Abstract
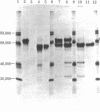
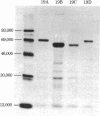
Carboxypeptidase Y, a vacuolar enzyme in Saccharomyces cerevisiae, is synthesized as a larger precursor whose apparent molecular mass is approximately 67,000 daltons. We have characterized a recessive mutation, pep4-3, that prevents maturation of this precursor. The accumulated precursor does not possess enzymatic activity. We have shown that the precursor accumulating in the pep4-3 mutant is not produced in a doubly mutant strain that also bears a mutation in the carboxypeptidase Y structural gene that eliminates production of carboxypeptidase Y. We have also shown that a nonsense fragment of carboxypeptidase Y is processed. Although there is evidence that proteinase B can catalyze the conversion of the precursor to a mature form in vitro, nonsense mutations in the structural gene for proteinase B, PRB1, do not affect the levels of carboxypeptidase Y activity, and strains bearing these mutations produce a carboxypeptidase Y of apparently normal size. Hence, proteinase B is not essential for the maturation of carboxypeptidase Y precursor in vivo. The pep4-3 mutation affects at least five vacuolar enzymes. This suggests that there is a processing event common to all of these enzymes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer H., Sigarlakie E. Localization of alkaline phosphatase in Saccharomyces cerevisiae by means of ultrathin frozen sections. J Ultrastruct Res. 1975 Feb;50(2):208–215. doi: 10.1016/s0022-5320(75)80052-9. [DOI] [PubMed] [Google Scholar]
- Bellamy G., Bornstein P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1138–1142. doi: 10.1073/pnas.68.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer P., Steyn-Parvé E. P. Isolation and purification of an acid phosphatase from baker's yeast (Saccharomyces cerevisiae). Biochim Biophys Acta. 1966 Nov 15;128(2):400–402. doi: 10.1016/0926-6593(66)90189-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cabib E., Ulane R., Bowers B. Yeast chitin synthetase. Separation of the zymogen from its activating factor and recovery of the latter in the vacuole fraction. J Biol Chem. 1973 Feb 25;248(4):1451–1458. [PubMed] [Google Scholar]
- Campbell P. N., Blobel G. The role of organelles in the chemical modification of the primary translation products of secretory proteins. FEBS Lett. 1976 Dec 31;72(2):215–226. doi: 10.1016/0014-5793(76)80973-8. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., Macgregor R. R., Chu L. L., Kimmel J. R., Hamilton J. W. Calcemic fraction-A: biosynthetic peptide precursor of parathyroid hormone. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1521–1525. doi: 10.1073/pnas.69.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIE E. W., RATNOFF O. D. WATERFALL SEQUENCE FOR INTRINSIC BLOOD CLOTTING. Science. 1964 Sep 18;145(3638):1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Early events in the biosynthesis of the lysosomal enzyme cathepsin D. J Biol Chem. 1979 Dec 10;254(23):11771–11774. [PubMed] [Google Scholar]
- Gregory R. A., Tracy H. J. Isolation of two "big gastrins" from Zollinger-Ellison tumour tissue. Lancet. 1972 Oct 14;2(7781):797–799. doi: 10.1016/s0140-6736(72)92151-4. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Müller H., Holzer H. Compartmentation of the tryptophan-synthase-proteolyzing system in Saccharomyces cerevisiae. Eur J Biochem. 1974 Oct 1;48(1):117–117. doi: 10.1111/j.1432-1033.1974.tb03748.x. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Biosynthesis of carboxypeptidase Y in yeast. Evidence for a precursor form of the glycoprotein. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1430–1436. doi: 10.1016/s0006-291x(76)80173-8. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Biosynthesis of the vacuolar yeast glycoprotein carboxypeptidase Y. Conversion of precursor into the enzyme. Eur J Biochem. 1978 Apr 17;85(2):599–608. doi: 10.1111/j.1432-1033.1978.tb12275.x. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Carbohydrate moiety of carboxypeptidase Y and perturbation of its biosynthesis. Eur J Biochem. 1978 Nov 15;91(2):567–575. doi: 10.1111/j.1432-1033.1978.tb12710.x. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Inhibition of the apparent rate of synthesis on the vacuolar glycoprotein carboxypeptidase Y and its protein antigen by turicamycin in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1976 Sep;10(3):402–410. doi: 10.1128/aac.10.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R., Aibara S., Hata T. A unique carboxypeptidase activity of yeast proteinase C. Biochim Biophys Acta. 1970 Aug 15;212(2):359–361. doi: 10.1016/0005-2744(70)90218-4. [DOI] [PubMed] [Google Scholar]
- Hayashi R., Moore S., Stein W. H. Carboxypeptidase from yeast. Large scale preparation and the application to COOH-terminal analysis of peptides and proteins. J Biol Chem. 1973 Apr 10;248(7):2296–2302. [PubMed] [Google Scholar]
- Hellerstrom C., Howell S. L., Edwards J. C., Andersson A. An investigation of glucagon biosynthesis in isolated pancreatic islets of guinea pigs. FEBS Lett. 1972 Oct 15;27(1):97–101. doi: 10.1016/0014-5793(72)80418-6. [DOI] [PubMed] [Google Scholar]
- Hemmings B. A., Zubenko G. S., Jones E. W. Proteolytic inactivation of the NADP-dependent glutamate dehydrogenase in proteinase-deficient mutants of Saccharomyces cerevisiae. Arch Biochem Biophys. 1980 Jul;202(2):657–660. doi: 10.1016/0003-9861(80)90475-0. [DOI] [PubMed] [Google Scholar]
- Hershko A., Fry M. Post-translational cleavage of polypeptide chains: role in assembly. Annu Rev Biochem. 1975;44:775–797. doi: 10.1146/annurev.bi.44.070175.004015. [DOI] [PubMed] [Google Scholar]
- Hickman S., Neufeld E. F. A hypothesis for I-cell disease: defective hydrolases that do not enter lysosomes. Biochem Biophys Res Commun. 1972 Nov 15;49(4):992–999. doi: 10.1016/0006-291x(72)90310-5. [DOI] [PubMed] [Google Scholar]
- Jones E. W. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977 Jan;85(1):23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Potts J. T., Jr, Rich A. Proparathyroid hormone: identification of a biosynthetic precursor to parathyroid hormone. Proc Natl Acad Sci U S A. 1972 Mar;69(3):643–647. doi: 10.1073/pnas.69.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Tunicamycin--an inhibitor of yeast glycoprotein synthesis. Biochem Biophys Res Commun. 1974 May 7;58(1):287–295. doi: 10.1016/0006-291x(74)90925-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampen J. O. External enzymes of yeast: their nature and formation. Antonie Van Leeuwenhoek. 1968;34(1):1–18. doi: 10.1007/BF02046409. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lenney J. F., Matile P., Wiemken A., Schellenberg M., Meyer J. Activities and cellular localization of yeast proteases and their inhibitors. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1378–1383. doi: 10.1016/0006-291x(74)90350-7. [DOI] [PubMed] [Google Scholar]
- MACFARLANE R. G. AN ENZYME CASCADE IN THE BLOOD CLOTTING MECHANISM, AND ITS FUNCTION AS A BIOCHEMICAL AMPLIFIER. Nature. 1964 May 2;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- MCLELLAN W. L., Jr, LAMPEN J. O. The acid phosphatase of yeast. Localization and secretion by protoplasts. Biochim Biophys Acta. 1963 Feb 12;67:324–326. doi: 10.1016/0006-3002(63)91832-8. [DOI] [PubMed] [Google Scholar]
- Marriott M., Tanner W. Localization of dolichyl phosphate- and pyrophosphate-dependent glycosyl transfer reactions in Saccharomyces cerevisiae. J Bacteriol. 1979 Aug;139(2):566–572. doi: 10.1128/jb.139.2.566-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Neumann N. P., Lampen J. O. The glycoprotein structure of yeast invertase. Biochemistry. 1969 Sep;8(9):3552–3556. doi: 10.1021/bi00837a010. [DOI] [PubMed] [Google Scholar]
- Neurath H., Walsh K. A. Role of proteolytic enzymes in biological regulation (a review). Proc Natl Acad Sci U S A. 1976 Nov;73(11):3825–3832. doi: 10.1073/pnas.73.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe B. D., Bauer G. E. Evidence for glucagon biosynthesis involving a protein intermediate in islets of the anglerfish (Lophius americanus). Endocrinology. 1971 Sep;89(3):642–651. doi: 10.1210/endo-89-3-642. [DOI] [PubMed] [Google Scholar]
- Opheim D. J. alpha-D-Mannosidase of Saccharomyces cerevisiae. Characterization and modulation of activity. Biochim Biophys Acta. 1978 May 11;524(1):121–130. doi: 10.1016/0005-2744(78)90110-9. [DOI] [PubMed] [Google Scholar]
- Rigopoulou D., Valverde I., Marco J., Faloona G., Unger R. H. Large glucagon immunoreactivity in extracts of pancreas. J Biol Chem. 1970 Feb 10;245(3):496–501. [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTTON D. D., LAMPEN J. O. Localization of sucrose and maltose fermenting systems in Saccharomyces cerevisiae. Biochim Biophys Acta. 1962 Jan 29;56:303–312. doi: 10.1016/0006-3002(62)90567-x. [DOI] [PubMed] [Google Scholar]
- Skudlarek M. D., Swank R. T. Biosynthesis of two lysosomal enzymes in macrophages. Evidence for a precursor of beta-galactosidase. J Biol Chem. 1979 Oct 25;254(20):9939–9942. [PubMed] [Google Scholar]
- Steiner D. F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967 Aug 11;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- Susani M., Zimniak P., Fessl F., Ruis H. Localization of catalase A in vacuoles of Saccharomyces cerevisiae: evidence for the vacuolar nature of isolated "yeast peroxisomes". Hoppe Seylers Z Physiol Chem. 1976 Jul;357(7):961–970. doi: 10.1515/bchm2.1976.357.2.961. [DOI] [PubMed] [Google Scholar]
- Tager H. S., Steiner D. F. Isolation of a glucagon-containing peptide: primary structure of a possible fragment of proglucagon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2321–2325. doi: 10.1073/pnas.70.8.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. H., Tiller G. E., Jr, Reynolds L. W., Miller C. S., Bace J. W. Increased levels of sialic acid associated with a sialidase deficiency in I-cell disease (mucolipidosis II) fibroblasts. Biochem Biophys Res Commun. 1976 Jul 12;71(1):188–195. doi: 10.1016/0006-291x(76)90267-9. [DOI] [PubMed] [Google Scholar]
- Tung A. K., Zerega F. Biosynthesis of glucagon in isolated pigeon islets. Biochem Biophys Res Commun. 1971 Oct 15;45(2):387–395. doi: 10.1016/0006-291x(71)90831-x. [DOI] [PubMed] [Google Scholar]
- Wolf D. H., Weiser U. Studies on a carboxypeptidase Y mutant of yeast and evidence for a second carboxypeptidase Activity. Eur J Biochem. 1977 Mar 1;73(2):553–556. doi: 10.1111/j.1432-1033.1977.tb11350.x. [DOI] [PubMed] [Google Scholar]
- Woodbury W., Spencer A. K., Stahman M. A. An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem. 1971 Nov;44(1):301–305. doi: 10.1016/0003-2697(71)90375-7. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. And now, "big, big" gastrin. Biochem Biophys Res Commun. 1972 Jul 25;48(2):391–395. doi: 10.1016/s0006-291x(72)80063-9. [DOI] [PubMed] [Google Scholar]
- Zubenko G. S., Mitchell A. P., Jones E. W. Septum formation, cell division, and sporulation in mutants of yeast deficient in proteinase B. Proc Natl Acad Sci U S A. 1979 May;76(5):2395–2399. doi: 10.1073/pnas.76.5.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]