Abstract
PEGylated dendron coils (PDCs) were investigated as a novel potential nanocarrier platform. PDCs self-assembled into micelles at lower CMCs than linear copolymer counterparts by 1–2 orders of magnitude, due to the unique architecture of dendrons. MD simulations also supported thermodynamically favourable self-assembly mediated by dendrons.
Self-assembled molecular nanoconstructs with controllable physical, chemical, and biological properties represent one of the most versatile platforms for drug delivery.1 Above their critical micelle concentrations (CMCs), linear, branched, and hyperbranched amphiphilic block copolymers can assemble into thermodynamically stable supramolecular structures of different sizes, morphologies, and properties.2 Among those copolymers, dendron-coils (DC) have attracted a great deal of scientific interest due to their unique structure and properties. A DC is comprised of a dendron, a branch of a dendrimer, and flexible hydrophilic and/or hydrophobic linear polymers, which allows us to engineer its amphiphilicity in a form suitable for self-assembly and molecular delivery.3 The monodisperse, highly branched molecular architecture of the dendron imparts a precise control over the peripheral functional groups and multivalency, as in dendrimers.4 Uniquely, DCs have been reported to self-assemble into micelles at CMCs as low as in the order of 10−8 M, which are expected to be significantly lower than CMCs of linear-block copolymers with similar hydrophilic–lipophilic balances (HLBs).5 The high HLB is important for a nanocarrier to achieve a large surface coverage by a hydrophilic layer, e.g., poly(ethylene glycol) (PEG), to maximize its in vivo circulation time while minimizing its non-specific interactions with biological components.6 Although DC-based micelles are ideally suited for nanocarriers, the role of dendrons should be explored by systematic and quantitative studies.
In this study, we perform a systematic quantitative study of the dendron role during the self-assembly of supramolecular structures, by comparing the micelle morphology and CMCs when formed from DCs and linear polymers with the same HLBs. We support our experiments with detailed atomistic molecular dynamics (MD) simulations to clarify the self-assembly conditions. Our results indicate that the DC-based micelles exhibit a significantly greater thermodynamic stability and surface coverage of hydrophilic layers than the linear polymer-based micelles, demonstrating great potential as a nanocarrier platform.
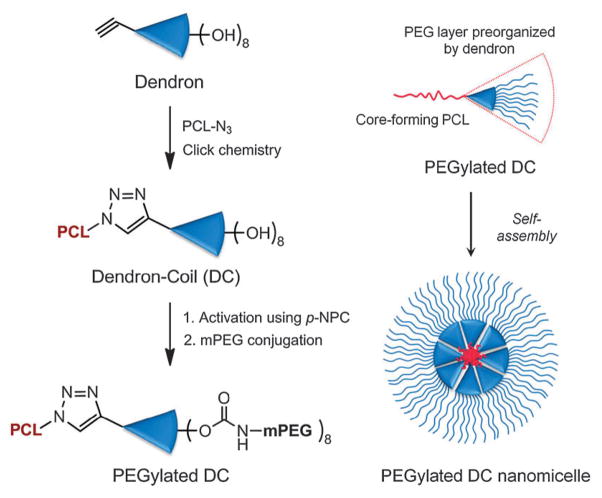
We have synthesized four novel PEGylated DCs (PDCs) that are designed to be suitable as drug delivery vehicles. Our biocompatible PDCs consist of three functional components: (1) poly(ε-caprolactone) (PCL), used as a hydrophobic, biodegradable core-forming block; (2) biodegradable 2,2-bis(hydroxyl-methyl)propionic acid generation 3 (G3) dendron with an acetylene core, chosen to mediate the core- and shell-forming blocks through selective click chemistry and to achieve a localized high density of peripheral functional groups; and (3) biocompatible methoxy-terminated PEG (mPEG) forming the hydrophilic corona. We have also chosen two different molecular weights of PCL and mPEG (3.5 and 14 kDa for PCL; 2 and 5 kDa for mPEG) to vary the HLB values of the resulting PDCs in a wide range. The synthetic route to produce the PDCs is summarized in Fig. 1 (see details in ESI†). Similarly, the linear-block copolymer counterparts were prepared. All 8 amphiphilic copolymers (4 dendron-based and 4 linear as listed in Table 1) were successfully synthesized with low polydispersity indices (PDIs lower than 1.4), as confirmed using FT-IR, 1H-NMR, and GPC at each reaction step (Fig. S3–S7 and Table S1, ESI†).
Fig. 1.
Schematic diagram of preparation of a nanomicelle from PEGylated DCs synthesized through click chemistry between PCL and G3 dendron, followed by mPEG conjugation.
Table 1.
Critical micelle concentrations (CMCs) of the amphiphilic copolymers with various hydrophilic–lipophilic balances (HLBs)
| Sample | HLBa | HL-ratiob | CMC/mg L−1 | CMC (10−7 M) |
|---|---|---|---|---|
| PCL3.5K-mPEG2K | 7.27 | 36 : 64 | 1.32 | 2.40 |
| PCL3.5K-mPEG5K | 11.76 | 59 : 41 | 3.29 | 3.75 |
| PCL14K-mPEG2Kc | 2.50 | 13 : 87 | — | — |
| PCL14K-mPEG5K | 5.26 | 26 : 74 | 1.62 | 0.82 |
| PCL3.5K-G3-mPEG2K | 16.56 | 77 : 23 | 4.87 | 3.02 |
| PCL3.5K-G3-mPEG5K | 18.42 | 91 : 9 | 12.59 | 3.52 |
| PCL14K-G3-mPEG2K | 10.93 | 52 : 48 | 1.62 | 0.65 |
| PCL14K-G3-mPEG5K | 14.90 | 74 : 26 | 4.74 | 1.17 |
HLB = 20 MH/(MH + ML), where MH is the mass of the hydrophilic block and ML is the mass of the lipophilic block.9 The dendron is considered hydrophilic.
Hydrophilic–lipophilic ratio.
PCL14K-mPEG2K could not be tested due to its poor water solubility.
To directly assess the thermodynamic stability of the molecular assemblies, the CMC of each amphiphilic copolymer was measured as shown in Fig. S8 (ESI†).7 A low CMC is particularly important for a nanocarrier in the bloodstream, due to an immediate, large dilution factor upon injection. Table 1 summarizes the measured CMCs, HLBs, and hydrophilic– lipophilic (HL) ratios of the 8 copolymers. The CMCs of the linear-block copolymers are in good agreement with the previous reports5d,8 and are comparable in magnitude to those of the PDCs, which have 2–4 fold higher HLBs.
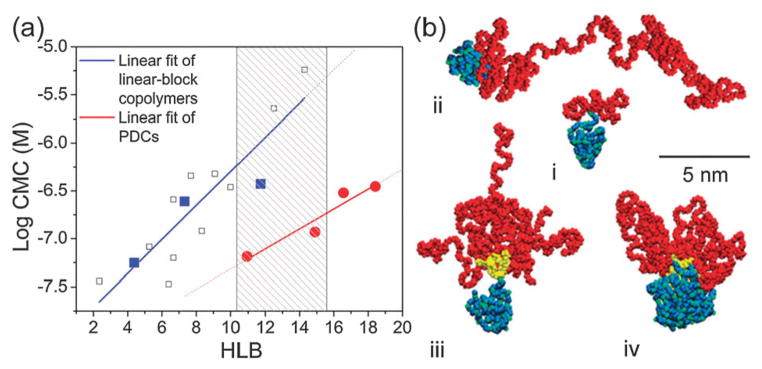
Fig. 2a shows a nearly linear correlation between CMC and HLB, observed for both linear and dendron-based copolymers. CMCs for linear-block copolymers composed of the same polymer blocks are included10 to illustrate the large differences (1–2 orders of magnitude) between CMC values observed for PDC micelles and linear-block copolymer micelles at similar HLBs. These data provide solid evidence that the pre-organized molecular architecture of the multiple PEGs and a single PCL mediated by a dendron facilitates the formation of remarkably stable PDC self-assemblies with large hydrophilic proportions. This is well illustrated on the PDC PCL3.5K-G3-mPEG5K (CMC of 3.5 × 10−7 M and HLB of 18.4) that is almost twice more hydrophilic than its linear counterpart PCL3.5K-mPEG5K (CMC of 3.8 × 10−7 M and HLB of 11.8). By measuring both dendron and linear copolymers at large HLBs (16–18), the CMCs of the linear-block copolymers (~10−5 M)5c,d are estimated to be up to two orders of magnitude larger than those of the PDCs (~10−7 M).
Fig. 2.
(a) Linear relationship between CMC and HLB for (●) PDCs and (■) linear-block copolymers. Additional data points for the linear-block copolymers (□) were acquired from the literature that used the same polymer blocks. Note that, at the same HLB (10–15), the PDC self-assemblies maintain CMCs that are 1–2 orders of magnitude lower than the linear counterparts, as highlighted in the shaded region. (b) MD simulations of (i) PCL3.5K-mPEG2K, (ii) PCL3.5K-mPEG16K, (iii) PCL3.5K-G3-mPEG2K, and (iv) PCL14K-G3-mPEG2K molecules after 5 ns in water (PCL: blue, G3-dendron: yellow, PEG: red). Water is not shown.
We performed MD simulations of the copolymers to clarify how their molecular architectures influence the micelle selfassembly. Fig. 2b illustrates the structures of individual PCL3.5K-mPEG2K, PCL3.5K-mPEG16K, PCL3.5K-G3-mPEG2K, and PCL14K-G3-mPEG2K copolymers obtained after 5 ns of equilibration in water at T = 300 K. All the hydrated copolymers have a compact PCL block and relatively extended conformations of the PEG blocks. PCL3.5K-G3-mPEG2K shows a relatively stable conical shape, compared to PCL3.5K-mPEG16K with the identical HLB, since the dendron always keeps the PEG blocks close to the folded PCL block. The pre-organization of multiple PEG blocks attached to the surface of each PDC is favourable in the micelle self-assembly, giving a very small entropic cost in the self-assembly (see details in ESI†). Moreover, PDCs with largely conical structures also have large enthalpic contributions to the coupling Gibbs free energy (Fig. S10, ESI†). In general, geometric constraints placed on amphiphilic molecules decrease their coupling Gibbs free energies, as necessary in their self-assembly.2a,11
We further investigated the PDC and linear-block copolymer micelles in terms of their size and morphology using transmission electron microscopy (TEM) and dynamic light scattering (DLS). Fig. 3a shows that all PDC and linear-block copolymer micelles were largely spherical in shape with narrow size distributions (Fig. S11, ESI†). The average diameters of all the self-assembled structures in the TEM and DLS measurements were smaller than 50 nm, except PCL14K-mPEG5K micelles that were ~100 nm in diameter with a broader size distribution. The slight discrepancy in diameters between the TEM images and DLS measurements is because TEM visualizes the hydrophobic core of dried micelles with a minor contribution from the collapsed PEG shell.12 Moreover, the polydisperse nature of polymers contributes to the variations in the measured diameters. Nonetheless, it is clear that PDC micelles form self-assembled structures with predominantly spherical shape and narrow size distribution, as compared to the linear polymer-based micelles, further supporting the superiority of PDCs in self-assembly. The micelles formed from linear-block copolymers and PDCs also have different surface coverage of the hydrophilic PEG layers. Fig. 3b shows that the hydrophobic core is visible in the linear PCL3.5K-mPEG2K micelle, whereas it is fully covered by the PEG layer in the PCL3.5K-G3-mPEG2K PDC micelle. Interestingly, the core of the PCL14K-G3-mPEG2K PDC micelle is not completely covered by PEG, due to the long PCL chains, indicating that molecular weights of each polymer component in a PDC are also important to manipulate the surface coverage.
Fig. 3.
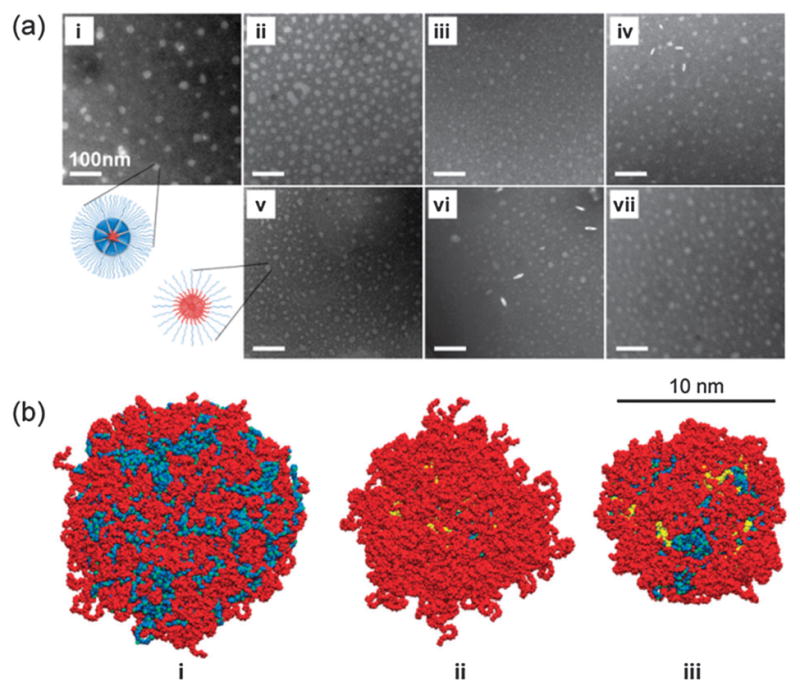
(a) TEM images of micelles self-assembled from PDCs: (i) PCL3.5K-G3-mPEG2K, (ii) PCL3.5K-G3-mPEG5K, (iii) PCL14K-G3-mPEG2K, (iv) PCL14K-G3-mPEG5K; and from the linear copolymers: (v) PCL3.5K-mPEG2K, (vi) PCL3.5K-mPEG5K, and (vii) PCL14K-mPEG5K. Scale bar = 100 nm. (b) MD simulations of micellar structures formed from (i) 128 PCL3.5K-mPEG2K, (ii) 14 PCL3.5K-G3-mPEG2K, and (iii) 10 PCL14K-G3-mPEG2K (PCL: blue, G3-dendron: yellow, PEG: red). Water is not shown.
For the simulated micelles, we used the reported “magic” aggregation number (Nagg) of 14 to construct the PCL3.5K-G3-mPEG2K PDC micelle containing 112 PEG chains (Fig. 3b–ii),11a and 128 was used to construct the linear-block copolymer micelle to match the number of PEG chains (Fig. 3b–i). Ten PCL14K-G3-mPEG2K molecules (80 PEGs) were used to match the size of the PCL3.5K-G3-mPEG2K PDC micelle (Fig. 3b–iii). Using geometrical relationships developed by Nagarajan,13 it is obvious that PDCs have a smaller Nagg than linear-block copolymers with the same length of linear polymer components, which is consistent with our MD results (Table S2, ESI†). Further, the high flexibility and number of PEG on the exterior of each PDC can promote the dense packing of the polymer chains, which can result in a CMC decrease.14 All the results presented show that PDC-based micelles are more thermodynamically stable than those formed of linear block copolymers at the same HLBs.
We have designed and investigated PDCs that are modular, enabling a potential mix-and-match approach to prepare multifunctional nanocarriers. As a preliminary study for the drug delivery application of the PDCs, we have assessed biocompatibility and the drug release of the micelles. Indomethacin (IMC) was encapsulated intomicelles at high efficiency (Table S3, ESI†), and the controlled release profiles were observed over 6 days. In general, copolymers with PEG2K resulted in a more efficient encapsulation, indicating that the lower HLB gives higher encapsulation efficiency.15 As shown in Fig. S12 (ESI†), no significant differences in IMC release profiles were observed between PDC and linear-block copolymer micelles. However, a slower release of IMC was observed from the micelles with PCL14K over the first 24 h. This is likely due to increased hydrophobic interactions between PCL and IMC, leading to a slower rate of diffusion. The cytotoxicity of all copolymers was evaluated on KB cells after 24 h incubation using an MTS assay (Fig. S13, ESI†). None of the tested copolymers exhibited noticeable cytotoxicity at concentrations up to 100 μM.
In summary, we report the design, synthesis, and self-assembly of four new PDCs with different HLBs. The PDC micelles were compared with those formed from linear-block copolymer counterparts. Our observations clearly illustrate that the pre-organized conical dendron architecture facilitates molecular assemblies with ultra low CMCs at high HLBs. The MD simulations also provide molecular level details of the self-assembly process. The highly stable supramolecular assemblies with homogeneous sizes and morphologies, highly hydrophilic PEG surfaces, biocompatibility, and controlled release profiles all prove that PDCs have great potential to provide a novel, versatile drug delivery platform.
Supplementary Material
Acknowledgments
This work was partially supported by Vahlteich Award from University of Illinois Foundation and was conducted in a facility constructed with support from NIH (grant # C06RR15482). The calculations were performed on the NERSC supercomputer networks.
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c1cc14331j
Notes and references
- 1.(a) Wiradharma N, Zhang Y, Venkataraman S, Hedrick JL, Yang YY. Nano Today. 2009;4:302. [Google Scholar]; (b) Harada A, Kataoka K. Prog Polym Sci. 2006;31:949. [Google Scholar]
- 2.(a) Israelachvili JN, Mitchell DJ, Ninham BW. J Chem Soc, Faraday Trans 2. 1976;72:1525. [Google Scholar]; (b) Whitesides GM, Mathias JP, Seto CT. Science. 1991;254:1312. doi: 10.1126/science.1962191. [DOI] [PubMed] [Google Scholar]
- 3.Rosen BM, Wilson CJ, Wilson DA, Peterca M, Imam MR, Percec V. Chem Rev. 2009;109:6275. doi: 10.1021/cr900157q. [DOI] [PubMed] [Google Scholar]
- 4.(a) Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, Jr, Banaszak Holl MM. Chem Biol. 2007;14:107. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]; (b) McNerny DQ, Kukowska-Latallo JF, Mullen DG, Wallace JM, Desai AM, Shukla R, Huang B, Banaszak Holl MM, Baker JR., Jr Bioconjugate Chem. 2009;20:1853. doi: 10.1021/bc900217h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Papp I, Sieben C, Ludwig K, Roskamp M, Bottcher C, Schlecht S, Herrmann A, Haag R. Small. 2010;6:2900. doi: 10.1002/smll.201001349. [DOI] [PubMed] [Google Scholar]; (d) Kostiainen MA, Szilvay GR, Lehtinen J, Smith DK, Linder MB, Urtti A, Ikkala O. ACS Nano. 2007;1:103. doi: 10.1021/nn700053y. [DOI] [PubMed] [Google Scholar]
- 5.(a) Tian L, Hammond PT. Chem Mater. 2006;18:3976. [Google Scholar]; (b) Poon Z, Chen S, Engler AC, Lee HI, Atas E, von Maltzahn G, Bhatia SN, Hammond PT. Angew Chem, Int Ed. 2010;49:7266. doi: 10.1002/anie.201003445. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lu CF, Guo SR, Liu L, Zhang YQ, Li ZH, Gu JR. J Polym Sci, Part B: Polym Phys. 2006;44:3406. [Google Scholar]; (d) Liu JB, Zeng FQ, Allen C. Eur J Pharm Biopharm. 2007;65:309. doi: 10.1016/j.ejpb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Mol Interventions. 2006;6:98. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 7.Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. J Controlled Release. 2005;109:169. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 8.(a) Kim SY, Shin ILG, Lee YM, Cho CS, Sung YK. J Controlled Release. 1998;51:13. doi: 10.1016/s0168-3659(97)00124-7. [DOI] [PubMed] [Google Scholar]; (b) Forrest ML, Won CY, Malick AW, Kwon GS. J Controlled Release. 2006;110:370. doi: 10.1016/j.jconrel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Becher P, Schick MJ. Nonionic Surfactants Physical Chemistry. Marcel Dekker; New York: 1987. [Google Scholar]
- 10.Zhou GB, Smid J. Langmuir. 1993;9:2907. [Google Scholar]
- 11.(a) Chen T, Zhang Z, Glotzer SC. Proc Natl Acad Sci U S A. 2007;104:717. doi: 10.1073/pnas.0604239104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kellermann M, Bauer W, Hirsch A, Schade B, Ludwig K, Bottcher C. Angew Chem, Int Ed. 2004;43:2959. doi: 10.1002/anie.200353510. [DOI] [PubMed] [Google Scholar]; (c) Kratzat K, Finkelmann H. Langmuir. 1996;12:1765. [Google Scholar]
- 12.Zeng F, Liu J, Allen C. Biomacromolecules. 2004;5:1810. doi: 10.1021/bm049836a. [DOI] [PubMed] [Google Scholar]
- 13.Nagarajan R. Langmuir. 2002;18:31. [Google Scholar]
- 14.Suek NW, Lamm MH. Langmuir. 2008;24:3030. doi: 10.1021/la703006w. [DOI] [PubMed] [Google Scholar]
- 15.Shuai X, Ali H, Nasongkla N, Kim S, Gao J. J Controlled Release. 2004;98:415. doi: 10.1016/j.jconrel.2004.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.