AS, Wong BS, Camilleri M, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549–1558. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 27.Fonseca VA, Handelsman Y, Staels B. Colesevelam lowers glucose and lipid levels in type 2 diabetes: the clinical evidence. Diabetes Obes Metab. 2010;12:384–392. doi: 10.1111/j.1463-1326.2009.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prawitt J, Caron S, Staels B. Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr Diab Rep. 2011 Mar 24; doi: 10.1007/s11892-011-0187-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knop FK. Bile-induced secretion of glucagon-like peptide-1: pathophysiological implications in type 2 diabetes? Am J Physiol. 2010;299:E10–E13. doi: 10.1152/ajpendo.00137.2010. [DOI] [PubMed] [Google Scholar]