Abstract
Antenna systems of plants and green algae are made up of pigment-protein complexes belonging to the light-harvesting complex (LHC) multigene family. LHCs increase the light-harvesting cross-section of photosystems I and II and catalyze photoprotective reactions that prevent light-induced damage in an oxygenic environment. The genome of the moss Physcomitrella patens contains two genes encoding LHCb9, a new antenna protein that bears an overall sequence similarity to photosystem II antenna proteins but carries a specific motif typical of photosystem I antenna proteins. This consists of the presence of an asparagine residue as a ligand for Chl 603 (A5) chromophore rather than a histidine, the common ligand in all other LHCbs. Asparagine as a Chl 603 (A5) ligand generates red-shifted spectral forms associated with photosystem I rather than with photosystem II, suggesting that in P. patens, the energy landscape of photosystem II might be different with respect to that of most green algae and plants. In this work, we show that the in vitro refolded LHCb9-pigment complexes carry a red-shifted fluorescence emission peak, different from all other known photosystem II antenna proteins. By using a specific antibody, we localized LHCb9 within PSII supercomplexes in the thylakoid membranes. This is the first report of red-shifted spectral forms in a PSII antenna system, suggesting that this biophysical feature might have a special role either in optimization of light use efficiency or in photoprotection in the specific environmental conditions experienced by this moss.
Keywords: Carotenoid, Energy Metabolism, Membrane Proteins, Photosynthesis, Photosynthetic Pigments, Physcomitrella patens, Antenna, Light-harvesting Complex, Photoprotection, Red Forms
Introduction
Light energy powers photosynthesis. Sunlight is absorbed by chlorophyll (Chl)2 and carotenoid molecules bound to protein supercomplexes embedded in thylakoid membranes, called photosystem I and II (PSI and PSII). Each photosystem has two moieties: (i) the core complex and (ii) the peripheral antenna system. The core complex contains mostly plastid-encoded subunits, which are responsible for charge separation and for the first steps of electron transport and are also active in light harvesting. An additional antenna system, made of nucleus-encoded subunits binding Chl a, Chl b, and xanthophylls, is localized peripherally in photosystems and is responsible for most light harvesting, transfer of excitation energy to the reaction centers, and photoprotective reactions like ROS scavenging and quenching of triplet and singlet excited states (1–3). Reaction center protein sequences are widely conserved among organisms, and only small differences are found in sequences of oxygenic organism as far apart as higher plants and cyanobacteria (4, 5). Antenna systems are instead more variable, and in land plants and green algae, they are composed of multiple copies of light-harvesting complex (LHC) proteins (4) assembled around core complexes (6). Clustering analysis of LHC protein sequences clearly distinguishes members associated with PSI (LHCa or LHCI) from those belonging to PSII (LHCb or LHCII) (7). In Arabidopsis thaliana, six different polypeptides were identified for PSI (LHCa1 to -6) and a total of eight for PSII (LHCb1 to -8), including the more recent proposed addition of LHCb7 and LHCb8 (7, 8). In the genome of the moss Physcomitrella patens, two sequences encoding an additional LHCb protein, named LHCb9, were also identified (9).
The fluorescence emission spectrum of plant thylakoids at low temperature shows two main emission peaks (i.e. 685 and 735 nm), which originate from the grana domains containing PSII-LHCII complexes and the stroma-exposed domains containing PSI-LHCI, respectively. The PSI emission originates from Chl a molecules absorbing at wavelengths higher than 700 nm, thus strongly red-shifted with respect to Chl a molecules in organic solvents. For this reason, they are often called “red Chls” or “red (spectral) forms.” Red Chls have been localized in the PSI antenna system, particularly in LHCa3 and LHCa4 (10–13). Mutational analysis showed that red forms in LHCa complexes originate from two interacting Chls, 603 and 609 (also known as A5 and B5 in a previous nomenclature)3 (17, 18). The nature of the ligand of Chl 603 plays a key role in the origin of “red” forms by determining the distance between the α-carbon chain and the bound chlorophyll. In fact, an asparagine coordinates Chl 603 in LHCa3 and LHCa4 and it holds the chromophore in a position allowing its strong interaction with Chl 609, which leads to formation of a charge transfer state and the appearance of red emission forms (18–20). In LHCa1 and LHCa2, the coordination by the bulkier histidine puts Chls 603 away from Chl 609, leading to a weaker interaction and a strong reduction of red forms (18). LHCb1 to -8 polypeptides, composing the antenna system of photosystem II, all have a histidine as Chl 603 ligand and do not show red-shifted emissions. LHCb9 is an exception because it carries an asparagine residue as the ligand for this chlorophyll (9). In this work, we report on the characterization of the two LHCb9 isoforms encoded in the P. patens genome. The cDNA sequence was overexpressed in bacteria and reconstituted in vitro with pigments. The holoprotein showed a fluorescence emission peak red-shifted with respect to any other LHCb protein thus far described. We also show that LHCb9 is found in P. patens thylakoids and is indeed associated with PSII, in agreement with its overall sequence similarity to LHCb proteins. The possible function of red-shifted spectral forms in PSII and the reason for their presence in P. patens is discussed with reference to the adaptation of antenna systems to environmental growth conditions.
EXPERIMENTAL PROCEDURES
Sequence Retrieval and Analysis
LHCb9 and other LHC sequences from P. patens were retrieved from expressed sequence tag (PHYSCObase) and genome (Department of Energy Joint Genome Institute) databases as described (9, 21). Sequence information from the National Center for Biotechnology Information and The Plant Transcript Assemblies were mainly used for TBLASTN searches. Sequence alignments were generated by ClustalW and manually corrected using BioEdit. The regions of the three transmembrane helices were considered in the analysis, as described previously in similar works (22, 23). Phylogenetic trees were inferred using parsimony, neighbor-joining distance, and maximum likelihood approaches using the Phylogenetic Inference Package (PHYLIP) version 3.67 and the PHYML program (24, 25), as described previously (9).
Reconstitution in Vitro
LHCb9.1 (XM_001756491) and LHCb9.2 (XM_001779101) were amplified by PCR from total P. patens cDNA and cloned in a modified pET-28a(+) (26). The apoprotein was overexpressed in E. coli and purified as inclusion bodies. Pigment-protein complexes were refolded in vitro and purified from excess free pigments (27). The N164H mutant for LHCb9.2 (numbers refer to the precursor sequence) was obtained using the QuikChangeTM site-directed mutagenesis kit (Stratagene).
Spectroscopy and Pigment Analysis
Absorption spectra were recorded using a Cary 300 (Varian Inc.) spectrophotometer, in 10 mm HEPES, pH 7.5, 0.2 m sucrose, and 0.06% n-dodecyl-β-d-maltopyranoside. Low temperature fluorescence emission spectra were measured using a Cary Eclipse (Varian Inc.) and corrected for the instrumental response. Samples were excited at 440, 475, and 500 nm. The spectral bandwidth was 5 nm (excitation) and 3 nm (emission). Chlorophyll concentration was about 0.02 μg/ml in 60% glycerol, 10 mm HEPES, and 0.03% n-dodecyl-β-d-maltopyranoside. Chlorophyll/carotenoid ratio and Chl a/b ratio were independently measured by fitting the spectrum of acetone extracts with the spectra of individual purified pigments (28) and by HPLC analysis (29).
Physcomitrella Growth and Thylakoid Isolation
Protonemal tissue of P. patens, Gransden wild-type strain, was grown on PpNO3 minimum medium (30) solidified by 1% purified agar agar (Euromedex, Mundolsheim, France). Plants were propagated under sterile conditions on minimum medium in 9-cm Petri dishes overlaid with a cellophane disk (Cannings, Bristol, UK) as described (31). Plates containing plant samples were placed in a growth chamber under controlled conditions: 22 °C day/21 °C night temperature, 16 h light/8 h dark photoperiod, and a light intensity of 40 microeinsteins m−2 s−1 (10 microeinsteins m−2 s−1 for low light and 400 microeinsteins m−2 s−1 for high light). Thylakoids from 2-week-old plants (protonemal tissue) were prepared following the same protocol used for higher plants with minor modifications. Tissues were harvested and freshly homogenized in cold extraction buffer (0.5% milk powder, 0.4 m NaCl, 20 mm Tricine-KOH, pH 7.8, and 1 mm ϵ-aminocaproic acid). After filtration, samples were precipitated by centrifugation at 4 °C at 1500 × g for 15 min and then resuspended in an ipotonic buffer (15 mm NaCl, 5 mm MgCl2, and 20 mm Tricine-KOH, pH 7.8). After centrifugation for 15 min at 4 °C at 10,000 × g, thylakoids were resuspended in a buffer containing 50% glycerol, 15 mm NaCl, 5 mm MgCl2, and 10 mm Hepes-KOH, pH 7.5. Thylakoids were frozen in liquid nitrogen and stored at −80 °C until use. Purification of pigment-binding proteins from thylakoids was performed by sucrose gradient ultracentrifugation upon solubilization of membranes with final 0.8% n-dodecyl-α-d-maltopyranoside.
SDS-PAGE Electrophoresis, Western Blotting Analysis, and Stoichiometry Calculations
SDS-PAGE analyses were performed as in Ref. 32. After SDS-PAGE, polypeptides were transferred onto a nitrocellulose membrane (Sartorious AG, Gottingen, Germany) using a blot system from Bio-Rad and detected with specific homemade antibodies (supplemental Fig. 1). To verify the identity of the LHCb9 band, antibody was preincubated for 1 h with total 35 μg of LHCb9.1 and LHCb9.2 inclusion bodies (17.5 μg each). In order to evaluate protein stoichiometry, we loaded 0.05, 0.03, 0.02, and 0.01 μg of Chl reconstituted protein together with 1.5, 1, 0.75, and 0.5 μg of Chl from thylakoids. Antibody signal was quantified by densitometry after checking its linearity with gel loading as in Ref. 33. In order to estimate LHCb9/PSII stoichiometry, we assumed that LHCb9 binds 10 ± 2 Chls/monomer. We also estimated Physcomitrella PSII antenna size using fluorescence induction in 3-(3,4-dichlorophenyl)-1,1-dimethylurea, whose kinetics are known to depend on the PSII functional antenna size. As a comparison, we used barley plants, WT and clorina f2 mutants, where PSII antenna size is known to be 300 and 50 Chls/PSII, respectively (34). With this method, we could estimate PSII antenna size in Physcomitrella to be 529 ± 89 Chls/PSII. Final estimation is derived from green gels, like the ones in Ref. 9, showing that 68% of thylakoid Chls are associated with PSII core or antenna complexes. The latter two values led to the calculation that in Physcomitrella thylakoids, there are 788 ± 132 Chls present for each PSII.
Molecular Modeling
The LHCII structure from Ref. 14, Protein Data Bank accession code 1RWT, was mutated using Swiss-PdbViewer (35). After mutating histidine into asparagine, the distance between Chl and protein backbone was manually corrected and fixed equal to Chl 612, which is also coordinated by asparagine.
RESULTS
A New LHC Polypeptide Identified in P. patens
LHCb9 was identified in the P. patens genome as carrying peculiar sequence properties with respect to any other previously known LHC isoform (9). The polypeptide sequence showed a similarity to PSII antenna polypeptides and was thus called LHCb9. We identified two isoforms, LHCb9.1 and LHCb9.2, with 80% sequence identity. We observed that the number of expressed sequence tag clones identified in P. patens databases is particularly high for both LHCb9 isoforms; LHCb9.1 is the most abundant transcript among antenna proteins (9). Assuming that the number of expressed sequence tag clones retrieved is roughly indicative of gene expression levels (8), LHCb9.1 is thus presumably a highly expressed LHC gene in P. patens, suggesting a relevant functional role for the corresponding polypeptide in the moss photosynthetic apparatus.
In Fig. 1, the sequence of LHCb9 is compared with those of other LHCb proteins. In the phylogenetic tree, LHCb9 sequences are located between monomeric LHCb proteins (LHCb4, LHCb5, and LHCb6) and the components of the major LHCII antenna complex (named LHCbm1 to -13 in P. patens). In fact, although LHCb9 sequences cluster with major antenna polypeptides, the consistency of this configuration is low, ranging from 32 to 56–100, depending on the algorithm employed. In fact, LHCb9.1 has 50% similarity and 30% identity with both sequences encoding major antenna polypeptides (i.e. PpLHCbm1 and -2 and PpLHCb3) and the minor antenna protein PpLHCb5. Similarity is instead lower when compared with PpLHCb4 and PpLHCb6 sequences.
FIGURE 1.
Phylogenetic analysis of LHC polypeptides. The evolutionary tree comparative analysis includes protein sequences from A. thaliana (At), Populus trichocarpa (Pt), Oryza sativa (Os), P. patens (Pp), C. reinhardtii (Cr), and Ostreococcus tauri (Ot). Sequences obtained from expressed sequence tag databases of a further algal species (Mesostigma viride (Mv)) were also included. LHCa3 from A. thaliana, P. patens, and C. reinhardtii was included as an external outgroup. The tree was built using a maximum likelihood approach. Bootstrap values reported in brackets were obtained from maximum likelihood, neighbor-joining distance, and maximum parsimony approaches, respectively. For clarity, these values are not shown when consistency is low or when nodes are not significant for isoform discrimination.
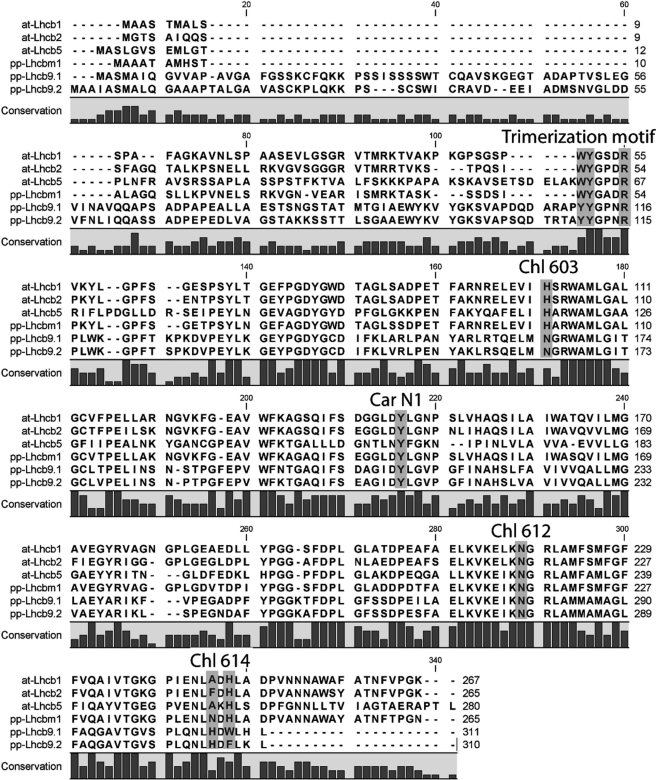
Sequence analysis is useful to infer information on protein biochemistry and activity. We therefore looked closer at Chl-binding residues highly conserved in LHCs (14, 15, 36) and found that they are all conserved in LHCb9 (Fig. 2). An LHCb9 peculiarity is that Chl 603 is coordinated by an asparagine rather than a histidine as in all other LHCb polypeptides. Because both asparagine and histidine can coordinate the Mg2+ in Chl rings, pigment binding is probably unaffected (Fig. 2). A further characteristic of LHCb9 is the non-conservation of the histidine residue, as reported for LHCb6 polypeptide (7). It should be noticed, however, that an extra histidine is found in a nearby position in helix D and could coordinate Chl 614 according to the hypothesis that helix D assumes a different orientation than in other LHC proteins (Fig. 2).
FIGURE 2.
Chlorophyll and carotenoid binding sites in LHCb9 sequence. LHCb9.1 and LHCb9.2 polypeptide sequences have been aligned with LHCb1, LHCb2, and LHCb5 from A. thaliana and LHCbm1 from P. patens. The putative trimerization motif as well as residues known to be involved in coordination of Chl and carotenoid (Car) are indicated and discussed under “Results.”
Xanthophyll coordination in LHC proteins is most likely due to multiple interactions with the polypeptide, and sequence motifs for carotenoid binding are not well defined yet. Sites L1 and L2 are conserved in all LHC members analyzed so far (37, 38) and are likely to be present in LHCb9 as well. A conserved tyrosine residue in LHCb1, LHCb4, and LHCb5 has been shown to be fundamental for neoxanthin binding to site N1 (26). This tyrosine is also conserved in LHCb9 (position 209; Fig. 2), suggesting that it binds neoxanthin.
The capacity of LHCII polypeptides to form trimers depends on the presence of residues in the N-terminal domain WYR (indicated as a trimerization motif in Fig. 2) (39). A WYR motif is also found in LHCb5, although this is normally associated with PSII as a monomer (40) and only trimerizes in LHCII-depleted plants (41). In LHCb9, the first tryptophan of the motif is substituted by a tyrosine, suggesting a low probability of finding trimers, including LHCb9, in P. patens WT thylakoids.
In Vitro Reconstitution of LHCb9 Recombinant Protein
The presence of an asparagine replacing the more common histidine as a ligand for Chl 603 is typical of LHC members associated with PSI, such as A. thaliana LHCa3 and LHCa4 (9, 42), and LHCa2, LHCa4, and LHCa9 in Chlamydomonas reinhardtii (43). The asparagine in PSI antenna is responsible for red-shifted Chl absorption (18), and up to now, LHCb9 is the only case where this ligand for Chl 603 is found outside the LHCa subfamily.
In order to verify if the asparagine ligand can induce red-shifted Chl spectral forms even within a LHCb-like sequence, we cloned and expressed the two LHCb9 isoforms in E. coli using total P. patens cDNA as a template. LHCb9 apoproteins were purified from inclusion bodies and refolded in vitro in the presence of pigments using a well established procedure (27). Both LHCb9 polypeptides yielded stable monomeric pigment-binding proteins. Pigment compositions of the reconstituted complexes are reported in Table 1, together with reference data from Hordeum vulgare LHCb2 reconstituted in vitro using the same procedure (44). HvLHCb2 was chosen because its primary sequence is the most similar to LHCb9 among the LHCb isoforms characterized previously. Chl a/b ratios are very similar in all complexes (from 1.4 to 1.5; Table 1), suggesting that LHCb9 has a high affinity for Chl b, similar to LHCII members and different from monomeric LHCs, which have a higher Chl a/b ratio (i.e. a Chl a/b ratio of 2.0–2.2 and 3.0 for LHCb5 and LHCb4, respectively) (26, 45). In contrast, xanthophyll-binding properties of LHCb9 are more similar to those of minor LHCbs because it preferentially binds violaxanthin and lutein, whereas neoxanthin content is low with respect to LHCb2, a component of the major LHCII trimeric antenna.
TABLE 1.
Pigment binding properties of LHCb9 reconstituted in vitro
Chl and carotenoid binding properties are reported for reconstituted LHCb9.1 and LHCb9.2. Carotenoid data are normalized to 100 Chls (a + b) because Chl/polypeptide stoichiometry is not known for LHCb9. Maximum S.D. is 0.05 for Chl a/b and 0.5 for carotenoids.
| Chl a/Chl b | Carotenoids | Neoxanthin | Violaxanthin | Lutein | |
|---|---|---|---|---|---|
| PpLHCb9.1 | 1.35 | 24.9 | 3.7 | 4.4 | 16.7 |
| PpLHCb9.2 | 1.34 | 27.4 | 5.3 | 5.3 | 16.8 |
| HvLHCb2 | 1.36 | 23.6 | 8.2 | 1.2 | 14.1 |
Chl/protein binding stoichiometry of LHCb9 is not known, but it is most likely comprised between 9 and 12 Chls/monomer, as found experimentally in LHCb5 and LHCb2, respectively, reconstituted in vitro. For all values within this interval, normalization of data in Table 1 to Chl stoichiometry suggests the presence of three carotenoid binding sites, one specific for lutein, one binding lutein or violaxanthin, and the last one specific for neoxanthin. This is similar to all LHCb1 to -5 proteins (26, 45) and is consistent with the conserved motifs from sequence analysis.
Absorption spectra showed a Chl a Qy peak at 675.5 nm (Fig. 3, A and B), and an efficient energy transfer from xanthophylls and Chl b to Chl a, typical for all functional LHC proteins, was observed by the constancy of fluorescence emission spectra upon excitation at 430 nm (Chl a), 470 nm (Chl b), and 500 nm (xanthophylls) (not shown). LHCb9 absorption spectra are similar to that of HvLHCb2 (Fig. 3A), in agreement with their similar pigment content (44). However, the red-most part of the spectra showed differences, and both LHCb9 isoforms have a more red-shifted absorption with respect to HvLHCb2 (Fig. 3B). The fitting of the Qy region of the spectrum with spectral forms of Chl a in a protein environment yields information on pigment composition and protein structure (28). In order to reconstruct LHCb9.1 and LHCb9.2 spectra, at least one red-shifted spectral form at 685–690 nm had to be introduced in order to obtain a satisfactory fitting (Fig. 3C). This is different from all other LHCbs, where the red-most Chl is consistently found around 680 nm using the same analysis (45). Consistent with this red-shifted absorption, the low temperature fluorescence emission spectra peaked at 683 and 687 nm, respectively, for LHCb9.1 and LHCb9.2 with respect to the 679 nm of HvLHCb2 (Fig. 3D). Such a property is unique among LHCb proteins, which all show very similar fluorescence emission peaks at 679–680 nm (44, 45).
FIGURE 3.
Spectroscopic properties of LHCb9 reconstituted in vitro. A, comparison of LHCb9.1 and LHCb9.2 absorption spectra (black and red, respectively) with barley HvLHCb2 (blue). B, red region of spectra shown in A. C, fitting of LHCb9.2 absorption spectrum (black) with Chl absorption forms. Chl b and Chl a forms are shown in cyan and green, respectively. The two red-most Chl a forms, with a maximum at 683 and 690 nm, are shown in blue. D, low temperature fluorescence spectra upon excitation at 500 nm of LHCb9.1, LHCb9.2, and barley HvLHCb2, shown in black, red, and blue, respectively. All spectra are normalized to the maximum value in the Qy region. a.u., arbitrary units.
Site-directed Mutagenesis on Chl 603-binding Residue of LHCb9.2
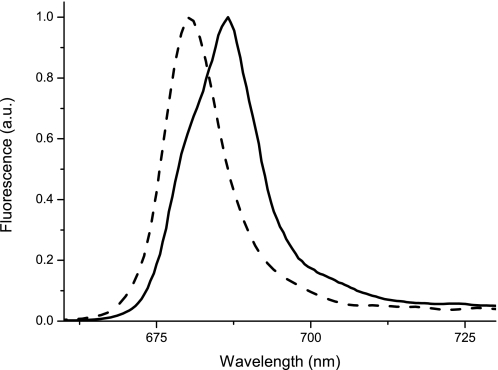
In order to verify experimentally if the red-shifted emission in LHCb9 originated from the Chl 603 as in the PSI antenna, we generated site-directed mutants substituting the asparagine ligand with a histidine, which was effective in reducing red forms in Lhca (18). The Asn to His mutation does not alter significantly the Chl binding properties, as suggested by the invariance of the Chl a/b ratio (1.29 ± 0.07 in the mutant versus 1.36 ± 0.05 in WT). Fluorescence emission is instead influenced and is clearly blue-shifted from 686 to 681 nm (Fig. 4). It is worth pointing out that the fluorescence emission spectrum of the Asn to His mutant is now very similar to that of other LHCb proteins, demonstrating the key role of this amino acid substitution in inducing the spectral shift.
FIGURE 4.
Role of Chl 603 ligand in LHCb9 red-shifted forms. Low temperature fluorescence spectra upon excitation at 500 nm of LHCb9.2 WT and N164H mutant are shown by a solid and dashed line, respectively. All spectra are normalized to the maximum value. a.u., arbitrary units.
Localization of LHCb9 in P. patens Thylakoids
In order to locate the LHCb9 polypeptides in P. patens thylakoids, we produced a polyclonal antibody using recombinant LHCb9.1 polypeptide as antigen. Among P. patens thylakoid proteins separated by SDS-PAGE, the anti-LHCb9 antibody recognized a band with apparent molecular mass of 29,300 Da (i.e. the mass of LHCb9 mature protein). Other bands are also highlighted in the 20–30 kDa range, where LHCs are expected to migrate (supplemental Fig. 1) and are probably due to binding to conserved LHC epitopes. To confirm this attribution, we repeated the Western blotting analysis by preincubating the antibody with LHCb9.1 and LHCb9.2 inclusion bodies. As shown in supplemental Fig. 1, the band attributed to LHCb9 is depleted by the preincubation differently from other bands with lower apparent molecular mass, implying that the upper band was indeed LHCb9. Moreover, we verified that antibodies against LHCb1 to -3, LHCb4, LHCb5, and LHCb6 (9) recognized bands with lower apparent molecular weight with respect to that identified as LHCb9 (not shown). It should be noted that the anti-LHCb9.1 did recognize both recombinant LHCb9.1 and LHCb9.2 isoforms with similar affinity, as expected from their high sequence similarity (supplemental Fig. 1).
We then proceeded to LHCb9 immunotitration in P. patens thylakoids in order to evaluate its approximate stoichiometry with respect to other thylakoid proteins. With this aim, we first determined the affinity of the antibody for LHCb9.1 by measuring the signal obtained from gels loaded with different amounts of recombinant LHCb9.1 reconstituted in vitro with pigments. On the same gel, we also loaded different amounts of thylakoid membranes (for details, see “Experimental Procedures”). Densitometric quantification showed that the same immunoblotting signal was obtained by loading 407 ± 53 Chls from thylakoids and one Chl from recombinant LHCb9.1 protein. This value can be used to estimate a stoichiometry of 0.19 ± 0.06 LHCb9 copies/PSII, by using the Chl/LHCb9 monomer and Chl/PSII values of 10 ± 2 and 788 ± 132, respectively (see “Experimental Procedures” for details). This result must be taken with some caution because it depends on several estimations; nevertheless, it suggests that LHCb9 is a significant component of P. patens thylakoids.
We also used the anti-LHCb9 antibody to assess if protein accumulation is modulated by light intensity during plant growth. We found that LHCb9 is more abundant in control conditions with respect to both low light and high light conditions (Fig. 5). Antenna proteins have normally similar regulatory patterns; the isoform with a preferential role in light harvesting is normally induced in low light, whereas proteins involved in photoprotection as LHCSR or PSBS are induced in strong light (8, 46, 47). Instead, here LHCb9 has a unique regulation with an increased expression in intermediate light conditions.
FIGURE 5.
LHCb9 accumulation in different light conditions. Western blotting against LHCb9 in P. patens thylakoids purified from plants grown under different light regimes. Different dilutions of the same sample were loaded to ensure signal linearity. As indicated, to achieve similar signal intensities, different Chl amounts of low light (LL), control light (CL), and high light (HL) thylakoids have been loaded. a. u., arbitrary units.
Localization of LHCb9 in PSII-LHCII Supercomplexes
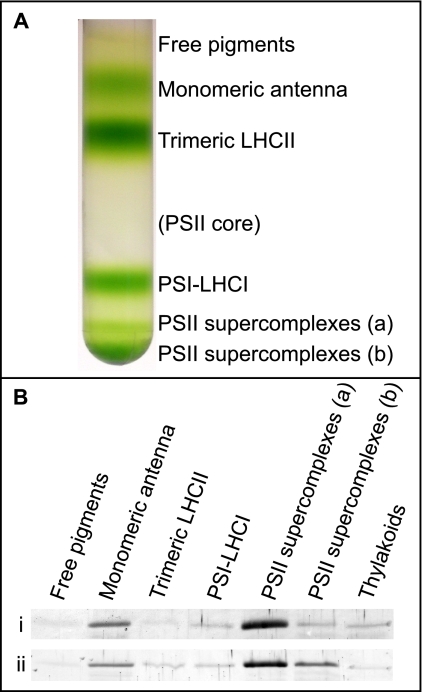
Data presented so far showed that LHCb9.1 and/or LHCb9.2 are indeed present in P. patens thylakoids. Red-shifted Chl forms have not been described before in a PSII antenna protein, and this might suggest that LHCb9 is associated with PSI, despite its sequence similarity with LHCb proteins. In order to assess whether LHCb9 is associated with PSI or PSII, we fractionated thylakoid pigment-binding complexes by sucrose gradient ultracentrifugation upon solubilization with a mild detergent (Fig. 6A). Green bands were identified from their migration, absorption spectra, and SDS-PAGE profile. PSI and PSII antenna complexes are well separated by this method. In fact, although LHCas are stably associated with the reaction center complex of PSI upon detergent solubilization, LHCbs are easily dissociated in two major bands corresponding to monomeric and trimeric antenna proteins, although a relevant fraction of LHCb proteins in P. patens migrates at the bottom of the gradient as PSII-LHCII supercomplexes (9). We tested these sucrose gradient bands for the presence of LHCb9 and found that this protein is enriched in monomeric antennas and PSII supercomplexes (Fig. 6B), strongly suggesting its association with PSII.
FIGURE 6.
LHCb9 distribution in P. patens thylakoids. A, sucrose gradient ultracentrifugation pattern from solubilized thylakoids purified from plants grown in control light conditions. Pigments are distributed in six bands corresponding (from the top) to free pigments, monomeric and trimeric antenna proteins, and PSI-LHCI and PSII-LHCII supercomplexes of two different sizes. A seventh expected band corresponding to the PSII core is very faint. B, Western blotting against LHCb9 on different bands from sucrose gradient. In the first row (i), 1 μg of Chl was loaded in each lane, whereas in the second row (ii), the same band volume was loaded. 1 μg of Chl of thylakoids was always loaded as reference.
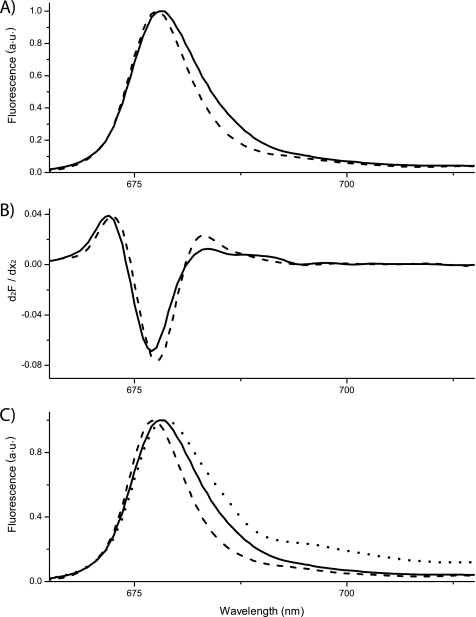
A further support for the association of LHCb9 to PSII reaction center was found by exploiting its peculiar emission at low temperature (i.e. red shift with respect to all other known LHCb polypeptides). This feature allows identification of this polypeptide even when it is mixed with other PSII antenna proteins. The 77 K fluorescence emission spectrum from the monomeric antenna fraction (sucrose band 2; Fig. 6A), enriched in LHCb9, is wider than spectra from the trimeric fraction, and a shoulder can be resolved at 685 nm in the former fraction, consistent with the LHCb9 emission (Fig. 7, A and B).
FIGURE 7.
Spectroscopic evidence for the presence of LHCb9 among P. patens antennas. A, LT fluorescence spectra of band 2, containing monomeric LHCbs and trimeric LHCII, shown as solid and dashed lines, respectively. B, second derivative of spectra shown in A. C, comparison of LT spectra of bands from a sucrose gradient containing PSII-LHCII supercomplexes (dotted line) and monomeric (solid line) and trimeric (dashed line) antenna fractions from P. patens. All spectra were normalized to the peak value. Excitation was set at 500 nm. a.u., arbitrary units.
The second derivative analysis of the spectra (Fig. 7B) allowed highlighting of the presence of a contribution peaking at 685 nm in the monomeric fractions, which is completely missing in the trimer antenna fraction. We also noticed that the monomeric and trimeric fractions purified from A. thaliana thylakoids have very similar fluorescence emission spectra consistent with the absence of LHCb9 in this plant. In particular, the red-most contribution is missing, thus further strengthening the correlation between LHCb9 and the red-shifted fluorescence emission signal.
In Fig. 7C, the LT fluorescence of P. patens PSII supercomplexes is shown together with LHCb trimers and monomers, used as a reference for the absence and the presence of LHCb9, respectively. In the supercomplex spectrum, the main peak corresponds to the emission from antenna proteins, whereas a shoulder at around 695 nm is due to PSII core emission. In these samples, emissions in the 725–740 nm interval is also visible due to a small contamination from PSI-LHCI supercomplexes. The blue-most peak from the antenna, however, is clearly wider than that from LHCII trimers alone and very similar to that from monomeric antennas, consistent with a red-shifted contribution from LHCb9 in PSII supercomplexes, confirming its association with PSII-LHCII supercomplexes suggested by immunoblotting.
DISCUSSION
LHCb9 Is a New Photosystem II Antenna Polypeptide
In this work, we characterized LHCb9, a new LHC polypeptide with peculiar properties in the moss P. patens. Antenna polypeptides associated with PSI and PSII can be distinguished based on their amino acid sequence because of their early divergence during the evolution of plants (4, 48). We first defined this polypeptide as a photosystem II antenna because of the high similarity with LHCb antenna proteins (9). LHCb9 sequence and pigment binding prediction showed similarity to both monomeric (LHCb5) and trimeric (LHCbm) PSII antenna proteins, and thus LHCb9 could not be assigned to one or the other subgroups. Immunoblotting with an antibody directed against LHCb9, however, localized this protein in fractions containing monomeric LHCbs (LHCb4, LHCb5, and LHCb6) rather than in those containing the trimeric peripheral LHCII. Consistently, LHCb9 was found to be part of the PSII-LHCII large supercomplex (Figs. 6 and 7). Thus, although the possibility that LHCb9 transiently associates to photosystem I during state transition cannot be excluded, its identification as a genuine PSII antenna is consistent with all experimental results. Monomeric LHCbs have been localized between the PSII core complex and the peripheral antenna LHCII (49, 50). In P. patens, genes encoding monomeric LHCb4, LHCb5, and LHCb6 subunits are all transcribed, and the corresponding polypeptides accumulate (9). Thus, LHCb9 does not substitute completely one or more of LHCb4 to -6 in PSII-LHCII architecture. It is, however, possible that LHCb9 might take the place of LHCb4 to -6 in a fraction of PSII supercomplexes, consistent with its substoichiometric abundance with respect to the PSII core complex. Alternatively, it might bind to the PSII core in parallel with LHCb4 to -6 on the peripheral antenna layer.
Possible Physiological Role of LHCb9
LHCb9 is the only known case of a PSII antenna having an asparagine as Chl 603 ligand instead of histidine. Asparagines coordinate chlorophylls as well as histidines, but the size of the lateral chain is smaller, bringing the ligand closer to the protein backbone. In this case, Chl 603 also becomes closer to Chl 609. In this context, the strength of the Chl-Chl interaction is higher and leads to the formation of a charge transfer state and of red-shifted Chl absorption forms (18–20). In the case of LHCb9.2, this effect can be shown by the 6-nm shift observed in fluorescence emission between the WT and Asn to His mutant (Fig. 4).
Thus, our data show that red-shifted forms are indeed present in LHCb9. It is worth underlining that although transition energies in this complex are higher than in the PSI antenna, these are nevertheless relevant because they are associated with PSII, where the reaction center energy (P680) is higher than in PSI (P700). Furthermore, trapping time is much slower in PSII than PSI, and thus any exciton delocalization is expected to have a stronger effect on photochemistry here with respect to the case of PSI. Certainly, LHCb9 significantly affects energy distribution in PSII-LHCII supercomplexes as shown by the LT fluorescence spectra; here, in fact, the LHCb9 effect can be detected despite the fact that this protein is present in far lower amounts with respect to other non-red-shifted antenna subunits (Fig. 7).
Red forms in PSI have been suggested to increase light absorption, providing an advantage in a spectrally filtered light caused by self-absorption and shading (51–53). Alternatively, red forms of PSI have been proposed to play a relevant role in PSI photoprotection by concentrating excitation energy in protein domains specialized for Chl singlet/triplet quenching (54, 55). Because an LHCb9 homologous has not yet been found in any other plant but mosses, we might speculate that this protein plays a role related to specific environmental conditions experienced by P. patens. Mosses grow in a dim light environment, where red-shifted forms can provide some advantage in a shade environment when the light spectrum is altered by self-absorption (52).
As for the photoprotection role, it is worth mentioning that plants growing shaded by overhanging canopies are exposed to sudden light increase during sun flecks, a highly stressful condition leading to oxidative stress (56). It has been recently shown that the interaction between Chl 603 and Chl 609 is needed for the formation of a carotenoid radical cation whose fast recombination yields heat dissipation (57). We can thus hypothesize that red forms within the PSII antenna system of P. patens may also function in focusing excitons within a low energy sink, where they can be safely dissipated. As in the case of PSI, thus also for LHCb9, red-shifted Chls may play a role both in light harvesting and photoprotection, and according to analysis of protein accumulation, its effect is more helpful in intermediate light conditions. In order to verify LHCb9, this hypothesis on their physiological role, a reverse genetic analysis of the two LHCb9 isoforms is required that can be performed thanks to the unique property of P. patens of performing homologous recombination, as recently exploited for the determination of the function of LHCSR and PSBS proteins (58).
Supplementary Material
Acknowledgment
We thank Stefano Caffarri (Université de la Méditerranée, Marseille, France) for help with LT fluorescence spectra.
This work was supported by the Cassa di Risparmio di Padova e Rovigo (CaRiPaRo) Foundation, Università di Padova Grant CPDA089403, EEC Project Harvest and Fondo per gli Investimenti della Ricerca di Base-Parallelomics Grant RBIP06CTBR.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
Different nomenclatures have been proposed for Chl binding sites in LHC proteins. Here we use the one from Ref. 14 instead of the older one from Ref. 15 because the latter suggests occupancy of the site by Chl a or b which is not a general property through the protein family. We also do not use the one from Ref. 16 because it refers specifically to plant PSI antennas and could not be generalized to other homologous proteins.
- Chl
- chlorophyll
- LHC
- light-harvesting complex
- Lhca and -b
- light-harvesting complex of photosystem I and II, respectively
- LHCII
- major light harvesting complex of photosystem II
- PSI and PSII
- photosystem I and II, respectively
- Tricin
- N-[Tris-(hydroxymethyl)-methyl]glycine
- LT
- low temperature.
REFERENCES
- 1. Mozzo M., Dall'Osto L., Hienerwadel R., Bassi R., Croce R. (2008) J. Biol. Chem. 283, 6184–6192 [DOI] [PubMed] [Google Scholar]
- 2. Jahns P., Latowski D., Strzalka K. (2009) Biochim. Biophys. Acta 1787, 3–14 [DOI] [PubMed] [Google Scholar]
- 3. Horton P., Ruban A. (2005) J. Exp. Bot. 56, 365–373 [DOI] [PubMed] [Google Scholar]
- 4. Green B. R., Durnford D. G. (1996) Annu. Rev. Plant Physiol Plant Mol. Biol. 47, 685–714 [DOI] [PubMed] [Google Scholar]
- 5. Nelson N., Ben-Shem A. (2005) BioEssays 27, 914–922 [DOI] [PubMed] [Google Scholar]
- 6. Busch A., Hippler M. (2011) Biochim. Biophys. Acta 1807, 864–877 [DOI] [PubMed] [Google Scholar]
- 7. Jansson S. (1999) Trends Plant Sci. 4, 236–240 [DOI] [PubMed] [Google Scholar]
- 8. Klimmek F., Sjödin A., Noutsos C., Leister D., Jansson S. (2006) Plant Physiol. 140, 793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alboresi A., Caffarri S., Nogue F., Bassi R., Morosinotto T. (2008) PLoS ONE 3, e2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang H., Goodman H. M., Jansson S. (1997) Plant Physiol. 115, 1525–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmid V. H., Cammarata K. V., Bruns B. U., Schmidt G. W. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 7667–7672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmid V. H., Potthast S., Wiener M., Bergauer V., Paulsen H., Storf S. (2002) J. Biol. Chem. 277, 37307–37314 [DOI] [PubMed] [Google Scholar]
- 13. Castelletti S., Morosinotto T., Robert B., Caffarri S., Bassi R., Croce R. (2003) Biochemistry 42, 4226–4234 [DOI] [PubMed] [Google Scholar]
- 14. Liu Z., Yan H., Wang K., Kuang T., Zhang J., Gui L., An X., Chang W. (2004) Nature 428, 287–292 [DOI] [PubMed] [Google Scholar]
- 15. Kühlbrandt W., Wang D. N., Fujiyoshi Y. (1994) Nature 367, 614–621 [DOI] [PubMed] [Google Scholar]
- 16. Ben-Shem A., Frolow F., Nelson N. (2003) Nature 426, 630–635 [DOI] [PubMed] [Google Scholar]
- 17. Morosinotto T., Mozzo M., Bassi R., Croce R. (2005) J. Biol. Chem. 280, 20612–20619 [DOI] [PubMed] [Google Scholar]
- 18. Morosinotto T., Breton J., Bassi R., Croce R. (2003) J. Biol. Chem. 278, 49223–49229 [DOI] [PubMed] [Google Scholar]
- 19. Romero E., Mozzo M., van Stokkum I. H., Dekker J. P., van Grondelle R., Croce R. (2009) Biophys. J. 96, L35–L37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Croce R., Chojnicka A., Morosinotto T., Ihalainen J. A., van Mourik F., Dekker J. P., Bassi R., van Grondelle R. (2007) Biophys. J. 93, 2418–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rensing S. A., Lang D., Zimmer A. D., Terry A., Salamov A., Shapiro H., Nishiyama T., Perroud P. F., Lindquist E. A., Kamisugi Y., Tanahashi T., Sakakibara K., Fujita T., Oishi K., Shin-I T., Kuroki Y., Toyoda A., Suzuki Y., Hashimoto S., Yamaguchi K., Sugano S., Kohara Y., Fujiyama A., Anterola A., Aoki S., Ashton N., Barbazuk W. B., Barker E., Bennetzen J. L., Blankenship R., Cho S. H., Dutcher S. K., Estelle M., Fawcett J. A., Gundlach H., Hanada K., Heyl A., Hicks K. A., Hughes J., Lohr M., Mayer K., Melkozernov A., Murata T., Nelson D. R., Pils B., Prigge M., Reiss B., Renner T., Rombauts S., Rushton P. J., Sanderfoot A., Schween G., Shiu S. H., Stueber K., Theodoulou F. L., Tu H., Van de Peer Y., Verrier P. J., Waters E., Wood A., Yang L., Cove D., Cuming A. C., Hasebe M., Lucas S., Mishler B. D., Reski R., Grigoriev I. V., Quatrano R. S., Boore J. L. (2008) Science 319, 64–69 [DOI] [PubMed] [Google Scholar]
- 22. Durnford D. G., Deane J. A., Tan S., McFadden G. I., Gantt E., Green B. R. (1999) J. Mol. Evol. 48, 59–68 [DOI] [PubMed] [Google Scholar]
- 23. Six C., Worden A. Z., Rodríguez F., Moreau H., Partensky F. (2005) Mol. Biol. Evol. 22, 2217–2230 [DOI] [PubMed] [Google Scholar]
- 24. Guindon S., Gascuel O. (2003) Syst. Biol. 52, 696–704 [DOI] [PubMed] [Google Scholar]
- 25. Guindon S., Lethiec F., Duroux P., Gascuel O. (2005) Nucleic Acids Res. 33, W557–W559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caffarri S., Passarini F., Bassi R., Croce R. (2007) FEBS Lett. 581, 4704–4710 [DOI] [PubMed] [Google Scholar]
- 27. Giuffra E., Cugini D., Croce R., Bassi R. (1996) Eur. J. Biochem. 238, 112–120 [DOI] [PubMed] [Google Scholar]
- 28. Croce R., Canino G., Ros F., Bassi R. (2002) Biochemistry 41, 7334–7343 [DOI] [PubMed] [Google Scholar]
- 29. Gilmore A. M., Yamamoto H. Y. (1991) Plant Physiol. 96, 635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ashton N. W., Grimsley N., Cove D. J. (1979) Planta 144, 427–435 [DOI] [PubMed] [Google Scholar]
- 31. Trouiller B., Schaefer D. G., Charlot F., Nogué F. (2006) Nucleic Acids Res. 34, 232–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ballottari M., Govoni C., Caffarri S., Morosinotto T. (2004) Eur. J. Biochem. 271, 4659–4665 [DOI] [PubMed] [Google Scholar]
- 33. Ballottari M., Dall'Osto L., Morosinotto T., Bassi R. (2007) J. Biol. Chem. 282, 8947–8958 [DOI] [PubMed] [Google Scholar]
- 34. Melis A. (1989) Phil. Trans. R. Soc. Lond. B 323, 397–409 [Google Scholar]
- 35. Guex N., Peitsch M. C. (1997) Electrophoresis 18, 2714–2723 [DOI] [PubMed] [Google Scholar]
- 36. Bassi R., Croce R., Cugini D., Sandonà D. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 10056–10061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morosinotto T., Caffarri S., Dall'Osto L., Bassi R. (2003) Physiol. Plant. 119, 347–354 [Google Scholar]
- 38. Schmid V. H. (2008) Cell Mol. Life Sci. 65, 3619–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hobe S., Förster R., Klingler J., Paulsen H. (1995) Biochemistry 34, 10224–10228 [DOI] [PubMed] [Google Scholar]
- 40. Dainese P., Hoyer-hansen G., Bassi R. (1990) Photochem. Photobiol. 51, 693–703 [DOI] [PubMed] [Google Scholar]
- 41. Ruban A. V., Solovieva S., Lee P. J., Ilioaia C., Wentworth M., Ganeteg U., Klimmek F., Chow W. S., Anderson J. M., Jansson S., Horton P. (2006) J. Biol. Chem. 281, 14981–14990 [DOI] [PubMed] [Google Scholar]
- 42. Elrad D., Grossman A. R. (2004) Curr. Genet. 45, 61–75 [DOI] [PubMed] [Google Scholar]
- 43. Mozzo M., Mantelli M., Passarini F., Caffarri S., Croce R., Bassi R. (2010) Biochim. Biophys. Acta 1797, 212–221 [DOI] [PubMed] [Google Scholar]
- 44. Caffarri S., Croce R., Cattivelli L., Bassi R. (2004) Biochemistry 43, 9467–9476 [DOI] [PubMed] [Google Scholar]
- 45. Ballottari M., Mozzo M., Croce R., Morosinotto T., Bassi R. (2009) J. Biol. Chem. 284, 8103–8113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peers G., Truong T. B., Ostendorf E., Busch A., Elrad D., Grossman A. R., Hippler M., Niyogi K. K. (2009) Nature 462, 518–521 [DOI] [PubMed] [Google Scholar]
- 47. Gerotto C., Alboresi A., Giacometti G. M., Bassi R., Morosinotto T. (2011) Plant Cell Environ. 34, 922–932 [DOI] [PubMed] [Google Scholar]
- 48. Neilson J. A., Durnford D. G. (2010) Photosynth. Res. 106, 57–71 [DOI] [PubMed] [Google Scholar]
- 49. Boekema E. J., van Roon H., Calkoen F., Bassi R., Dekker J. P. (1999) Biochemistry 38, 2233–2239 [DOI] [PubMed] [Google Scholar]
- 50. Boekema E. J., Van Roon H., Van Breemen J. F., Dekker J. P. (1999) Eur. J. Biochem. 266, 444–452 [DOI] [PubMed] [Google Scholar]
- 51. Trissl H. W. (1993) Photosynth. Res. 35, 247–263 [DOI] [PubMed] [Google Scholar]
- 52. Rivadossi A., Zucchelli G., Garlaschi F. M., Jennings R. C. (1999) Photosynth. Res. 60, 209–215 [Google Scholar]
- 53. Matsubara S., Morosinotto T., Osmond C. B., Bassi R. (2007) Plant Physiol. 144, 926–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carbonera D., Agostini G., Morosinotto T., Bassi R. (2005) Biochemistry 44, 8337–8346 [DOI] [PubMed] [Google Scholar]
- 55. Alboresi A., Ballottari M., Hienerwadel R., Giacometti G. M., Morosinotto T. (2009) BMC Plant Biol. 9, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Watling J. R., Robinson S. A., Woodrow I. E., Osmond C. B. (1997) Aust. J. Plant Physiol. 24, 17–25 [Google Scholar]
- 57. Ahn T. K., Avenson T. J., Ballottari M., Cheng Y. C., Niyogi K. K., Bassi R., Fleming G. R. (2008) Science 320, 794–797 [DOI] [PubMed] [Google Scholar]
- 58. Alboresi A., Gerotto C., Giacometti G. M., Bassi R., Morosinotto T. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 11128–11133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.