Abstract
Although there is growing evidence for a role of the innate immune response in Parkinson's disease, the nature of any humoral response in dopaminergic degeneration is uncertain. Here we report on a protracted N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of dopaminergic death that potentially allows a more full adaptive humoral response to develop. Rag2 mutant mice that lack the full adaptive response (deficient in both T and B cells) are resistant to dopaminergic death and behavioral deficiencies in this model. These mice are resensitized after reconstitution with WT splenocytes. To more directly provide evidence for humoral/IgG involvement, we show that deficiency of Fcγ receptors, which are critical for activation of macrophages/microglia by binding to IgGs, is also protective in this protracted model. FcγR-deficient mice display improved behavior and impaired microglial activation. Interestingly, however, Rag2 mutant but not FcγR-deficient mice are resistant to a more standard N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine paradigm where death is more rapid. Taken together, these data indicate that, provided sufficient time, the humoral arm of the adaptive immune system can play a critical functional role in modulating the microglial response to dopaminergic degeneration and suggest that this humoral component may participate in degeneration in Parkinson's disease.
Keywords: Humoral Response, Immunodeficiency, Lymphocyte, Neurodegeneration, Parkinson's Disease
Introduction
Degeneration of dopamine (DA)2 neurons of the substancia nigra pars compacta (SNpc) is a key hallmark of Parkinson's disease (PD). However, the mechanism by which this death occurs is not completely clear (1–3). Growing evidence indicates that oxidative stress, mitochondrial damage, and ubiquitin-proteasomal dysfunction participate in the degenerative process of DA neurons (4). Interestingly, an increasing number of observations also indicate the importance of the immune response in PD and models of PD. Most of this evidence, however, has centered around the innate neuroinflammatory response. For example, microgliosis-mediated DA cell death, particularly via oxidative insult, has been reported in different models of PD (5). NADPH oxidase, the largest superoxide-producing enzyme in microglia, and inducible nitric oxide synthase are involved in N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine -induced death of DA neurons (6–8). Moreover, the classic inflammagen lipopolysaccharide causes neurodegeneration of DA neurons through Toll-like receptor 4-dependent pathways (9–11). More recently, however, increasing evidence also suggests a role for the adaptive immune response in models of PD.
The first correlative evidence suggesting a role for a cellular adaptive response was the observations of infiltrating T cells in a number of PD-related contexts. For example, a substantial number of T cells were detected in the SNpc of PD patients (12, 13). Similarly, MPTP treatment results in infiltration of T cells to the SNpc and striatum as well as an increase in expression of microglial MHC class II antigen (14, 15). The required role of T cells, in particular CD4-positive T helper cells, was highlighted by studies that show that Rag, Scid, and CD4-negative mice are resistant to dopaminergic loss induced by MPTP (2, 13). The role of the humoral response, however, is unclear. Antibodies to DA neurons have been detected in the cerebral spinal fluid of PD patients. These antibodies were found in 78% of patients with clinical PD versus 3% of control patients (16). In addition, the expression of CD23, a type of Fc receptor, is detected in the substancia nigra and striatum of PD patients but not in control patients (17). Likewise, MPTP has been shown to increase Fcγ receptor expression (14, 15, 17). However, the role/participation of FcγR and the humoral response in DA degeneration is unknown.
Here we report a novel protracted degeneration paradigm to study the adaptive immunopathogenic mechanisms in a mouse model of dopaminergic loss. With this model, we provide evidence that the adaptive immune response, in particular the FcγRs, can play a critical role in DA loss in vivo.
EXPERIMENTAL PROCEDURES
Experimental Animals
The following strains were obtained from Taconic Laboratory (Germantown, NY): Black 6+/+ (C57BL/6Ntac), Rag2−/− (B6.129S6-Rag2tm1FwaN12), and Fcer1g−/− (B6.129P2-Fcer1gtmRavN12) mice. All mice were backcrossed with C57BL/6 mice for 12 generations and intercrossed to homozygosity. Animals were housed in a barrier facility specialized for immunocompromised mice because of their high susceptibility to infection. Mice were maintained on a 12-h light/dark cycle with lights on at 6 am and a room temperature of 21 °C. Animals had ad libitum access to slightly acidified water and mouse chow from Ralston Purina (St. Louis, MO). KO and WT mice were interbred to produce littermate controls. All experimental procedures met the Canadian Council on Animal Care guidelines and were approved by the University of Ottawa Committee for Animal Care.
Immunohistochemistry
Mice were anesthetized and intracardially perfused with 4% paraformaldehyde. Brains were removed, fixed overnight with 4% paraformaldehyde and subsequently cryoprotected in 10% sucrose before cryosectioning into 14- to 40-μm free-floating sections. Striatal and substancia nigra sections were immunostained with rat anti-CD11b (1:200, Serotec, Oxford, UK), rat anti-dopamine transporters (DAT, 1:2000; Millipore, Bedford, MA), rat anti-ΔFosB (1:1000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or mouse anti-tyrosine hydroxylase (TH, 1:10,000, Immunostar, Hudson, WI). Primary antibodies were visualized with diaminobenzidine or cy3.
Neurochemical Analysis
Mice were decapitated, and their striatum was obtained through micropunches with a 1-mm-diameter biopsy needle and immediately stored in a freezer (−80 °C). Measurements of DA and its metabolite (HVA) were determined using HPLC methods.
N-Methyl-4-phenylpyridinium Ion (MPP+) Measurements
HPLC analysis was used to measure the MPP+ ion 90 min after MPTP administration as described previously (18).
MPTP Administration
Seven- to ten-week old mice were used for injections. All animals were injected in the regular animal care facility with extra precautions to prevent infection, such as using a portable fume hood during injections and handling these mice first during all wellness assessments. MPTP (Sigma) was administered intraperitoneally using the standard subchronic or more protracted paradigm. The subchronic standard paradigm consists of injecting 30 mg/kg of MPTP once daily for 5 consecutive days and sacrificing 14 days after the start of injections. For the protracted treatment, we injected 10 mg/kg of MPTP on the first and third day of each week for 4 consecutive weeks. These mice were sacrificed 5 weeks after the start of injections. Control mice received an equal volume of saline (0.9%).
Passive Transfer
Single-cell suspensions were prepared from spleens of MPTP-treated WT mice. Recipient Rag2−/− mice received two i.v. injections, 4 h apart, of 107 total splenocytes in 0.2 ml of phosphate buffered saline solution. After a 4-week reconstitution period, mice were treated with the protracted MPTP paradigm or received an equal volume of saline (0.9%) solution, as described above.
Pole Test
The time of descend to the ground was recorded after mice were individually placed upright near the top of a 2-foot pole. The pole test was administered on the sixth day of every week during chronic MPTP treatment. Mice have a natural tendency to avoid heights, and their descend to the ground offers a unique perspective into motor output.
Quantification of Neuronal Survival
DA neurons in the SNc were assessed for survival using the dopaminergic cell marker TH. A minimum of two sections from each of five levels around the medial terminal nucleus region of the SNc (from −2.92 to −3.52 relative to the bregma) were estimated for the number of TH-positive neurons. The average total number of TH-positive neurons was determined by Abercrombie's correction. Quantification of TH immunoreactivity was substantiated by a second group of mice interbred to produce littermate controls. The average total number of TH-positive neurons was estimated using Stereo investigator (version 6, MicrobrightField, Williston, VT) and applying optical fractionation to 40-μm sections from the entire SNc (-2.54 to −3.88 relative to the bregma).
Quantification of Striatal Immunohistochemistry
Density of striatal DA fiber, DAT, and ΔFosB was quantified using an Eclipse densitometry analysis (18). Each tissue density was compared relative to its own background.
Statistical Analysis
All data are expressed as mean ± S.E. Analyses of all values were performed using one-way ANOVA followed by Tukey's post-hoc test, to determine differences among normal distribution of means.
RESULTS
A More Protracted Model of MPTP-induced Degeneration
We examined dopaminergic loss induced by the neurotoxin MPTP as a prototypical toxin model of degeneration (19). In this paradigm, the metabolite of MPTP, MPP+, is selectively taken up by dopamine neurons and targets complex 1 of the mitochondria, resulting in increased oxidative stress and eventual dopaminergic loss (4, 20–22). It is a widely utilized model of DA loss, made even more relevant because genetic depletion of known PD genes do not yet result in substantial dopamine death in mice (23). It is important to note, however, that the MPTP model has obvious caveats in its relevance to PD. However, it likely provides important insights into how DA neurons may die in vivo. To test the potential role of the adaptive response, in particular the FcγR in dopaminergic loss, we reasoned that we required a more protracted model of dopamine loss to allow time for a humoral response to develop. Typical acute (20 mg × 4 in 1 day) or subchronic (30 mg/kg × 5 days) lead to significant dopaminergic loss within 1 week of treatment (13, 24). We chose a more protracted dosing paradigm consisting of an intraperitoneal injection (10 mg/kg) twice a week for a month and sacrificing 5 weeks after the start of MPTP injections. Fig. 1 illustrates the timing of DA neuron loss with this latter dosage. To ensure that the bulk of dopamine loss did not occur early, we examined degeneration at the 2-week time point. No significant loss was observed at this time, whereas a significant loss (43.93%) was detected after 5 weeks of MPTP treatment. These results indicate a more protracted time frame of loss that would potentially allow time for the humoral component of the immune response to participate in the degeneration of MPTP-treated mice.
FIGURE 1.
Chronic MPTP elicits DAergic neuron death. A, representative photomicrographs of TH+ cells in the SNpc of the indicated non-littermate treatment groups. The SNc is demarcated. B, quantification of dopaminergic cell bodies 2 or 5 weeks after the start of chronic MPTP intoxication. Scale bars = 250 μm. Error bars represent mean ± S.E. ANOVA, **, p < 0.01; n = five to seven animals per group.
The Rag2-deficient Mice Are Resistant to Chronic MPTP
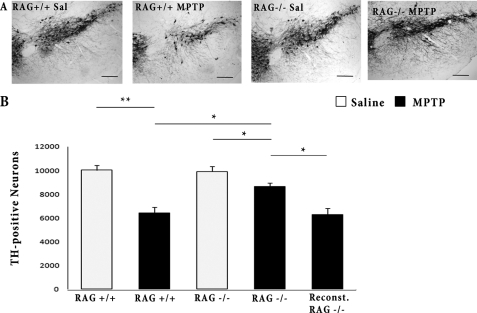
We first tested whether any aspects of the adaptive immune response might play a role in the observed dopaminergic loss in this more protracted model of loss. We examined the contribution of both T cells and B cells to death by evaluating Rag2−/− mice in our chronic paradigm. Chronic MPTP induces significantly less DA neurodegeneration in Rag2−/− mice compared with their Rag2+/+ littermates (12.83% versus 35.94%) (Fig. 2). To ensure that these differences were not due to differences in the production of MPP+, the active metabolite of MPTP, we measured MPP+ production by HPLC analyses. There was no detectable difference in MPP+ levels in WT and Rag2-deficient mice (data not shown). We also performed a rescue experiment whereby we passively transferred splenocytes from Rag2+/+ mice treated with MPTP to Rag2−/− mice. One month later, we looked for resensitization in reconstituted Rag2−/− mice. Indeed, reconstituted Rag2−/− mice are more susceptible to MPTP-induced DA neuron death compared with non-reconstituted littermates (Fig. 2), further supporting the importance of the immune system in dopaminergic loss.
FIGURE 2.
Rag2−/− mice display attenuated TH+ cell loss after chronic MPTP administration. A, representative photomicrographs of TH+ cells in the SNpc of the indicated littermate treatment groups. B, quantification of dopaminergic cell bodies 5 weeks after the start of saline (Sal) or chronic MPTP intoxication by stereology, as described under “Experimental Procedures”. Scale bars = 250 μm. Error bars represent mean ± S.E. ANOVA, *, p < 0.05; **, p < 0.01; n = five to seven animals per group.
We next examined the effects of Rag2 deficiency in the target region of the SNc, the striatum that contains the terminals from the dopamine neurons in the SNc. We analyzed for the expression of TH and DAT as markers for these terminals. Chronic MPTP induced greater TH-reactive nerve terminal loss in Rag2+/+ mice compared with Rag2−/− littermates (Fig. 3). Likewise, the DAT expression profile mirrors that of DA fiber density. DAT density is less in RAG+/+ mice treated with MPTP than in RAG−/− littermates (Fig. 3). Finally, we examined levels of DA and its metabolite HVA in striatal punches by HPLC analyses. There was a dramatic loss in both DA and HVA levels following chronic MPTP treatment at 5 weeks in the Rag 2+/+ mice, 73.1% and 82.1%, respectively (Fig. 4). These reductions were ameliorated in the Rag2−/− mice (47.2% and 43.1%, respectively).
FIGURE 3.
Dopamine nerve terminals are protected in Rag2−/− mice treated with chronic MPTP. A, representative photomicrographs showing TH immunoreactivity from the striatum of indicated littermate treatment groups. Sal, saline. B, optical density quantification of striatal TH+ fiber density. C, representative photomicrographs of striatal sections stained for DATs from indicated littermate groups. D, optical density quantification of striatal DATs. Scale bars = 50 μm (A) and 200 μm (C). Error bars represent mean ± S.E. ANOVA, *, p < 0.05; ***, p < 0.001; n = six to eight animals per group.
FIGURE 4.
DA and HVA levels in striatum of Rag2−/− mice. Levels of DA and HVA were measured using HPLC from striatal punch biopsies of the indicated littermate treatment groups. Sal, saline. Error bars represent mean ± S.E. ANOVA, *, p < 0.05; **, p < 0.01; n = five to six animals per group.
Previous work from a number of groups indicates that damage to the nigrostriatal dopaminergic pathway results in compensatory responses in the postsynaptic neurons of the striatum. Although this response is complex, we have shown one marker that changes is ΔFosB, which is induced postsynaptically, presumably as an indication of hyperactivation mediated by denervation of the dopaminergic tracts, to compensate for reduced dopaminergic neurotransmission. We have used this measure as an indication of altered basal ganglion circuitry (25–27). As shown in Fig. 5, Rag2+/+ mice intoxicated with MPTP display a significantly higher density in striatal ΔFosB expression, suggesting that nigrostriatal tract degeneration sufficiently alters basal ganglia function. In Rag2−/− mice, in contrast, this response is blunted.
FIGURE 5.
Rag2−/− mice treated with chronic MPTP exhibit reduced expression of striatal Δ-Fos B, a marker for postsynaptic changes in the denervated striatum. A, representative photomicrographs illustrating ΔFosB immunoreactivity in the striatum. B, quantification of striatal Δ-Fos B optical density. Sal, saline. Scale bars = 100 μm. Error bars represent mean ± S.E. ANOVA, **, p < 0.01; ***, p < 0.001; n = six to eight animals per group.
Microgliosis plays an important process in the adaptive immune response. Alongside DA neuron death, we examined for markers of neuroinflammation using the microglia-specific CD11b receptor, which is up-regulated upon microgliosis (28). Rag2+/+ mice treated with chronic MPTP display significantly higher CD11b expression within the MTN region of the SNpc (Fig. 6). However, in the Rag2-deficient mice, MPTP did not elicit any notable microglial activation.
FIGURE 6.
Rag2−/− mice exhibit less neuroinflammation. A, representative photomicrographs of microglia stained with anti-CD 11b. B, quantification of morphologically activated microglia around the MT nucleus of the ventral SNpc. Sal, saline. Scale bars = 100 μm. Error bars represent mean ± S.E. ANOVA, **, p < 0.01; n = six to eight animals per group.
Finally, we evaluated the behavioral consequences of chronic MPTP toxicity and DA loss in WT and Rag2-deficient littermate controls. Initially, we examined for any changes in general locomotor activity using beam break cages. However, no reproducible changes in locomotor behavior could be detected (data not shown). We hypothesized that perhaps a more challenging test was required to unmask deficits brought on by the observed dopaminergic loss because typically more than a 60–80% loss is required to see major differences in general locomotion. The pole test measures the time it takes for mice to descend a 2-foot pole. The first 2 weeks of testing after initiation of the MPTP challenge did not show any detectable differences in any groups. However, at week 3, differences between WT non-treated and MPTP-treated mice could start to be detected. At 4 and 5 weeks, these differences became accentuated. This time course of the motor deficit is consistent with the observed destruction of the nigrostriatal axis. Importantly, Rag2-deficient mice displayed significantly improved behavior when compared with WT MPTP- treated counterpart. Rag2−/− mice do not develop impairments when compared with its untreated counterpart even after 5 weeks of starting chronic MPTP treatment (Fig. 7). This evidence suggests that neuroprotection afforded by Rag2 deficiency translates into motor movement preservation and that surviving DA neurons are indeed functional.
FIGURE 7.
Rag2−/− mice are resistant against MPTP-induced motor movement impairments, unlike Rag2+/+ littermate mice. The pole test was given to mice throughout the dosing paradigm, as described under “Experimental Procedures.” The time of descend to the ground was recorded after mice were individually placed upright near the top of a 2-foot pole. Sal, saline. Error bars represent mean ± S.E. ANOVA, *, p < 0.05; **, p < 0.01; n = six to eight animals per group.
Fcγ Receptors Play a Role in the Protracted Paradigm of MPTP Induced Loss
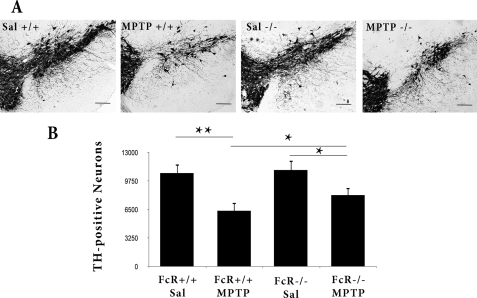
The evidence above supports a role for the adaptive immune system. However, the contribution of any humoral response cannot be ascertained using Rag mice because both T and B cells are affected. To more carefully evaluate the potential role of the humoral response, we next examined the role of FcγRs in our protracted MPTP paradigm. Importantly, chronic MPTP treatment induces less DA cell body degeneration in FcγR−/− mice compared with their FcγR+/+ littermates (Fig. 8). However, this protection was less than that afforded by Rag2 deficiency (Fig. 2).
FIGURE 8.
FcγR−/− mice exhibit attenuated DA cell loss after chronic MPTP treatment. A, representative photomicrographs of TH+ cells in the SNpc of the indicated littermate treatment groups. B, quantification of dopaminergic cell bodies 5 weeks after the start of saline (Sal) or chronic MPTP intoxication by stereology, as described under “Experimental Procedures.” Scale bars = 250 μm. Error bars represent mean ± S.E. ANOVA, *, p < 0.05; **, p < 0.01; n = five to seven animals per group.
This partial neuroprotection afforded in the SNpc extends down the nigrostriatal axis, as demonstrated by our immunohistochemical analysis of the striatum for dopamine terminal loss. Striatal density of both TH and DAT were significantly increased in FcγR−/− mice when compared with WT littermate controls (Fig. 9). Similarly, improvements in both dopamine and HVA levels as measured by HPLC were observed in the FcγR-deficient mice (Fig. 10). This protection, particularly with the dopamine, was relatively mild, however. Striatal ΔFosB staining was also attenuated in FcγR-deficient mice treated with chronic MPTP when compared with WT MPTP-treated controls (Fig. 11). Finally, CD11b expression was also attenuated with FcγR deficiency in response to chronic MPTP when compared with WT controls (Fig. 12).
FIGURE 9.
Dopamine nerve terminals are protected in FcγR−/− mice treated with chronic MPTP. A, representative photomicrographs showing TH+ fiber density from the striatum of indicated littermate treatment groups. Sal, saline. B, optical density quantification of striatal TH+ fiber density. C, representative photomicrographs of striatal sections stained for DATs from indicated littermate groups. D, optical density quantification of striatal DATs. Scale bars = 50 μm in (A) and 200 μm (C). Error bars represent mean ± S.E. ANOVA, *, p < 0.05; **, p < 0.01; ***, p < 0.001; n = six to eight animals per group.
FIGURE 10.

DA and HVA levels in striatum of FcγR−/− mice. Levels of DA and HVA were measured using HPLC from striatal punch biopsies of the indicated littermate treatment groups. Sal, saline. Error bars represent mean ± S.E. ANOVA, *, p < 0.05; **, p < 0.01; n = five to six animals per group.
FIGURE 11.
FcγR−/− mice treated with chronic MPTP exhibit reduced expression of striatal Δ-Fos B. A, representative photomicrographs illustrating ΔFosB immunoreactivity in the striatum. Sal, saline. B, quantification of striatal ΔFosB optical density. Scale bars = 100 μm. Error bars represent mean ± S.E. ANOVA, *, p < 0.05; **, p < 0.01; n = six to eight animals per group.
FIGURE 12.
FcγR−/− mice exhibit less neuroinflammation. A, representative photomicrographs of microglia stained with anti-CD11b. Sal, saline. B, quantification of morphologically activated microglia around the MT nucleus of the ventral SNpc. Scale bars = 100 μm. Error bars represent mean ± S.E. Representative photomicrographs of TH+ cells in the SNpc of the indicated littermate treatment groups. ANOVA, *, p < 0.05; **, p < 0.01; n = six to eight animals per group.
The pole test was administered to the FcγR mice throughout the dosing paradigm to corroborate the histological findings. After 3 to 5 weeks of chronic MPTP administration, FcγR+/+ mice developed motor movement deficits (Fig. 13). Consistent with our histological protection and ΔFosB results, FcγR−/− mice were significantly protected from these deficits at weeks 3 and 4. However, the behavioral improvement was transient because the deficit was again observed at week 5. Other tests, such as the 24-h beam break locomotion test, showed no difference in any groups as described with the Rag2 mice above (data not shown).
FIGURE 13.
FcγR−/− mice are resistant against early MPTP-induced motor movement impairments, unlike FcγR+/+ littermates mice. Pole test analysis of mice treated with saline (Sal) or MPTP during the 5-week paradigm. Error bars represent mean ± S.E. ANOVA, *, p < 0.05; **, p < 0.01; n = six to eight animals per group.
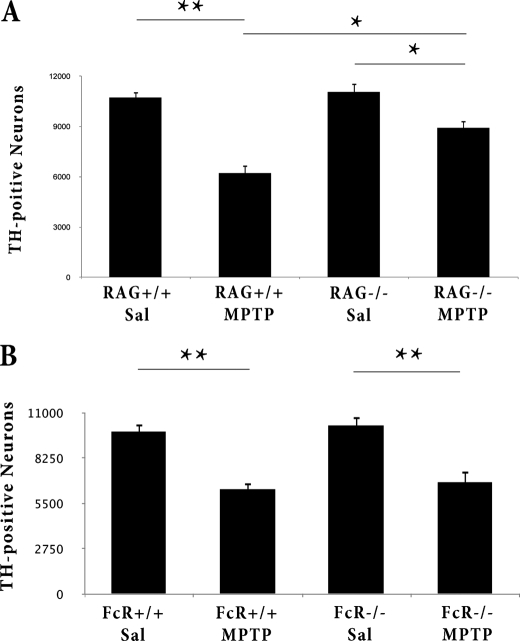
Rag2- but Not FcγR-deficient Mice Are Resistant to More Acute MPTP Toxicity
Our results above with the FcγR−/− mice support a role of the humoral arm of the adaptive immune response in chronic MPTP-induced loss. To further support this notion, we reasoned that the FcγR under a more acute MPTP paradigm of death will not functionally participate in DA neuron death. Consistent with this model, FcγR+/+ mice and their FcγR−/− littermates exhibit equal DA neurotoxicity after acute MPTP treatment (Fig. 14). Note that this is not to imply that the more acute MPTP paradigm does not activate the humoral response. Indeed, acute MPTP treatment has been reported to elicit antibody generation to selective epitopes (2). However, the functional consequences of such activation are diminished likely because of the more rapid onset of DA loss.
FIGURE 14.
Rag2−/− mice but not FcγR−/− mice display attenuated TH+ cell loss after acute MPTP administration. Quantification of dopaminergic cell bodies in the SNpc of Rag2 mice (A) and FcγR mice (B) after acute MPTP intoxication by stereology, as described under “Experimental Procedures.” Error bars represent mean ± S.E. ANOVA, **, p < 0.01; n = five to six animals per group.
We also tested Rag2-deficient mice in this acute paradigm. Consistent with previous reports of T- and B cell-deficient mice (2, 13), these Rag2−/− mice were also significantly protected from acute MPTP induced loss, similar to our more chronic paradigm (Fig. 14).
DISCUSSION
Growing evidence suggested the participation of the humoral response in PD. These data include the observations of IgGs from PD patients reacting with dopamine neurons (16) and IgG deposition in lewy bodies (29). Altered peripheral humoral function has also been reported in PD patients, and elevated levels of IgGs to heat shock proteins were described (30). B cell and T cell infiltrates have also been demonstrated in an α-synuclein overexpression model of PD (31). We set out to provide more functional data on the required nature of this response as well as shed light on which antibody effector function may promote dopaminergic degeneration. To achieve this, we developed a more protracted/chronic MPTP model potentially more conducive to participation of a humoral response in DA degeneration. Histological analysis of wild-type mice indicates that 1 month of protracted MPTP treatment elicits significant DA neuron loss in the SNpc. DA neuron injury is observed later in the dosing paradigm. The loss of DA cell bodies was accompanied by nerve terminal degeneration and compensatory changes in postsynaptic striatal neurons. Moreover, mice incur motor movement impairments late in the MPTP dosing paradigm. This delayed and progressive neurodegenerative nature is consistent with the temporal expression of the adaptive immune response. Likewise, it more closely resembles PD pathogenesis than traditional acute MPTP treatment, which elicits more rapid neurotoxicity.
Using this model, we first detected Rag2−/− mice to have less dopaminergic death than littermate WT controls. Rag2-deficient mice have a disruption in the recombination activating gene 2. This gene produces an enzyme critical in the generation of T and B cell receptors. Therefore, these mice are devoid of mature T and B cells (the major cellular components of the adaptive immune response) and are unable to produce antibodies (32, 33). In addition, this protection was associated with reduced terminal loss, significant preservation of dopamine levels, improvement in markers of postsynaptic function, and improved behavioral outcomes. These results are consistent with previous reports of protection mediated by a number of immune-compromised mice following more acute paradigms of MPTP exposure (2, 13).
After confirming lymphocytic involvement in our chronic model, we next looked at potential downstream effectors, in particular FcγR function, because of its involvement in antibody-microglia interactions and its importance in adaptive immunity (1). The FcγR−/− mice we used are deficient in a γ chain that is required for receptor assembly and signal transduction of FcγRIII and FcϵRI receptors (34). Accordingly, FcγR deficiency leads to a functional impairment of macrophages/microglia, neutrophils, mast cells, basophils, and NK cell binding capabilities for IgGs (34). As with the Rag mice, FcγR-deficient mice also showed significant protection in all the parameters examined. However, the protection observed in FcγR−/− mice was qualitatively less than that observed with Rag2−/− mice. For example, although behavioral improvements were observed with FcγR deficiency, this protection was transient. The potential reasons for this are several. Rag2 deficiency eliminates multiple arms of the adaptive immune response, whereas FcγR deficiency is obviously more limited in this respect. Relevant to this, FcγR involvement does not occur in more acute paradigms of MPTP, whereas Rag2 deficiency is protective in both acute and chronic models. Nonetheless, our results clearly demonstrate the involvement of FcγR in dopaminergic loss induced in a system where the adaptive immune system is allowed to develop fully.
Our observations of FcγR involvement in MPTP-induced toxicity is consistent with other reports that FcγR is up-regulated in postmortem PD brains (35) and following MPTP treatment in experimental animal models (14, 15, 17). IgGs produced against DA neurons could promote neurodegeneration via the FcγR (31, 35, 36). Upon engaging antibodies in the CNS, FcγR causes the activation of immune cells, secretion of neurotoxic and neuroinflammatory factors, and stimulates phagocytosis in processes termed antibody-dependent cell-mediated cytotoxicity and antibody-mediated phagocytosis, respectively (37, 38). The role of FcγRs on microglia activation are a particularly interesting possibility, provided the large number of resident immune cells in the nigral region and a large number of reports suggesting the importance of these cells in nigral degeneration (39). An initial microglial response as part of the innate immune response is consistent with a role of inflammation in PD. However, the humoral immune response may interact with the innate inflammatory system, leading to a more sustained response (1, 40). Consistent with this view, we showed that FcγR−/− mice demonstrate less microglial activation when compared with their control littermates.
Our results, as well as those of others with a variety of immune-compromised animal models, clearly suggest the importance of the cellular adaptive immune response following MPTP treatment. Helper T cells likely play a major role in this degeneration because CD4-deleted mice specifically show decreased degeneration (13). How T cells are activated in the context of MPTP is unclear. MPTP treatment has been shown to not only elicit lymphocytic infiltration but also to increase expression of microglial MHC class II antigen (14, 15). The nature of potential antigens eliciting an adaptive response is unclear. However, there have been numerous proposals of “neopeptide” generation initiated by oxidative stress. For example, microglia generate superoxide and nitric oxide that can rapidly interact to form the peroxynitrite (ONOO-), a highly reactive oxidative species capable of traversing cell membranes and causing lipid peroxidation, DNA damage, mitochondrial inhibition, and nitrotyrosine formation (41, 42). DA quinine has also been shown to be equally reactive. These oxidized molecules may serve as epitopes stimulating an adaptive response.
One proposed neopeptide is nitrosine-modified synuclein. A single injection of MPTP in squirrel monkeys was sufficient to cause a buildup of α-synuclein in midbrain DA neurons, which stained positive for nitratred and phosphorylated α-synuclein (43). MPTP treatment also causes nitrosine-modified α-synuclein buildup in cervical lymph nodes of mice (2). These mice also mice generate antibodies to native and nitrated α-synuclein. We propose that the generation of the humoral response such as that observed above and consequent activation of Fcγ receptor complexes is critical to mediate both the microglial response and dopaminergic degeneration in more chronic models of MPTP toxicity.
Taken together, these data further support the critical nature of the adaptive immune system in DA neuron injury in a mouse model of PD. The discovery of this role has profound implications for how we view PD as a neurodegenerative disease that relies, at least in part, on both the innate and adaptive immune response to mediate degeneration.
Acknowledgments
We thank Dr. Harry Atkins and Dr. Serge Przedborski for help in editing this manuscript.
This work was supported by grants from the Canadian Institutes of Health Research (CIHR), the Heart and Stroke Foundation of Ontario (HSFO), the Parkinson's Disease Foundation (PDF), the Parkinson's Society of Canada (PSC), Michael J. Fox Foundation, Neuroscience Canada, the Centre for Stroke Recovery (CSR) Canadian Stroke Network, and World Class University program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology, South Korea Grant R31-2008-000-20004-0.
- DA
- dopamine
- SNpc
- substancia nigra pars compacta
- PD
- Parkinson's disease
- DAT
- dopamine transporter
- TH
- tyrosine hydroxylase
- MPTP
- N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP+
- N-methyl-4-phenylpyridinium ion
- ANOVA
- analysis of variance
- Fcr
- Fc gamma
- FcrR
- Fc gamma receptor
- SNc
- Substantia Nigra Compacta
- HVA
- homovanillic acid.
REFERENCES
- 1. Cao S., Theodore S., Standaert D. G. (2010) Mol. Neurodegener. 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benner E. J., Banerjee R., Reynolds A. D., Sherman S., Pisarev V. M., Tsiperson V., Nemachek C., Ciborowski P., Przedborski S., Mosley R. L., Gendelman H. E. (2008) PLoS ONE 3, e1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGeer P. L., Itagaki S., Boyes B. E., McGeer E. G. (1988) Neurology 38, 1285–1291 [DOI] [PubMed] [Google Scholar]
- 4. Dauer W., Przedborski S. (2003) Neuron 39, 889–909 [DOI] [PubMed] [Google Scholar]
- 5. Gao H. M., Hong J. S., Zhang W., Liu B. (2002) J. Neurosci. 22, 782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Almer G., Vukosavic S., Romero N., Przedborski S. (1999) J. Neurochem. 72, 2415–2425 [DOI] [PubMed] [Google Scholar]
- 7. Liberatore G. T., Jackson-Lewis V., Vukosavic S., Mandir A. S., Vila M., McAuliffe W. G., Dawson V. L., Dawson T. M., Przedborski S. (1999) Nat. Med. 5, 1403–1409 [DOI] [PubMed] [Google Scholar]
- 8. Wu D. C., Teismann P., Tieu K., Vila M., Jackson-Lewis V., Ischiropoulos H., Przedborski S. (2003) Proc. Natl. Acad. Sci. 100, 6145–6150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arai H., Furuya T., Yasuda T., Miura M., Mizuno Y., Mochizuki H. (2004) J. Biol. Chem. 279, 51647–51653 [DOI] [PubMed] [Google Scholar]
- 10. De Pablos R. M., Herrera A. J., Villarán R. F., Cano J., Machado A. (2005) FASEB J. 19, 407–409 [DOI] [PubMed] [Google Scholar]
- 11. Lehnardt S., Massillon L., Follett P., Jensen F. E., Ratan R., Rosenberg P. A., Volpe J. J., Vartanian T. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 8514–8519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Appel S. H., Beers D. R., Henkel J. S. (2010) Trends Immunol. 31, 7–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brochard V., Combadière B., Prigent A., Laouar Y., Perrin A., Beray-Berthat V., Bonduelle O., Alvarez-Fischer D., Callebert J., Launay J. M., Duyckaerts C., Flavell R. A., Hirsch E. C., Hunot S. (2009) J. Clin. Invest. 119, 182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurkowska-Jastrzebska I, Wrońska A, Kohutnicka M, Czlonkowski A, Czlonkowska A. (1999) Acta Neurobiol. Exp. (Wars.) 59, 1–8 [DOI] [PubMed] [Google Scholar]
- 15. Kurkowska-Jastrzebska I., Wrońska A., Kohutnicka M., Czlonkowski A., Czlonkowska A. (1999) Exp. Neurol. 156, 50–61 [DOI] [PubMed] [Google Scholar]
- 16. Carvey P. M., McRae A., Lint T. F., Ptak L. R., Lo E. S., Goetz C. G., Klawans H. L. (1991) Neurology 41, 53–60 [DOI] [PubMed] [Google Scholar]
- 17. Hunot S., Dugas N., Faucheux B., Hartmann A., Tardieu M., Debré P., Agid Y., Dugas B., Hirsch E. C. (1999) J. Neurosci. 19, 3440–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crocker S. J., Smith P. D., Jackson-Lewis V., Lamba W. R., Hayley S. P., Grimm E., Callaghan S. M., Slack R. S., Melloni E., Przedborski S., Robertson G. S., Anisman H., Merali Z., Park D. S. (2003) J. Neurosci. 23, 4081–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Serra P. A., Pluchino S., Marchetti B., Desole M. S., Miele E. (2008) Parkinsonism Relat. Disord. 14, S189-S193 [DOI] [PubMed] [Google Scholar]
- 20. Storch A., Ludolph A. C., Schwarz J. (2004) J. Neural Transm. 111, 1267–1286 [DOI] [PubMed] [Google Scholar]
- 21. Yokoyama H., Kuroiwa H., Yano R., Araki T. (2008) Neurol. Sci. 29, 293–301 [DOI] [PubMed] [Google Scholar]
- 22. Banerjee R., Starkov A. A., Beal M. F., Thomas B. (2009) Biochim. Biophys. Acta 1792, 651–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dawson T. M., Ko H. S., Dawson V. L. (2010) Neuron 66, 646–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mount M. P., Lira A., Grimes D., Smith P. D., Faucher S., Slack R., Anisman H., Hayley S., Park D. S. (2007) J. Neurosci. 27, 3328–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dragunow M., Butterworth N., Waldvogel H., Faull R. L., Nicholson L. F. (1995) Brain Res. Mol. Brain. Res. 30, 393–396 [DOI] [PubMed] [Google Scholar]
- 26. Doucet J. P., Nakabeppu Y., Bedard P. J., Hope B. T., Nestler E. J., Jasmin B. J., Chen J. S., Iadarola M. J., St-Jean M., Wigle N., Blanchet P., Grondin R., Robertson G. S. (1996) Eur. J. Neurosci. 8, 365–381 [DOI] [PubMed] [Google Scholar]
- 27. Fasano S., Brambilla R. (2002) Curr. Mol. Med. 2, 649–665 [DOI] [PubMed] [Google Scholar]
- 28. Zeng H. Y., Zhu X. A., Zhang C., Yang L. P., Wu L. M., Tso M. O. (2005) Invest. Ophthalmol. Vis. Sci. 46, 2992–2999 [DOI] [PubMed] [Google Scholar]
- 29. Neff F., Wei X., Nölker C., Bacher M., Du Y., Dodel R. (2008) Autoimmun. Rev. 7, 501–507 [DOI] [PubMed] [Google Scholar]
- 30. Fiszer U., Fredrikson S., Czlonkowska A. (1996) J. Neurol. Sci. 139, 66–70 [PubMed] [Google Scholar]
- 31. Theodore S., Cao S., McLean P. J., Standaert D. G. (2008) J. Neuropathol. Exp. Neurol. 67, 1149–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schatz D. G., Baltimore D. (2004) Cell 116, S103-S106 [DOI] [PubMed] [Google Scholar]
- 33. Petiniot L. K., Weaver Z., Barlow C., Shen R., Eckhaus M., Steinberg S.M., Ried T., Wynshaw-Boris A., Hodes R. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 6664–6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takai T., Li M., Sylvestre D., Clynes R., Ravetch J. V. (1994) Cell 76, 519–529 [DOI] [PubMed] [Google Scholar]
- 35. Orr C. F., Rowe D. B., Mizuno Y., Mori H., Halliday G. M. (2005) Brain 128, 2665–2674 [DOI] [PubMed] [Google Scholar]
- 36. He Y., Le W. D., Appel S. H. (2002) Exp. Neurol. 176, 322–327 [DOI] [PubMed] [Google Scholar]
- 37. Ulvestad E., Williams K., Matre R., Nyland H., Olivier A., Antel J. (1994) J. Neuropathol. Exp. Neurol. 53, 27–36 [DOI] [PubMed] [Google Scholar]
- 38. Ulvestad E., Williams K., Vedeler C., Antel J., Nyland H., Mørk S., Matre R. (1994) J. Neurol. Sci. 121, 125–131 [DOI] [PubMed] [Google Scholar]
- 39. Kim W. G., Mohney R. P., Wilson B., Jeohn G. H., Liu B., Hong J. S. (2000) J. Neurosci. 20, 6309–6316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stone D. K., Reynolds A. D., Mosley R. L., Gendelman H. E. (2009) Antioxid. Redox Signal. 11, 2151–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dringen R. (2005) Antioxid. Redox Signal. 7, 1223–1233 [DOI] [PubMed] [Google Scholar]
- 42. Ischiropoulos H., Beckman J. S. (2003) J. Clin. Invest. 111, 163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCormack A. L., Mak S. K., Shenasa M., Langston W. J., Forno L. S., Di Monte D. A. (2008) J. Neuropathol. Exp. Neurol. 67, 793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]