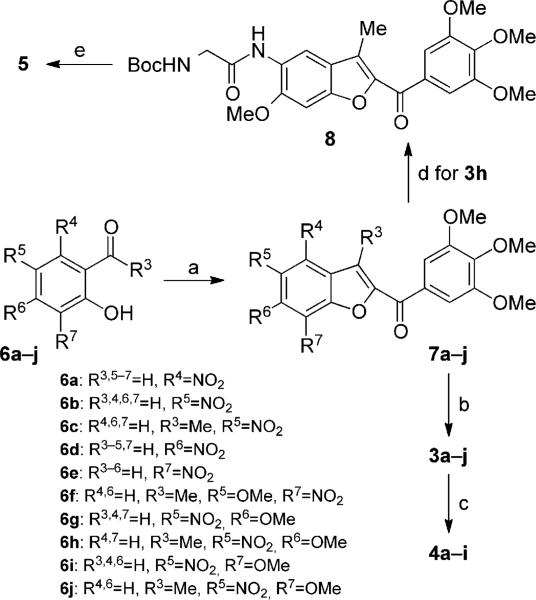
Scheme 1.
Synthesis of compounds 3a–j, 4a–i, and 5. Reagents and conditions: a) 1-(3,4,5-trimethoxyphenyl)-2-bromoethanone, K2CO3, (CH3)2CO, reflux, 18 h; b) Fe, HCl (37% in H2O), EtOH, reflux, 3 h; c) α-bromoacrylic acid, EDCI, HOBt, DMF, RT, 18 h; d) N-Boc-glycine, EDCI, HOBt, DMF, RT, 12 h; e) 3m HCl in EtOAc, RT, 3 h.