Abstract
It is widely assumed that G protein-coupled receptor kinase 2 (GRK2)-mediated specific inhibition of G protein-coupled receptors (GPCRs) response involves GRK-mediated receptor phosphorylation followed by β-arrestin binding and subsequent uncoupling from the heterotrimeric G protein. It has recently become evident that GRK2-mediated GPCRs regulation also involves phosphorylation-independent mechanisms. In the present study we investigated whether the histamine H2 receptor (H2R), a Gαs-coupled GPCR known to be desensitized by GRK2, needs to be phosphorylated for its desensitization and/or internalization and resensitization. For this purpose we evaluated the effect of the phosphorylating-deficient GRK2K220R mutant on H2R signaling in U937, COS7, and HEK293T cells. We found that although this mutant functioned as dominant negative concerning receptor internalization and resensitization, it desensitized H2R signaling in the same degree as the GRK2 wild type. To identify the domains responsible for the kinase-independent receptor desensitization, we co-transfected the receptor with constructions encoding the GRK2 RGS-homology domain (RH) and the RH or the kinase domain fused to the pleckstrin-homology domain. Results demonstrated that the RH domain of GRK2 was sufficient to desensitize the H2R. Moreover, disruption of RGS functions by the use of GRK2D110A/K220R double mutant, although coimmunoprecipitating with the H2R, reversed GRK2K220R-mediated H2R desensitization. Overall, these results indicate that GRK2 induces desensitization of H2R through a phosphorylation-independent and RGS-dependent mechanism and extends the GRK2 RH domain-mediated regulation of GPCRs beyond Gαq-coupled receptors. On the other hand, GRK2 kinase activity proved to be necessary for receptor internalization and the resulting resensitization.
Keywords: G Protein-coupled Receptors (GPCR), Receptor Desensitization, Receptor Endocytosis, Receptor Regulation, RGS Proteins, Signal Transduction, GRKs
Introduction
It has been classically described that G protein-coupled receptor (GPCR)2 desensitization involves rapid receptor phosphorylation by second messenger-dependent kinases and/or G protein-coupled receptor kinases (GRKs). GRK-mediated receptor phosphorylation promotes arrestin binding to the receptor followed by GPCR internalization in clathrin-coated pits. Therefore, the paradigmatic GRK major function is to enable arrestin binding to the receptor and uncoupling from the heterotrimeric G protein (1). However, GPCR signaling may also be specifically attenuated not only at the receptor level but also at the G protein level (2). In recent years, the intracellular regulator of G protein signaling (RGS) proteins have been discovered to serve additional, mostly negative modulatory roles, in G protein-mediated signal transduction (3). More than 30 proteins containing RGS or RGS homology (RH) domains have been described so far (4). Most of these proteins interact with Gαi or Gαq subunits, but there is also evidence for the interaction of RGS2 and the truncated form of RGS3 with the Gαs subunit (5, 6).
The seven GRKs proteins described so far contain an N-terminal RGS-homology domain (RH), a catalytic central domain (Kin), and a less conserved C-terminal region responsible for membrane localization that in the case of GRK2 is homologous to a pleckstrin domain (PH) (7). The involvement of the RH domain in the regulation of GPCR signaling was suggested by the use of GRK2K220R, a phosphorylation-inactive mutant with disrupted kinase activity unable to transfer phosphate from ATP to a substrate (8). This negative allele has been shown to compete with wild type GRK2, preventing phosphorylation of many GPCRs as the α1B-, β1-, and β2-adrenergic receptors, the type 1A angiotensin receptor, and the follicle-stimulating hormone receptor among others (8–12). However, in some instances GRK2K220R can attenuate GPCR signaling similarly to the wild type kinase (13, 14).
Histamine is a biogenic amine widely distributed in the body involved in the regulation of diverse biological actions, particularly in the central nervous system, gastric mucosa, mast cells, and basophils. Its effects are mediated by four well characterized receptor subtypes belonging to the large family of GPCRs. Particularly, the histamine H2 receptor (H2R) mediates hypotension, flushing, headache, increased gastric acid production, and enhanced vascular permeability (15, 16). This receptor subtype is coupled to a Gαs protein, and its activation leads to increase cAMP production and triggers rapid receptor desensitization (17).
We have previously reported that GRK2 is involved in both H2R phosphorylation and desensitization (18). We also showed an increased H2R responsiveness in U937 cells expressing a GRK2 antisense construct (19).
Furthermore, we described that H2R internalization and resensitization are dependent on arrestin, dynamin, and clathrin. However, the role of GRK2-mediated phosphorylation in H2R desensitization and internalization/resensitization remains presently unknown. The aims of the present work were to elucidate the phosphorylation-dependent and -independent regulation of the H2R by GRK2 and to identify the kinase domains crucial for such regulation.
Therefore, we evaluated the effects of the widely characterized GRK2K220R mutant on the desensitization and internalization/resensitization mechanisms. In this context we studied the impact of different constructs obtained from the GRK2K220R: GRK2D110A/K220R double mutant, RH domain, RH-PH or Kin-PH on these processes.
In three different cellular models we found that although the receptor phosphorylation was crucial for H2R internalization and resensitization, the kinase activity was not required to achieve H2R desensitization. Furthermore, we showed that only the RH-containing constructs desensitized the H2R and that mutation of residues involved in RH activity reversed receptor desensitization. These results minimize the steric hindrance impact that the mutant kinase could have in the coupling of the G protein to the receptor, suggesting that the RH domain contained in GRK2 is able to modulate a Gαs-associated signaling pathway. Present findings provide novel evidence for phosphorylation-dependent and -independent mechanisms in the regulation of the H2R by GRK2.
EXPERIMENTAL PROCEDURES
Materials
U937, HEK293T, and COS7 cells were obtained from the American Tissue Culture Collection. Cell culture medium, antibiotics, isobutylmethyl xanthine (IBMX), cAMP, prostaglandin E2 (PGE2), forskolin, G-418, anti-HA-agarose beads, protease inhibitors, and bovine serum albumin (BSA) were obtained from Sigma. Amthamine and tiotidine were from Tocris Cookson Inc. (Ballwin, MO). [3H]cAMP (∼31 Ci/mmol) and [3H]tiotidine (∼75 Ci/mmol) were purchased from PerkinElmer Life Sciences. Restriction enzymes, DNA ligase, reverse transcriptase, and Pfu were purchase from New England Biolabs, Inc. (Beverly, MA). Other chemicals used were of analytical grade and obtained from standard sources.
Plasmid Constructions
pBluescript-GRK2, pcDNA3-GRK2-K220R, and pcDNA3-GRK2-D110A/K220R were kindly provided by Dr. J. Benovic (Thomas Jefferson University, Microbiology and Immunology Department, Kimmel Cancer Center, Philadelphia). pcDNA-GRK2-D110A was kindly provided by Dr. R. Sterne-Marr (Biology Dept., Siena College, Loudonville, NY). GRK2 cDNA was subcloned into the pCEFL vector as previously described (18), and the GRK2 antisense construct was obtained as previously reported (19). The plasmids containing the different domains of GRK2 were constructed by PCR amplification of bovine GRK2K220R. To obtain the RH construct, the sequence coding for residues 46–179 was amplified and inserted into BamHI/EcoRI site of pCEFLHA. In the case of the RH-PH construct, the sequence coding for residues 46–179 was fused in the EcoRI site with PH sequence coding for residues 547-stop and then inserted into BamHI/XbaI site of pCEFLHA. Finally, Kin-PH construct was obtained by the amplification of residues 197-stop and inserted in BglII/XbaI site of pCEFLHA. The sequence integrity of each mutant was confirmed by automated DNA sequencing.
Cell Culture
U937 cells were cultured in a 5% CO2-humidified atmosphere at 37 °C in RPMI 1640 medium, and HEK293T and COS7 were cultured in Dulbecco's modified Eagle's medium; all were supplemented with 10% fetal calf serum and 5 μg/ml gentamicin.
Transient Transfection
For transient transfection, HEK293T and COS7 cells were grown to 80–90% confluency, and the cDNA constructs were transfected using Lipofectamine 2000. The transfection protocol was optimized as recommended by the supplier (Invitrogen). The expression of the constructs was confirmed by immunoblotting using specific antibodies. Assays were performed 48 h after transfection.
Stable Transfection
For stable transfection, U937 cells were harvested by centrifugation from cultures in exponential growth phase, washed in phosphate-buffered saline (PBS), and resuspended at 2 × 107 cells/ml in fresh RPMI medium on ice. pcDNA3-GRK2-K220R (10 μg) was linearized with SalI, then added to the cell suspension (250 μl) and kept on ice for 10 min. Cells and DNA were then subjected to a pulse of 200 V at a capacitance of 950 microfarads using a Gene Pulser (Bio-Rad); cells were returned to ice for 10 min and incubated in a non-selective medium overnight. Cells were then plated in a 48-well culture plate in 0.5 ml/well RPMI 1640 medium supplemented with 10% fetal calf serum and 50 μg/ml gentamicin containing 0.8 mg/ml G-418. After 2–3 weeks, the surviving clones were amplified. The expression of GRK2K220R was verified by RT-PCR using the following primers: forward 5′-CAAGATGAAGAACAAGCC-3′ and reverse 5′-ATTTAGGTGACACTATAG-3 (corresponding to SP6 sequence).
Coimmunoprecipitation Assays
Cross-linking of GRK2 mutants to the H2 receptor was done in intact COS-7 cells cotransfected with the HA-tagged H2R (18) and GRK2D110A/K220R or GRK2K220R. The cells were washed three times with PBS and incubated for 10 min at 37 °C in PBS with 10 μm amthamine. This medium was replaced by 3 ml of PBS with 2.5 mm dithiobis(succinimidyl propionate) (Pierce) with amthamine and incubated for 30 min at 25 °C. Then, cells were solubilized in 1 ml of radioimmune precipitation assay buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mm Tris, pH 7.4, 100 mm NaCl, 2 mm EDTA, 50 mm NaF, 1 mm phenylmethylsulfonyl, 5 μm aprotinin, 10 μm leupeptin, 5 μm pepstatin, 1 mm sodium vanadate) at 4 °C for 30 min, and immunoprecipitation of the HA-tagged receptor was done by overnight incubation at 4 °C with anti HA-agarose beads. Separation of immune complexes and cleavage of the cross-linker was done for 90 min at 37 °C in Laemmli buffer. Immunoprecipitated proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes. GRK2 was detected with a rabbit anti-GRK2 antiserum as described below.
Western Blot Assay
Cells were resuspended in lysis buffer (5 mm Tris-HCl, pH 8, 5 mm EDTA, 1% Triton X-100, 0.1% dithiothreitol, 1 mm phenylmethylsulfonyl, 5 μm aprotinin, 10 μm leupeptin, 5 μm pepstatin, 1 mm sodium vanadate). Samples were then incubated for 2 min in liquid nitrogen and for 3 min at 37 °C with vigorous agitation. Total proteins were quantified by the Bradford reaction. Sample buffer 5×(250 mm Tris-HCl, pH 6.8, 10% SDS, 500 mm 2-mercaptoethanol, 50% glycerol, and 0.25% bromphenol blue) and enough water to obtain a 2-μg/μl final protein concentration were added to the samples. They were then boiled for 5 min, resolved on SDS-polyacrylamide gel electrophoresis (12% or 15% gel), and transferred to nitrocellulose membranes for immunoblotting. The membranes were probed with 1 μg/ml rabbit anti-GRK2-specific antibodies or mouse anti-HA antibody (Santa Cruz Biotechnology) in PBS-Tween followed by subsequent washes with the same buffer. Reactivity was developed using an anti-rabbit and anti-mouse polyclonal antibody linked to horseradish peroxidase and enhanced chemiluminescence reagents, according to the manufacturer's instructions (Amersham Biosciences). Densitometry analyses of bands were performed by Scion Image (Scion Corp., Frederick, MD).
cAMP Assay
Concentration-response assays were performed by incubating the cells for 3 min in basal culture medium supplemented with 1 mm IBMX at 37 °C followed by 9 min of exposure to different amthamine concentrations. In time-course cAMP production/degradation studies, cells were resuspended in basal culture medium without IBMX at 37 °C and exposed to 10 μm amthamine at different time periods.
For desensitization assays cells were pretreated with 10 μm amthamine in the absence of IBMX at the times shown in the figures. Cells were then washed and resuspended in fresh medium containing 1 mm IBMX, incubated for 3 min, and exposed to 10 μm amthamine for 9 min to determine whether the system was able to generate cAMP. For resensitization assays, cells were first treated with 10 μm amthamine for 60 min, washed, and incubated in fresh medium at different times to evaluate the recovery of H2R active sites after the desensitizing stimulus.
In all cAMP assays, the reaction was stopped by ethanol addition followed by centrifugation at 2000 × g for 5 min. The ethanol phase was then dried, and the residue was resuspended in 50 mm Tris-HCl, pH 7.4, 0.1% BSA. cAMP content was determined by competition of [3H]cAMP for PKA, as previously described (20).
Radioligand Binding Assay
Triplicate assays were performed in 50 mm Tris-HCl, pH 7.4. Saturation studies were performed by incubating 106 U937 cells/tube, 2 × 104 HEK293T or 104 COS7 cells/p96 well for 40 min at 4 °C with increasing concentrations of [3H]tiotidine ranging from 0.4 up to 240 nm in the absence or presence of 1 μm unlabeled tiotidine. The incubation was stopped by dilution with 3 ml of ice-cold 50 mm Tris-HCl, pH 7.4. For U937 cells or derived clones, rapid filtration under reduced pressure onto Whatman GF/B glass-fibers filters followed by 3 washes with 3 ml of ice-cold buffer was performed. For HEK293T and COS7 cells, after 3 washes with 3 ml of ice-cold buffer, the bound fraction was collected in 200 μl of ethanol. Experiments on intact cells were carried out at 4 °C to avoid ligand internalization. The kinetic studies performed with 2 nm [3H]tiotidine at 4 °C showed that the equilibrium was reached at 30 min and persisted for 4 h (data not shown).
Receptor Internalization and Recovery
HEK293T, COS7, U937 cells, or derived clones were incubated at different times with 10 μm amthamine, and the number of receptor sites was analyzed by radioligand binding assay. The recovery of binding sites was evaluated by radioligand binding assay at different time points after washing the cells treated with 10 μm amthamine for 60 min.
Statistical Analysis
Binding data, sigmoidal dose-response, and desensitization fittings were performed with GraphPad Prism 4.00 for Windows, GraphPad Software (San Diego, CA). One-way analysis of variance followed by the Dunnett's post test was performed using GraphPad InStat Version 3.01, GraphPad Software (San Diego CA). Specific binding was calculated by subtraction of nonspecific binding from total binding.
RESULTS
Effect of GRK2K220R on H2R Signaling in U937 Cells
We have previously reported that the reduction of GRK2 expression in leukemic U937 cells by antisense technology attenuates the process of H2R desensitization (19). To evaluate whether receptor phosphorylation by GRK2 was required to achieve receptor desensitization, we examined the effect of expressing the catalytically inactive GRK2K220R dominant negative mutant (8) in U937 cells.
By stable transfection of U937 cells with the GRK2K220R mutant, three derived clones resistant to G418, arbitrarily named GB4, GE2, and GF5, were obtained. As evaluated by Western blot, GB4 and GE2 clones showed significantly higher GRK2 protein expression corresponding to both native and GRK2K220R (Fig. 1A). On the other hand, no modifications were observed in the GF5 clone, which was then used as an internal control. The specific expression of GRK2K220R in these clones was also analyzed by RT-PCR as detailed under “Experimental Procedures” (data not shown).
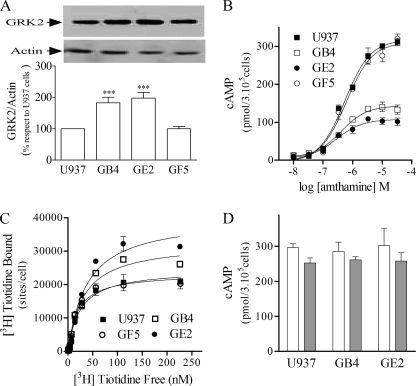
FIGURE 1.
Characterization of U937-derived clones. A, GRK2 expression in U937- and U937-derived clones is shown. Upper panel, whole-cell lysates were subjected to SDS-PAGE and analyzed by Western blot with anti-GRK2 or anti-actin antibodies. Lower panel, densitometry analysis was obtained by the Scion Image program. Data are the means ± S.E. (n = 3). 100% corresponds to GRK2 levels in U937 cells. ***, p < 0.01 with respect to U937 cells. B, H2R concentration-response curves are shown. U937, GB4, GE2, or GF5 cells were incubated for 9 min with increasing concentrations of amthamine at 37 °C in the presence of 1 mm IBMX, and cAMP levels were determined. Data were calculated as the means ± S.E. (n = 4). C, H2R membrane sites are shown. Saturation assays for [3H]tiotidine are shown. Similar results were obtained in at least three independent experiments. Bmax in sites/cell: U937, 24,579 ± 130; GF5, 23,374 ± 1,168; GB4, 32,507 ± 1,616** and GE2, 40,181 ± 2,193**. Data are the means ± S.E. (n = 3); **, p < 0.01 with respect to U937 cells. D, forskolin and PGE2 stimulated cAMP production in U937- and U937-derived clones. Cells were incubated with 100 μm forskolin (white bars) or 33 μm PGE2 (gray bars) for 9 min at 37 °C in the presence of 1 mm IBMX, and cAMP levels were determined. Data are the means ± S.E. (n = 3).
Concentration-response assays showed that GB4 and GE2 exhibited reduced cAMP formation in response to the H2R agonist amthamine as compared with GF5 and U937 control cells, although pEC50 values were similar in all cases (Fig. 1B). As the decrease in cAMP response to amthamine could result from a diminished number of H2R in the cell surface, we evaluated the density of receptors sites in the plasma membrane by [3H]tiotidine radioligand binding assay. Surprisingly, GB4 and GE2 clones presented significantly higher H2R binding sites (Fig. 1C).
With the aim to determine whether cAMP production was modified in an event upstream or downstream of adenylyl cyclase activation in GRK2K220R-expressing clones, we assessed cAMP production in response to forskolin (a direct adenylyl cyclase activator) and PGE2 (an agonist of other Gαs-coupled receptor known to be desensitized independently of GRK2 action (20)). Fig. 1D shows that cAMP production stimulated by forskolin and PGE2 was similar in GRK2K220R-expressing clones and U937 naïve cells. These results indicate that the decrease in amthamine-evoked cAMP response resulted from the presence of GRK2K220R and not from clonal variations.
When the time course of cAMP production/degradation balance after amthamine stimulation was evaluated, a decreased H2R-mediated cAMP response was also found in GB4 and GE2 cells (Fig. 2A). In desensitization assays a half-maximal desensitization time of 9.8 ± 0.7 min for U937 cells was observed as previously reported by us (17). The presence of the GRK2K220R mutant in the clones significantly modified the desensitization curve because GB4 and GE2 showed a left shift in the half-maximal desensitization time (Fig. 2B and Table 1). These results indicate that this kinase inactive mutant is somehow able to potentiate H2R desensitization and in consequence to reduce cAMP production in response to amthamine.
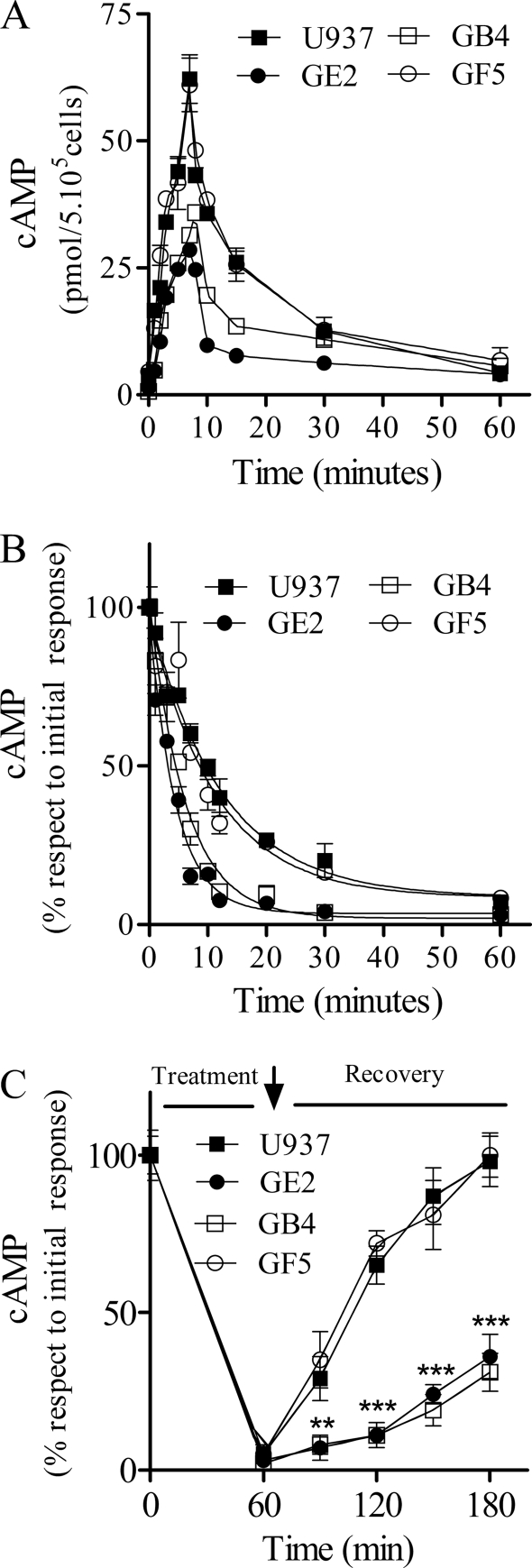
FIGURE 2.
Characterization of H2R-evoked cAMP response in U937- and U937-derived clones. A, kinetics of cAMP production in the absence of IBMX is shown. Cells were incubated at different time periods with 10 μm amthamine at 37 °C, and cAMP levels were determined. B, H2R desensitization kinetics. Cells were preincubated at different times with 10 μm amthamine, washed, and restimulated with 10 μm amthamine in the presence of 1 mm IBMX. Data are the means ± S.E. (n = 3). **, p < 0.01 with respect to U937 cells. C, resensitization of the H2R is shown. Cells were treated for 60 min with 10 μm amthamine, washed (↓), and further incubated in fresh medium at the indicated times. Data represent the percentage of cAMP measured after stimulation with 10 μm amthamine in the presence of 1 mm IBMX and are expressed as the means ± S.E. (n = 3). ***, p < 0.001 with respect to U937 cells.
TABLE 1.
Half-maximal desensitization times in U937 cells and derived clones
Data are the means ± S.E. (n = 3).
a p < 0.01 with respect to U937 cells.
To evaluate the recovery of cAMP response after 60 min of exposure to amthamine, cells were washed, and amthamine-stimulated cAMP production was assessed. Only U937 and GF5 cells were able to recover about 70% of the initial cAMP response to amthamine after 1 h. Conversely, GB4 and GE2 clones showed a similar response to that observed in pretreated cells, indicating the lack of recovery in these systems (Fig. 2C). These results support that the presence of GRK2K220R kinase inactive mutant restricts cell ability to restore the response to the agonist.
We previously reported that in both U937 and COS7 cells amthamine-induced H2R internalization is crucial for H2R resensitization and that this mechanism is dependent on β2 arrestin, dynamin, and clathrin (21). Therefore, it is possible to assume that the results obtained in Fig. 2C, where GB4 and GE2 did not resensitize, may be due to the lack of internalization and posterior recycling to the cell surface.
H2R Response Regulation by GRK2 Kinase Inactive Mutant in Heterologous Expression Systems
To further study the desensitization and resensitization dependence of the H2R on GRK2 kinase activity, we switched to the heterologous expression system of COS7 cells where we could transiently cotransfect the H2R and different GRK2 variants (wild type GRK2, an antisense GRK2 or GRK2K220R constructs).
As shown in Fig. 3A, Western blot analysis revealed that in COS7 cells, wild type or dominant negative mutant overexpressed after transfection, whereas the antisense construct led to a reduction in endogenous GRK2 expression as previously reported (19).
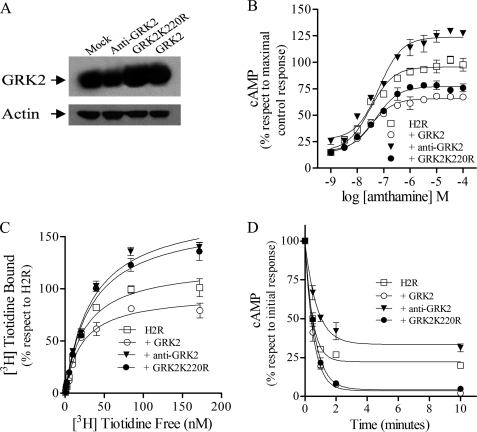
FIGURE 3.
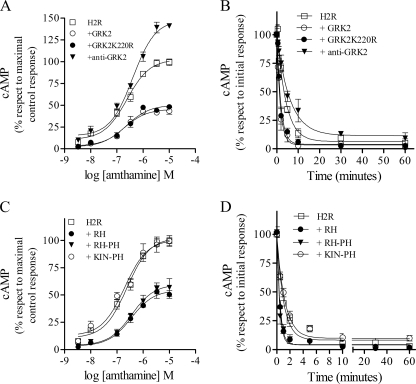
H2R regulation by GRK2 in COS7-transfected cells. COS7 cells were cotransfected with H2R and wild type GRK2, antisense GRK2, or GRK2K220R constructs. A, shown is analysis of GRK2 constructs expression in COS7-transfected cells. Cells were lysed, and equal amounts of proteins were subjected to SDS-PAGE and analyzed by Western blot with anti-GRK2 antibody. Data are representative of at least three independent experiments. B, shown are concentration-response curves for cAMP production. Cells were exposed for 9 min to increasing concentrations of amthamine at 37 °C in the presence of 1 mm IBMX, and cAMP levels were determined. C, H2R membrane sites are shown. Saturation assays for [3H]tiotidine. Bmax, H2R, 100 ± 4; +GRK2, 75 ± 4***; +anti-GRK2, 149 ± 7***; +GRK2K220R, 133 ± 6***. Data represent % Bmax fitted by nonlinear regression of [3H]tiotidine saturation assay, expressed as the means ± S.E. (n = 3). 100% corresponds to H2R-transfected cells. ***, p < 0.001 with respect to H2R-transfected cells. D, involvement of GRK2-mediated receptor phosphorylation in H2R desensitization is shown. Cells were preincubated at different times with 10 μm amthamine, washed, and restimulated with 10 μm amthamine in the presence of 1 mm IBMX. cAMP production was determined as described under “Experimental Procedures.” B–D, data are the means ± S.E. (n = 3).
H2R-transfected COS7 cells exposed to increasing amthamine concentrations (1 nm–10 μm) showed a concentration-dependent increase in cAMP content (Fig. 3B). However, a different cAMP maximal production for GRK2, GRK2K220R, and antisense construct was observed. Cells cotransfected with either wild type or mutated GRK2 exhibited significantly reduced cAMP production in response to H2R stimulation, whereas the antisense construct for GRK2 enhanced H2R response as previously reported (19) (Fig. 3B).
To determine whether the reduced H2R cAMP response in the presence of GRK2K220R resulted from diminished H2R sites in the cell surface, [3H]tiotidine binding assays were performed. Unexpectedly, GRK2K220R led to a 25% increase in H2R sites as compared with COS7 cells solely transfected with H2R (Fig. 3C). These results were similar to those obtained in U937-derived clones. On the other hand, the number of H2R sites in the presence of the GRK2 or the antisense construct for GRK2 correlated with the cAMP response observed with these GRK2 variants (Fig. 3B). These results indicate that the reduced cAMP response to amthamine, achieved in cells cotransfected with GRK2K220R, may be due to a higher rate of H2R desensitization rather than a reduction of H2R sites.
In an attempt to clarify this issue, desensitization experiments were carried out. Amthamine (10 μm)-pretreated cells for 0.5–10 min exhibited a time-dependent attenuation of cAMP production after a second exposure to the agonist (10 μm amthamine). This desensitization in H2R response was more pronounced when cells were cotransfected with GRK2K220R or GRK2 wild type but attenuated in the presence of the antisense construct for GRK2 (Fig. 3D and Table 2). Overall, these results clearly indicate that GRK2K220R is as effective as the wild type kinase at attenuating H2R signaling, suggesting the possibility that GRK2-mediated receptor phosphorylation is not necessary for H2R desensitization.
TABLE 2.
Half-maximal desensitization times in transfected COS7 cells
Data are means ± S.E. (n = 3).
| t½ Desensitization | |
|---|---|
| min | |
| H2R | 0.44 ± 0.05 |
| +GRK2 | 0.33 ± 0.03a |
| +anti-GRK2 | 0.53 ± 0.04a |
| +GRK2K220R | 0.34 ± 0.02a |
| +RH | 0.29 ± 0.04a |
| +RH-PH | 0.28 ± 0.03a |
| +KIN-PH | 0.46 ± 0.05 |
a p < 0.01 with respect to H2R-transfected cells.
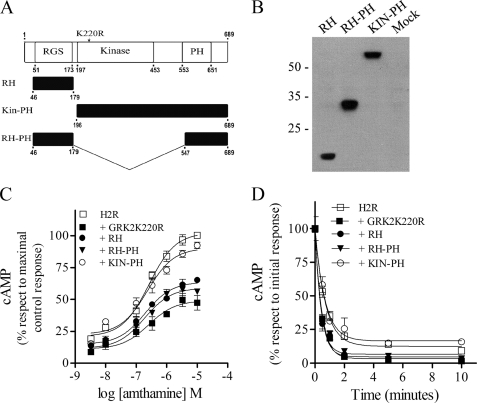
We next investigated the GRK2 domains responsible for this kinase activity-independent desensitization. The overall topology of the members of the GRK family consist of three well defined domains; 1) the conserved N-terminal region involved in receptor recognition, which exhibits sequence homology with RGS proteins, 2) the central domain, which is responsible for the kinase activity itself, and 3) the poorly conserved C-terminal region that mediates membrane targeting of the kinases and in the case of GRK2 encodes a pleckstrin homology domain. Therefore, based on these considerations, we subcloned the GRK2K220R RH and the RH or the kinase domain fused to the pleckstrin homology domain (RH-PH or Kin-PH) in a pCEFLHA vector (Fig. 4A). The correct expression of these constructs in COS7-transfected cells was verified by immunoblotting showing that the size of the bands corresponded to the subcloned fragments (Fig. 4B). Concentration-response curves were performed to examine the impact of coexpressing these constructs on H2R signaling. In cells that coexpressed RH or RH-PH, H2R response was dramatically reduced, showing a maximal cAMP production similar to that obtained in the presence of GRK2K220R (Fig. 4C). On the other hand, the cAMP production in cells expressing the Kin-PH construct did not significantly differ from that in cells with the receptor alone. In desensitization assays we found the same degree of signaling attenuation in the GRK2K220R dominant negative as well as in the RH and RH-PH constructs, whereas the Kin-PH construct did not potentiate H2R desensitization (Fig. 4D and Table 2). These studies showed that the expression of RH or RH-PH was as effective as GRK2K220R and, in consequence, as the wild type kinase at attenuating H2R signaling.
FIGURE 4.
H2R regulation by RGS in COS7-transfected cells. A, shown is a schematic representation of GRK2 functional domains. Shown are the N-terminal RH domain, the central catalytic domain, and the C-terminal pleckstrin homology domain. The point mutation at residue 220, essential for GRK2 catalytic activity, is identified. Also shown are the HA-tagged RH, KIN-PH, and RH-PH expression constructs. B–D, COS7 cells cotransfected with H2R and RH, KIN-PH, or RH-PH constructs are shown. B, shown is analysis of constructs expression in COS7-transfected cells. Cells were lysed, and equal amounts of proteins were subjected to SDS-PAGE and analyzed by Western blot with HA antibody. Data are representative of at least three independent experiments. C, shown are concentration-response curves for cAMP production. Cells were exposed for 9 min to increasing concentrations of amthamine at 37 °C in the presence of 1 mm IBMX, and cAMP levels were determined. D, desensitization kinetics are shown. Cells were preincubated at different times with 10 μm amthamine, washed, and restimulated with 10 μm amthamine in the presence of 1 mm IBMX. cAMP production was determined as described under “Experimental Procedures.” C and D, data are the means ± S.E. (n = 3).
We next addressed whether the results obtained in COS7 cells were reproduced in other cellular model expressing higher levels of GRKs and arrestins as HEK293T cells (22). Experiments carried out in HEK293T showed a lower H2R maximal response and a faster desensitization in GRK2K220R-cotransfected cells (Fig. 5, A and B, and Table 3), showing that the dominant negative mutant in these cells also behaves as the wild type allele regarding H2R signaling.
FIGURE 5.
H2R regulation by GRK2 in HEK293T-transfected cells. HEK293T cells cotransfected with H2R and Mock, GRK2, anti-GRK2, or GRK2K220R (A and B) or HEK293T cells cotransfected with H2R and Mock, RH, KIN-PH or RH-PH domains (C and D) are shown. A and C, concentration-response curves for cAMP production are shown. Cells were exposed for 9 min to increasing concentrations of amthamine at 37 °C in the presence of 1 mm IBMX, and cAMP levels were determined. B and D, shown is involvement of GRK2-mediated receptor phosphorylation in H2R desensitization. Cells were preincubated at different times with 10 μm amthamine, washed, and restimulated with 10 μm amthamine in the presence of 1 mm IBMX. cAMP production was determined as described under “Experimental Procedures.” A–D, data are the means ± S.E. (n = 3).
TABLE 3.
Half-maximal desensitization times in HEK293T cells
Data are means ± S.E. (n = 3).
| t½ Desensitization | |
|---|---|
| min | |
| H2R | 2.86 ± 0.11 |
| +GRK2 | 1.33 ± 0.09a |
| +anti-GRK2 | 4.25 ± 0.14a |
| +GRK2K220R | 1.27 ± 0.07a |
| +RH | 0.72 ± 0.07a |
| +RH-PH | 0.88 ± 0.09a |
| +KIN-PH | 2.53 ± 0.15 |
a p < 0.01 with respect to H2R-transfected cells.
The effect of GRK2K220R-derived constructs in concentration response and desensitization assays were assessed (Fig. 5, C and D, and Table 3). As observed in COS7 cells, the Kin-PH construct behaved similarly to the H2R alone in both assays, whereas RH and RH-PH mimicked GRK2K220R response. These results indicate that even in a system with different stoichiometry, the GRK2 dominant negative mutant desensitization of H2R signaling was dependent on the RH domain.
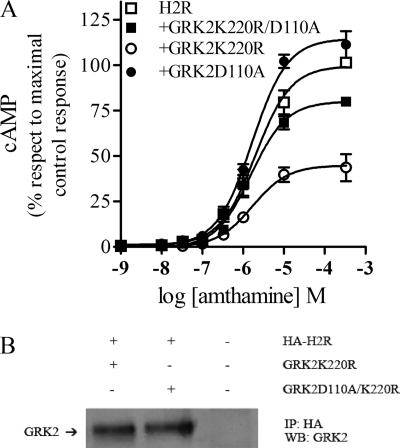
It has been described that the GRK2D110A mutant fails to bind and to regulate Gαq protein. To evaluate whether this residue was involved in Gαs regulation by the RH domain, concentration response assays were performed in the presence of GRK2D110A and GRK2D110A/K220R. The expression of the D110A mutant in COS7-transfected cells failed to desensitize the H2R, whereas the double mutant effectively relieved the inhibition of cAMP response caused by GRK2K220R (Fig. 6A). This result suggests that Asp-110 residue is involved in Gαs activity regulation. To evaluate whether the ability of GRK2 to desensitize the H2R correlates with the ability of interacting with the receptor, coimmunoprecipitation studies were performed. Fig. 6B shows that both GRK2 mutants coimmunoprecipitate with the receptor, showing that there is no correlation between GRK2 H2R desensitization and interaction.
FIGURE 6.
H2R regulation by GRK2 in COS7-transfected cells. COS7 cells cotransfected with H2R and GRK2K220R, GRK2D110A, or GRK2K220R-D110A constructs are shown. A, concentration-response curves for cAMP production are shown. Cells were exposed for 9 min to increasing concentrations of amthamine at 37 °C in the presence of 1 mm IBMX, and cAMP levels were determined. cAMP production was determined as described under “Experimental Procedures.” Data are the means ± S.E. (n = 3). B, coimmunoprecipitation of GRK2 mutants and H2R is shown. COS 7 cells cotransfected with HA-tagged H2R and GRK2 mutants or Mock were incubated for 10 min with 10 μm amthamine, and cross-linking with 2.5 mm dithiobis(succinimidyl propionate) was done as described under “Experimental Procedures.” Cells were lysed, and the receptor was immunoprecipitated (IP) with anti-HA antibodies. Coprecipitated GRK2 was detected by Western blot (WB).
H2R Internalization Regulation by GRK2 Kinase-inactive Mutant in COS7 Cells
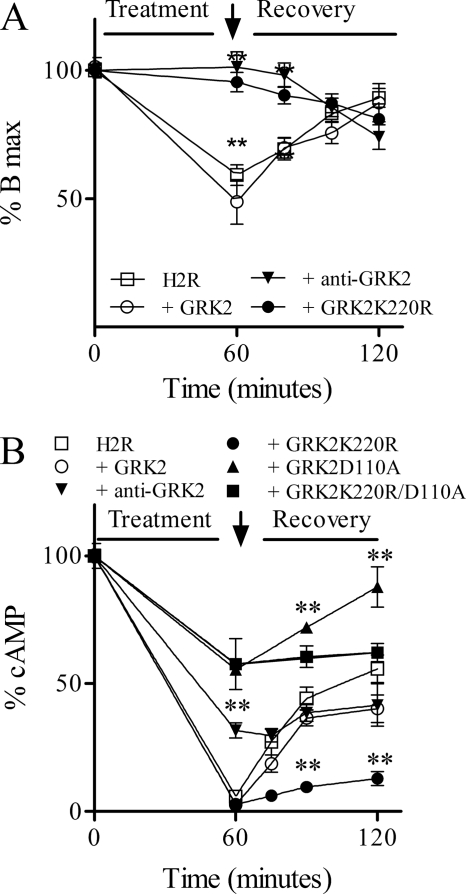
The role of receptor phosphorylation in the H2R internalization remains poorly understood. To determine the participation of GRK2-mediated H2R phosphorylation in receptor internalization, we compared the rate and extent of receptor internalization in COS7 cells expressing the H2R alone or coexpressing GRK2K220R, a wild type allele, or an antisense GRK2. After cell exposure to amthamine for 60 min, 40% H2R membrane sites were internalized, although this endocytosis was abolished in the presence of antisense GRK2 and GRK2K220R. Conversely, wild type GRK2 overexpression led to higher levels of both basal and amthamine-induced H2R internalization (Fig. 7A). These results show that the H2R required GRK2 kinase activity for its internalization. It is worth noting that when cAMP response was assessed, GRK2K220R behaved as the wild type kinase in its ability to dampen H2R-mediated cAMP response, but when the receptor was in the presence of the antisense construct to GRK2 or D110A GRK2 mutants, cAMP response was desensitized to a lesser extent. Even more resensitization was favored when kinase activity is conserved as in GRK2D110A mutant (Fig. 7B). These results indicate that GRK2K220R behaves as the antisense construct with respect to amthamine induced-H2R internalization and as the wild type kinase regarding H2R cAMP response. In conclusion, the kinase activity and, therefore, H2R phosphorylation, is required to achieve H2R internalization but not receptor desensitization.
FIGURE 7.
Involvement of GRK2-mediated phosphorylation in H2R desensitization, internalization, and resensitization. COS7 cells cotransfected with H2R and Mock, GRK2, anti-GRK2, GRK2D110A, or GRK2K220R constructs were exposed to 10 μm amthamine for 60 min, washed (↓), and incubated in fresh medium at the indicated times. A, internalization and recovery of H2R membrane sites are shown. Bmax was determined by nonlinear regression fit from a [3H]tiotidine saturation assay. B, resensitization of the H2R is shown. cAMP response was measured after stimulation with 10 μm amthamine in the presence of 1 mm IBMX. A and B, data are the means ± S.E. (n = 3). 100% corresponds to untreated H2R-transfected cells. **, p < 0.001 respect to H2R-transfected COS7 cells.
DISCUSSION
The present findings answer three mayor questions regarding the events involved in H2R regulation. Is agonist-induced GRK2-dependent receptor phosphorylation directly responsible for receptor-adenylyl cyclase uncoupling? Is it also responsible for receptor internalization? Which GRK2 domains promote H2R desensitization? Two experimental approaches were used to answer these questions; that is, the kinase-deficient mutant GRK2K220R and constructs containing different GRK2 domains.
The H2R cAMP response was already known to be profoundly dampened by GRK2 in both COS-7 and U937 cells (18, 19). Remarkably, in the present work we observed that in these cell lines as well as in HEK293T, the H2R was desensitized by the GRK2 kinase-inactive mutant K220R similarly to the wild type kinase, supporting the lack of phosphorylation-dependent mechanisms involved in H2R desensitization. The canonical model, originally described for the adrenergic receptor, asserts that receptor phosphorylation by GRKs allows arrestin binding to the receptor and subsequent uncoupling from the heterotrimeric G-protein and posterior receptor internalization (23). The experimental observation that the GRK2 kinase-inactive mutant desensitized the H2R agrees with our previous findings showing that arrestin 2 and 3 are not involved in H2R desensitization, although they are essential for its internalization (21).
Since the generation of GRK2K220R mutant by Kong et al. in 1994 (8), a large number of studies have reported that this kinase inactive mutant acts as a dominant negative mutant for the phosphorylation of different GPCRs (9, 12), thus, leading to diminished receptor desensitization (11, 24–26). Nevertheless, the overexpression of GRK2K220R in some instances results in increased attenuation of receptor signaling. In particular, GRK2K220R dampens second messenger production in the case of parathyroid, serotonin, endothelin, thyrotropin, and follicle-stimulating hormone receptors (13, 14, 27, 28). The recent elucidation of the GRK2 crystal structure indicates that it contains three functional domains, an N-terminal RGS homology domain, a central catalytic domain, and a C-terminal pleckstrin homology domain (29). Therefore, the phosphorylation-independent inhibition could be attributed either to a direct interaction between GRKs and the receptor or to a selective regulation of Gα by the RGS homology domain of this GRK (2). Based on the binding of a soluble bovine brain extract to a GRK2 affinity column, it was widely accepted that the RGS homology domain of GRK2 could solely interact with Gαq subunit to avoid G protein activation (30). Moreover, others studies reveal that the expression of the RH domain of GRK2 alone is sufficient for the signaling blockade of several GPCRs coupled to Gαq subunits (31, 32). However, more recent reports show evidence that the signaling of the Gαs-coupled follicle-stimulating hormone receptor and the Gαi-coupled D2 dopamine receptor is attenuated by GRK2K220R (27, 33); in these cases direct association between this kinase dead mutant and the receptors was suggested. In this context we found that the expression of the RH domain but not the Kin-PH domain of GRK2 was sufficient to mimic GRK2 wild type-mediated H2R desensitization. These observations lead us to speculate that a direct regulation of the Gαs activity by the RH domain could be involved in this phenomenon rather than GRK2K220R-mediated steric hindrance. Moreover, reversion of GRK2K220R-mediated H2R desensitization by mutation of the Asp-110 residue suggests that the mechanism by which the RH domain exerts its regulatory action on Gαs protein may be similar to Gαq. Coimmunoprecipitation studies show that K220R/D110A mutations in GRK2 do not prevent the interaction with the receptor. This result is consistent with previous reports where these mutations do not avoid the interaction between GRK2 and both receptor and non-receptor substrates (27, 34). Overall, these results exclude the possibility that the mere interaction of GRK2 and the receptor is responsible for the process of desensitization. Although direct association between Gαs and GRK2-RH domain is not provided in the present work and warrants further investigation, our findings support that GRK2-RH domain is sufficient to regulate Gαs-mediated response. Surprisingly, the present findings indicate that both RH and RH-PH constructs equally behave as GRK2K220R concerning H2R desensitization. These results suggest that βγ binding by the PH domain is not required to achieve adequate inhibition of the H2R response. In this regard, Namkung et al. (33) reported that in HEK293T cells, pretreatment with pertussis toxin, which prevents the activation of Gαi/o proteins and the release of βγ subunits, affects neither GRK2 binding nor the phosphorylation of the D2 dopamine receptor. These observations contrast with the canonical model, where receptor activation releases Gβγ subunits that in turn promote PH binding to Gβγ and translocation of GRK2 to the plasma membrane. Similarly to Namkung et al. (33), our results show that receptor activation rather than PH binding to Gβγ appears to be sufficient for GRK2 phosphorylation-independent desensitization of H2R.
To evaluate the role of GRK2 in H2R internalization, the effect of wild type GRK2 and GRK2K220R on the cell surface number of sites was evaluated before and after agonist stimulation. GRK2 overexpression further enhanced amthamine-induced receptor internalization, whereas GRK2 kinase dead mutant abolished receptor endocytosis and recycling. Our present and previous observations showing that GRK2 mediates H2R phosphorylation (18) clearly indicate that H2R phosphorylation by GRK2 is essential for promoting receptor internalization and recycling, probably by allowing subsequent arrestin binding to the phosphorylated receptor. This is in accordance with the classic GRK-β arrestin pathway and with our previous observations showing that arrestin and dynamin dominant negatives mutants also impair receptor internalization (21).
The overexpression of GRK2 led to a decreased number of H2R sites even in the absence of agonist stimulation. On the other hand, binding studies showed increased basal binding sites in the presence of GRK2K220R. Taken together these results suggest that the H2R is phosphorylated in the basal state, which conditions its basal turnover. However, other authors reported increased protein and mRNA levels for calcitonin receptor in CHO cells expressing GRK2K220R, suggesting the involvement of alternative mechanisms of receptor levels regulation (25).
Cell-type dependence of GPCR internalization was previously observed with the CXCR1 (35) and BLT1 receptors (36), likely as the result of differences in the content of essential signaling components. However, in the present work we found that despite the distinct amount of GRK2 present in COS7, HEK293T, and U937 cell lines, all systems behaved in a similar way as regarding H2R desensitization, internalization, and resensitization. The use of three distinct cellular models validates our final conclusion about the existence of phosphorylation-dependent and -independent regulatory mechanisms of H2R signaling. GRK2 desensitizes the H2R (a Gαs-coupled GPCR) by a phosphorylation-independent mechanism requiring only the RH domain, but phosphorylation of the H2R mediated by GRK2 is essential for H2R internalization.
Acknowledgment
We are sincerely grateful to Dr. L. Bianciotti for critical reading of the manuscript.
This work was supported by Universidad de Buenos Aires Grants UBACyT B050 and B808, Agencia Nacional de Promoción Científica y Tecnológica Grant PICT 38318, and Consejo Nacional de Investigaciones Científicas y Tecnológicas Grant CONICET-PIP 6110. This work was also supported by Consejo Nacional de Investigaciones Científicas y Técnicas fellowships (to M. N. A.).
This work is dedicated to the memory of Florinda Morici and Clarisa Muñoz.
- GPCR
- G protein-coupled receptor
- GRK
- GPCR kinase
- H2R
- histamine H2 receptor
- G-418
- Geneticin
- amthamine
- 2-amino-4-methylthiazole-5-ethanamine
- PGE2
- prostaglandin E2
- IBMX
- isobutylmethylxanthine
- RGS
- regulator of G protein signaling
- RH
- RGS-homology domain
- PH
- pleckstrin-homology domain.
REFERENCES
- 1. Krupnick J. G., Benovic J. L. (1998) Annu. Rev. Pharmacol. Toxicol. 38, 289–319 [DOI] [PubMed] [Google Scholar]
- 2. Ferguson S. S. G. (2007) Trends Pharmacol. Sci. 28, 173–179 [DOI] [PubMed] [Google Scholar]
- 3. Abramow-Newerly M., Roy A. A., Nunn C., Chidiac P. (2006) Cell. Signal. 18, 579–591 [DOI] [PubMed] [Google Scholar]
- 4. Riddle E. L., Schwartzman R. A., Bond M., Insel P. A. (2005) Circ. Res. 96, 401–411 [DOI] [PubMed] [Google Scholar]
- 5. Roy A. A., Baragli A., Bernstein L. S., Hepler J. R., Hébert T. E., Chidiac P. (2006) Cell. Signal. 18, 336–348 [DOI] [PubMed] [Google Scholar]
- 6. Chatterjee T. K., Eapen A. K., Fisher R. A. (1997) J. Biol. Chem. 272, 15481–15487 [DOI] [PubMed] [Google Scholar]
- 7. Jean-Baptiste G., Yang Z., Greenwood M. T. (2006) Cell. Mol. Life Sci. 63, 1969–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kong G., Penn R., Benovic J. L. (1994) J. Biol. Chem. 269, 13084–13087 [PubMed] [Google Scholar]
- 9. Diviani D., Lattion A. L., Larbi N., Kunapuli P., Pronin A., Benovic J. L., Cotecchia S. (1996) J. Biol. Chem. 271, 5049–5058 [DOI] [PubMed] [Google Scholar]
- 10. Freedman N. J., Liggett S. B., Drachman D. E., Pei G., Caron M. G., Lefkowitz R. J. (1995) J. Biol. Chem. 270, 17953–17961 [DOI] [PubMed] [Google Scholar]
- 11. Oppermann M., Freedman N. J., Alexander R. W., Lefkowitz R. J. (1996) J. Biol. Chem. 271, 13266–13272 [DOI] [PubMed] [Google Scholar]
- 12. Lazari M. F., Liu X., Nakamura K., Benovic J. L., Ascoli M. (1999) Mol. Endocrinol. 13, 866–878 [DOI] [PubMed] [Google Scholar]
- 13. Dicker F., Quitterer U., Winstel R., Honold K., Lohse M. J. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 5476–5481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freedman N. J., Ament A. S., Oppermann M., Stoffel R. H., Exum S. T., Lefkowitz R. J. (1997) J. Biol. Chem. 272, 17734–17743 [DOI] [PubMed] [Google Scholar]
- 15. Sachs G., Shin J. M., Vagin O., Munson K., Weeks D., Scott D. R., Voland P. (2002) Best Pract. Res. Clin. Gastroenterol. 16, 835–849 [DOI] [PubMed] [Google Scholar]
- 16. Spitaler M. M., Hammer A., Malli R., Graier W. F. (2002) Clin. Exp. Pharmacol. Physiol. 29, 711–716 [DOI] [PubMed] [Google Scholar]
- 17. Lemos Legnazzi B., Shayo C., Monczor F., Martin M. E., Fernandez N., Brodsky A., Baldi A., Davio C. (2000) Biochem. Pharmacol. 60, 159–166 [DOI] [PubMed] [Google Scholar]
- 18. Shayo C., Fernandez N., Legnazzi B. L., Monczor F., Mladovan A., Baldi A., Davio C. (2001) Mol. Pharmacol. 60, 1049–1056 [DOI] [PubMed] [Google Scholar]
- 19. Fernández N., Monczor F., Lemos B., Notcovich C., Baldi A., Davio C., Shayo C. (2002) Mol. Pharmacol. 62, 1506–1514 [DOI] [PubMed] [Google Scholar]
- 20. Shayo C., Legnazzi B. L., Monczor F., Fernández N., Riveiro M. E., Baldi A., Davio C. (2004) Biochem. Biophys. Res. Commun. 314, 798–804 [DOI] [PubMed] [Google Scholar]
- 21. Fernandez N., Monczor F., Baldi A., Davio C., Shayo C. (2008) Mol. Pharmacol. 74, 1109–1118 [DOI] [PubMed] [Google Scholar]
- 22. Ménard L., Ferguson S. S., Zhang J., Lin F. T., Lefkowitz R. J., Caron M. G., Barak L. S. (1997) Mol. Pharmacol. 51, 800–808 [PubMed] [Google Scholar]
- 23. Luttrell L. M., Lefkowitz R. J. (2002) J. Cell Sci. 115, 455–465 [DOI] [PubMed] [Google Scholar]
- 24. Pei G., Kieffer B. L., Lefkowitz R. J., Freedman N. J. (1995) Mol. Pharmacol. 48, 173–177 [PubMed] [Google Scholar]
- 25. Horie K., Insel P. A. (2000) J. Biol. Chem. 275, 29433–29440 [DOI] [PubMed] [Google Scholar]
- 26. Tiruppathi C., Yan W., Sandoval R., Naqvi T., Pronin A. N., Benovic J. L., Malik A. B. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 7440–7445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reiter E., Marion S., Robert F., Troispoux C., Boulay F., Guillou F., Crepieux P. (2001) Biochem. Biophys. Res. Commun. 282, 71–78 [DOI] [PubMed] [Google Scholar]
- 28. Sallese M., Mariggiò S., D'Urbano E., Iacovelli L., De Blasi A. (2000) Mol. Pharmacol. 57, 826–831 [PubMed] [Google Scholar]
- 29. Lodowski D. T., Barnhill J. F., Pyskadlo R. M., Ghirlando R., Sterne-Marr R., Tesmer J. J. G. (2005) Biochemistry 44, 6958–6970 [DOI] [PubMed] [Google Scholar]
- 30. Day P. W., Wedegaertner P. B., Benovic J. L. (2004) Methods Enzymol. 390, 295–310 [DOI] [PubMed] [Google Scholar]
- 31. Dhami G. K., Anborgh P. H., Dale L. B., Sterne-Marr R., Ferguson S. S. G. (2002) J. Biol. Chem. 277, 25266–25272 [DOI] [PubMed] [Google Scholar]
- 32. Sterne-Marr R., Dhami G. K., Tesmer J. J., Ferguson S. S. (2004) Methods Enzymol. 390, 310–336 [DOI] [PubMed] [Google Scholar]
- 33. Namkung Y., Dipace C., Urizar E., Javitch J. A., Sibley D. R. (2009) J. Biol. Chem. 284, 34103–34115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dhami G. K., Dale L. B., Anborgh P. H., O'Connor-Halligan K. E., Sterne-Marr R., Ferguson S. S. (2004) J. Biol. Chem. 279, 16614–16620 [DOI] [PubMed] [Google Scholar]
- 35. Barlic J., Khandaker M. H., Mahon E., Andrews J., DeVries M. E., Mitchell G. B., Rahimpour R., Tan C. M., Ferguson S. S., Kelvin D. J. (1999) J. Biol. Chem. 274, 16287–16294 [DOI] [PubMed] [Google Scholar]
- 36. Chen Z., Gaudreau R., Le Gouill C., Rola-Pleszczynski M., Stanková J. (2004) Mol. Pharmacol. 66, 377–386 [DOI] [PubMed] [Google Scholar]