Abstract
Spermatogenesis is a complex process involving an intrinsic genetic program composed of germ cell-specific and -predominant genes. In this study, we investigated the mouse Spink2 (serine protease inhibitor Kazal-type 2) gene, which belongs to the SPINK family of proteins characterized by the presence of a Kazal-type serine protease inhibitor-pancreatic secretory trypsin inhibitor domain. We showed that recombinant mouse SPINK2 has trypsin-inhibitory activity. Distribution analyses revealed that Spink2 is transcribed strongly in the testis and weakly in the epididymis, but is not detected in other mouse tissues. Expression of Spink2 is specific to germ cells in the testis and is first evident at the pachytene spermatocyte stage. Immunoblot analyses demonstrated that SPINK2 protein is present in male germ cells at all developmental stages, including in testicular spermatogenic cells, testicular sperm, and mature sperm. To elucidate the functional role of SPINK2 in vivo, we generated mutant mice with diminished levels of SPINK2 using a gene trap mutagenesis approach. Mutant male mice exhibit significantly impaired fertility; further phenotypic analyses revealed that testicular integrity is disrupted, resulting in a reduction in sperm number. Moreover, we found that testes from mutant mice exhibit abnormal spermatogenesis and germ cell apoptosis accompanied by elevated serine protease activity. Our studies thus provide the first demonstration that SPINK2 is required for maintaining normal spermatogenesis and potentially regulates serine protease-mediated apoptosis in male germ cells.
Keywords: Apoptosis, Protease, Protease Inhibitor, Reproduction, Sperm, Spermatogenesis, Fertility, Infertility, Serine Protease, Testis
Introduction
Male germ cell development, or spermatogenesis, is a complex process that involves the mitotic proliferation of spermatogonial stem cells, meiotic division of spermatocytes, and dramatic morphological changes from haploid spermatids to highly specialized sperm through spermiogenesis (1, 2). The tightly regulated nature of this process, which occurs in seminiferous tubules in testes, suggests the presence of a highly organized network of genes expressed in germ cells during spermatogenesis. The regulation of gene expression during spermatogenesis occurs at three levels: intrinsic, interactive, and extrinsic (3). The intrinsic program determines which genes are utilized and when the genes are expressed. The interactive process between germ cells and somatic cells is necessary for germ cell proliferation and progression. Extrinsic influences, such as steroid and peptide hormones, regulate the interactive process. The intrinsic component of the genetic program involves germ cell- and stage-specific gene expression patterns that constitute the unique features of male reproduction.
Proteases represent a large group of proteins (∼2% of all genes) that share the ability to catalyze the hydrolysis of peptide bonds. Proteases play crucial roles in controlling diverse biological processes such as tissue maintenance, repair, and development, and are essential for the survival of all organisms. Not surprisingly, given the central importance of proteases, deficiencies or alterations in the regulation of proteases underlie important human disease, including cancer, arthritis, and neurodegenerative and cardiovascular diseases (4–8). Accordingly, the activities of proteases must be finely tuned to maintain biological homeostasis. To regulate protease activities and avoid cellular damage, organisms produce protease inhibitors, which are widely distributed in organs. To date, 67 distinct protein families have been classified as protease inhibitors (9).
During the course of our study of unknown or unexplored genes with testis-specific or -predominant expression, we investigated the serine protease inhibitor Kazal-type 2 (Spink2)2 gene in mice. Previous comparative gene expression profiling between normal and abnormal human testes from patients diagnosed with azoospermia showed that SPINK2 expression was decreased 4-fold in abnormal testes compared with those from fertile men (10). Thus, this raises the possibility of SPINK2 involvement in male reproduction. SPINK2 belongs to the family of Kazal-type serine peptidase inhibitors, which have amino acid sequence homology to bovine pancreatic secretory trypsin inhibitor (11). The first member of the SPINK family, human SPINK1 (mouse Spink3), also known as pancreatic secretory trypsin inhibitor was discovered by Kazal (12). Previous studies and our in silico searches indicate that at least 13 SPINK family members are expressed in diverse tissues (13–18). SPINK2, the only SPINK family member expressed in testes, was first identified in humans (11, 19); however, information on mouse Spink2 has not yet been reported. In the present study, we report the first investigation of Spink2 in mice, providing comprehensive information on its expression and function. Notably, we generated mice carrying a gene trap mutation in Spink2 and found that mutant male mice with reduced SPINK2 levels exhibit impaired fertility.
EXPERIMENTAL PROCEDURES
Trypsin Inhibition Assays
Trypsin inhibition assays were performed by measuring the trypsin-cleaved products of the synthetic substrate (CBZ-Ile-Pro-Arg)2-Rhodamine110 (Molecular Probes). Purified His-tagged SPINK2 protein (0.1–30 μm) was preincubated with 42 nm trypsin (Sigma-Aldrich) at 22 °C for 10 min in HEPES-buffered saline (5 mm HEPES, 0.15 m NaCl, pH 7.35) containing 2 mm EDTA. Activity was determined by measuring the absorbance at 521 nm with a SPECTRAmax Gemini XS microplate reader (Molecular Devices).
Reverse Transcription PCR
The testis-specific expression of the Spink2 gene was verified by performing reverse transcription-PCR (RT-PCR) using cDNAs from nine different mouse tissues (testis, epididymis, ovary, brain, heart, kidney, lung, liver, and spleen), as well as cDNAs from the testes, and the epididymis of W/Wv mutant mice, which lack germ cells, and mature sperm. Total RNA was extracted using the TRIzol reagent (Molecular Research Center) according to the manufacturer's protocol, and cDNA was synthesized by random hexamer and oligo(dT) priming using Omniscript reverse transcriptase (Qiagen). A specific region of the Spink2 transcript was amplified with the primers 5′-GTG GGA TCC CCG ACT CTT CCG ATT C-3′ (forward) and 5′-CAT CAA AGA CGA GCC CTG AGA A-3′ (reverse) using the following PCR thermocycling conditions: 30 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s. The primers for glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and Protamine 1 (Prm1), used as a control, were amplified under the same conditions using the primers 5′-TGA AGG TCG GAG TCA ACG GAT TTG GT-3′ (forward) and 5′-CAT GTG GGC CAT GAG GTC CAC CAC-3′ (reverse) for Gapdh and 5′-GCC GCA GCA AAA GCA-3′ (forward) and 5′-CGG ACG GTG GCA TTT-3′ (reverse) for Prm1. Specific expression at different stages of spermatogenesis was established using total RNA obtained from testes of prepubertal and adult male mice (ages 8, 10, 12, 14, 16, 20, 30, and 84 days) for reverse transcription.
Antibodies
Polyclonal antisera against mouse SPINK2 was produced from rabbits immunized with antigens. Glutathione S-transferase (GST) fusion proteins were produced by generating PCR products corresponding to the hydrophilic region of SPINK2 protein (amino acids 21–84). After restriction digestion, the PCR products were ligated into a pGEX-5X-2 vector (GE Healthcare). The inserted DNA was sequenced prior to transformation of Escherichia coli BL21. After induction with 0.1 mm isopropyl-β-d-thiogalactopyranoside, GST-SPINK2 fusion proteins were subsequently isolated with glutathione-Sepharose 4B. A SPINK2 peptide corresponding to amino acids 70–86 was synthesized by AnyGen. GST-SPINK2 fusion proteins and the synthesized peptide were used as antigens for the production of rabbit polyclonal antibodies by Peptron. All antibodies were affinity-purified using their corresponding proteins and the AminoLink Immobilization kit (Pierce). Monoclonal anti-α-tubulin, anti-mouse ADAM2, and anti-rabbit caspase-3 were purchased from Sigma-Aldrich, Millipore, and Novus Biologicals, respectively. Horseradish peroxidase (HRP)-conjugated anti-rabbit and anti-mouse IgG secondary antibodies (Jackson ImmunoResearch) were used for immunoblot analyses.
Immunoblot Analysis
Proteins, denatured by boiling for 10 min in the presence of 3% SDS and 5% β-mercaptoethanol (1× SDS/β-mercaptoethanol sample buffer), were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (pore size, 0.2 μm; Bio-Rad Laboratories). Membranes were blocked in TBS-T (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.1% Tween 20) containing 5% nonfat dry milk for 1 h at room temperature and then washed and incubated with primary antibodies for 1 h. After washing three times with TBS-T (10 min each), membranes were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. Following three washes in TBS-T, immunoreactive proteins were detected using an enhanced chemiluminescence kit (Pierce).
Preparation of Testicular Cells, Testicular Sperm, and Epididymal Sperm from ICR Male Mice and Spink2 Mutant Mice
All animal investigations were carried out according to the guidelines for animal care and use of Gwangju Institute of Science and Technology. Testicular (spermatogenic) cells, and testicular sperm were isolated by suspending in 52% isotonic Percoll (GE Healthcare), centrifuging for 10 min (27,000 × g, 4 °C), and resuspending in Mg2+-HEPES buffer (20). Sperm from cauda epididymis and vas deferens were released directly into PBS. The sperm suspension was centrifuged twice at 800 × g for 3 min to remove contaminants. The collected cells and sperm were directly resuspended in 1× SDS/β-mercaptoethanol sample buffer followed by boiling for 10 min.
Immunohistochemistry
Paraffin sections of mouse testis (Zyagen) were deparaffinized using xylene and rehydrated with a graded series of 100, 95, 70% ethanol and PBS. After blocking with 3% BSA (Bovogen Biologicals) for 1 h, the sections were incubated with primary antibodies and rhodamine-conjugated secondary antibody (Jackson ImmunoResearch), and then stained with Hoechst 33342 dye (Sigma-Aldrich). Fluorescence signals were observed under a microscope (DMLB; Leica Microsystems).
Generation of Mutant Mice
A CMHD-GT_327A6-3 embryonic stem (ES) cell line containing a Spink2 gene trap allele was purchased from CMHD. The gene trap construct contained a splicing acceptor sequence, internal ribosome entry site, enhanced GFP gene, polyadenylation signal, neomycin resistance gene, splicing donor sequence, and β-galactosidase-neomycin resistance gene fusion. ES cells, derived from 129-strain mice, were injected into C57BL/6 blastocysts using standard procedures to produce chimeric mice. Chimeric males were mated with C57BL/6 females, and germ line transmission in pups was confirmed. The Spink2gt/gt line was obtained by crossing heterozygotes. Mice were genotyped by amplifying Spink2 and GFP in the gene trap vector using the following two sets of primers: 5′-AGA GAA AAG CGG ACC-3′ (forward) and 5′-GGA ATG GAA ACG GGG-3′ (reverse) for Spink2 and 5′-GGC AAC TAC AAG ACC-3′ (forward) and 5′-AGG TAG TGG TTG TCG-3′ (reverse) for enhanced GFP.
Phenotypic Analyses of Mutant Mice
For fertility test of Spink2 mutant males, each Spink2 mutant and wild-type (WT) male (8 weeks old) was placed with two C57BL/6 females. The females were checked for the presence of vaginal plugs and pregnancy. The number of pups was counted, and fertility rate was calculated. For sperm counting, sperm from cauda epididymis and vas deferens from 8-week-old Spink2 mutant and WT males was collected. Sperm cells were counted in a hemocytometer under a light microscope. In the analysis of testicular integrity, testes were fixed by immersion in Bouin's fixative for 24 h, embedded in paraffin, and sectioned using standard protocols. The morphology of testes was observed after hematoxylin and eosin staining of paraffin-embedded sections. The level of apoptosis in sections of testes was detected by TUNEL assay using the ApopTag Plus Peroxidase in Situ Apoptosis Detection kit (Chemicon), according to the manufacturer's instructions.
Determination of Proteolytic Activity of Testis Extracts
Testes from WT and Spink2 mutant males were collected. Then testis extracts were analyzed for serine protease activity with the synthetic substrate (CBZ-Ile-Pro-Arg)2-Rhodamine110 in 10 mm Tris, pH 7.5, containing 15% (v/v) ethanol, as suggested by the manufacturer (Molecular Probes). Proteolytic activity of testis lysates was monitored as an increase in fluorescence at 521 nm with a SPECTRAmax Gemini XS microplate reader (Molecular Devices).
Statistics
Results are presented as means ± S.E. or S.D. values. The statistical significance of differences between data means was determined using a two-tailed Student's t test.
RESULTS
Sequence and Phylogenetic Relationships of Mouse SPINK2
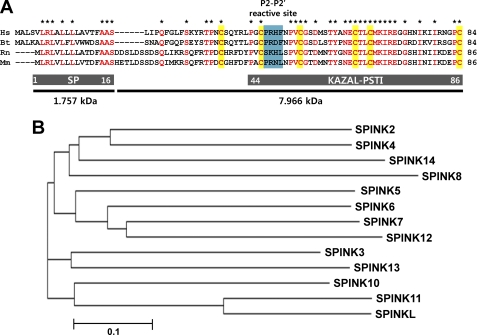
As an initial step in characterizing mouse Spink2, we performed sequence analyses using NCBI databases. The mouse Spink2 gene encodes an 86-amino acid protein (Fig. 1A) with a predicted molecular mass of 9.723 kDa. The SPINK2 protein is predicted to contain a signal peptide (residues 1–16, 1.757 kDa) and a Kazal-type serine protease inhibitor-pancreatic secretory trypsin inhibitor domain (residues 44–86). Pro47-Arg48-Asn49-Leu50 residues represent the P2-P2′ reactive site. The amino acid sequence of mouse SPINK2 shares 55, 82, and 53% similarity with that of human, rat, and bull, respectively. Notably, cysteine residues were conserved among these species. The phylogenetic relationships of SPINK2 to the other 12 SPINK members in mice are shown in Fig. 1B.
FIGURE 1.
Amino acid sequence, phylogenetic relationships, and domain structure of SPINK2. A, sequence alignment of SPINK2 among four species. Sequences were aligned using ClustalW2. Amino acids identical in all species are indicated with asterisks. The domain structure, amino acid number, and molecular mass are presented at the bottom. The conserved cysteine residues are shaded in yellow. The P2-P2′ reactive site is depicted by a blue box. The species names are as follows: Mm, Mus musculus; Hs, Homo sapiens; Rn, Rattus norvegicus; Bt, Bos taurus; SP, signal peptide; KAZAL-PSTI, Kazal-type pancreatic secretory trypsin inhibitor. GenBank accession numbers for SPINK2 are as follows: Mm, NP_899107; Hs, NP_066937; Rn, NP_001008870; Bt, NP_001108330. B, phylogenetic tree of mouse SPINK family members. The phylogenetic tree was created by the ClustalW2 method to evaluate the evolutionary relationship among SPINKs. The SPINKs that are most and least conserved with respect to SPINK2 are SPINK4 (NP_035593) and SPINK11 (NP_001041682.2), respectively.
Activity of SPINK2 in Vitro
To explore the functional characteristics of mouse SPINK2, we evaluated SPINK2 for serine protease-inhibitory activity in vitro, assaying the activity of His-tagged SPINK2 in the presence of trypsin and the synthetic substrate (CBZ-Ile-Pro-Arg)2-Rhodamine110. Upon enzymatic cleavage of the two oligopeptide side chains by the serine protease, the nonfluorescent substrate becomes fluorescent. This is a sensitive and selective substrate specific for the serine protease trypsin (21, 22). The results showed that recombinant SPINK2 protein reduced trypsin activity in a dose-dependent manner (Fig. 2A), confirming its protease-inhibitory activity. The half-maximal inhibitory concentration (IC50) was 2.73 μm (±0.45), and the maximal decrease in activity was detected in the presence of 30 μm SPINK2 protein.
FIGURE 2.
Activity of SPINK2 in vitro. Trypsin (42 nm) and His-tagged SPINK2 recombinant protein were incubated with the synthetic substrate (CBZ-Ile-Pro-Arg)2-Rhodamine110, and activity was determined by measuring absorbance at 521 nm. The addition of His-tagged SPINK2 recombinant protein decreased the activity of trypsin. Values are means ± S.D. (error bars, n = 3). Statistical significance (*, p < 0.05; **, p < 0.01) was assessed using Student's t test. Each experiment was repeated at least three times.
Spink2 Expression Pattern
To investigate the in vivo properties of Spink2, we first examined its expression profile at the transcriptional and protein level. The tissue distribution of Spink2 transcripts was investigated by RT-PCR using cDNAs from various mouse tissues. Spink2 was expressed strongly in the testis and weakly in the epididymis, but was not detected in other tissues (Fig. 3A). To determine which cells transcribe Spink2 in the testis, we performed RT-PCR with cDNA from the testes of W/Wv (c-kit) mutant mice, which lack germ cells. The results showed that Spink2 transcripts were absent in testes devoid of germ cells (Fig. 3B). We also investigated the Spink2 expression pattern in epididymis. Spink2 was expressed in the epididymis of W/Wv mutant mice lacking sperm. Mature sperm was also found to express Spink2, but the expression level was very low (Fig. 3B). Thus, epididymal expression of Spink2 is attributable in large to epididymal tissue, rather than sperm present in the epididymal tract.
FIGURE 3.
Distribution of Spink2 transcripts and protein. A, tissue distribution of Spink2, showing predominant transcription in testes. Complementary DNAs from various mouse tissues were amplified by PCR. Gapdh was included as a loading control. T, testis; E, epididymis; O, ovary; B, brain; H, heart; K, kidney; Li, liver; Lu, lung; Sp, spleen; Gapdh, glyceraldehyde-3-phosphate dehydrogenase. B, germ cell-specific expression of Spink2. RT-PCR was performed using testes and epididymis from WT and germ cell-lacking W/Wv mice and mature sperm. Protamine 1 (Prm1) was used as a control. C, developmental expression pattern of Spink2. Juvenile spermatogenesis consists of mitotic, meiotic, and postmeiotic phases. Stage-specific expression of Spink2 was determined from mouse testes on different days after birth (days 8, 10, 12, 14, 16, 20, 30, and 84). PL, preleptotene; L, leptotene; Z, zygotene; P, pachytene; D, diplotene; MI, meiotic division I; MII, meiotic division II. D, SPINK2 protein in WT and W/Wv testes. Total lysates from WT testes and germ cell-lacking testes from W/Wv mutant mice were immunoblotted with the anti-SPINK2 antibody. SPINK2 was only found in normal mouse testes. An anti-α-tubulin antibody was used as a control. E, stage-specific expression of SPINK2 during spermatogenesis examined by immunoblotting using total lysates obtained from prepubertal and adult male mice (days 7, 14, 21, 28, 35, 42, 49, and 56). SPINK2 was present from day 21, and the processed form was detected beginning on day 35. F, diagram of sperm development and maturation. Three populations of cells analyzed in the present study are shown: testicular cells (TC), testicular sperm (TS), and mature sperm (S). G, protein samples from TC, TS, and S immunoblotted with the anti-SPINK2 antibody. The 8-kDa protein is considered a processed form that lacks the signal peptide present in the 10-kDa protein. SPINK2 was present in all stages of germ cells. ADAM2 protein was included as a reference protein.
RT-PCR was additionally performed using testes from WT mice obtained at different times after birth (days 8, 10, 12, 14, 16, 20, 30, and 84). Most cells in the testis on days 8–10 are somatic, and the proportion of germ cells increases as spermatogenesis proceeds to produce (in order) spermatogonia, spermatocytes, and spermatids. If a particular gene is expressed in germ cells during spermatogenesis, a transcript for the gene will appear in the testis at a certain postpartum time point corresponding to a specific stage of spermatogenesis. Transcription of Spink2 was found to start at postnatal day 16, at a time that corresponds to pachytene spermatocytes (Fig. 3C). Taken together, our results indicate that Spink2 is expressed predominantly in the testis and exhibits germ cell-specific and developmentally regulated expression patterns.
To investigate the expression patterns of SPINK2 at the protein level, we generated a polyclonal antibody against a synthesized peptide (residues 70–86) and performed immunoblot analyses on testes from WT and W/Wv (germ cell-lacking) mutant mice. As shown in Fig. 3D, two bands with molecular sizes of 10 and 8 kDa were detected in WT testes, but no bands were detected in testes lacking germ cells, confirming the germ cell-specific expression pattern found in the RT-PCR analysis (Fig. 3B). Because SPINK2 has a signal peptide (Fig. 1B), it is highly likely that the 10-kDa band represents the intact precursor SPINK2 protein containing the signal peptide, whereas the 8-kDa protein corresponds to a processed form. Further immunoblot analyses, carried out using mouse testes obtained on different postnatal days, showed a developmentally regulated expression pattern (Fig. 3E), similar to the RT-PCR results (Fig. 3C). Finally, we examined the expression pattern of SPINK2 protein in germ cells during spermatogenesis. Immunoblot analyses were performed on cells from different stages during sperm development, such as testicular spermatogenic cells and testicular sperm, and mature sperm from the epididymis. The population of testicular cells includes spermatogenic cells corresponding to spermatogonia, spermatocytes, and round spermatids. Testicular sperm are a small portion of elongating and condensing spermatids and a larger fraction of fully developed sperm. Sperm represent posttesticular, mature sperm from the cauda epididymis and vas deferens (Fig. 3F). The result showed that SPINK2 was expressed in all cell types (Fig. 3G). Notably, the 10-kDa band present in testicular cells was absent from testicular sperm and mature sperm, suggesting that the signal peptide of SPINK2 is removed during spermatogenesis.
To establish the cellular localization of SPINK2 further, we carried out immunohistochemical analysis using paraffin sections of adult mouse testis. As a result, we observed the SPINK2 signal in the cytoplasmic region of round spermatids, but not in spermatogonia and spermatocytes (Fig. 4). In addition, as round spermatids differentiate into spermatozoa, the signal was detected in acrosomal regions. Our results collectively demonstrate that mouse SPINK2 protein is specific to germ cells in the testis and is developmentally regulated during spermatogenesis.
FIGURE 4.
Immunohistochemical analysis of SPINK2. Paraffin sections of mouse testis were stained with anti-SPINK2 antibody and normal rabbit serum. The nucleus was stained by Hoechst 33342 dye. Ab, specific antibody; Hoechst, Hoechst staining; Merge, merged images between antibody and Hoechst. Scale bar, 5 μm.
Generation of Spink2 mutant Mice
The trypsin-inhibitory activity and germ cell- and stage-specific expression of SPINK2 suggest that SPINK2 plays an important role in male reproduction. To investigate the in vivo role of Spink2 in the reproductive process, we generated Spink2 mutant mice by means of gene trap mutagenesis. Gene trapping is a high-throughput approach that randomly generates loss-of-function mutations by introducing insertional mutations across the mouse genome. Insertion of a gene trap vector with an upstream 3′ splice site and a downstream transcriptional termination sequence into an intron of an expressed gene results in a fusion transcript that encodes a truncated protein (23). We screened public gene trap databases for an ES cell line with insertion of the gene trap vector in the Spink2 gene, which generates an abnormal fusion transcript. Such an ES cell line was obtained, and the precise location of the genomic insertion was mapped by splinkerette PCR and DNA sequencing analyses. The integration site of the gene trap vector mapped to a position upstream of exon 1, producing a fusion transcript lacking exon 1 (Fig. 5A). Chimeric mice and heterozygous mice carrying the gene trap allele (Spink2+/gt) were generated using standard procedures. Homozygous (Spink2gt/gt) males were obtained by crossing with heterozygous mice. Spink2+/gt and Spink2gt/gt mice were verified by PCR genotyping based on sequence information (Fig. 5B). Spink2gt/gt mice were born live with the predicted Mendelian pattern of inheritance. The weights and growth rates of Spink2gt/gt newborn pups were not significantly different from those of WT (Spink2+/+) mice. To investigate SPINK2 protein expression in Spink2gt/gt mice, we performed immunoblot analyses on testes using the anti-SPINK2 antibody described above. This analysis revealed a reduction in SPINK2 levels in the testes from Spink2gt/gt mice compared with that in WT mice (Fig. 5C). The presence of SPINK2 protein in Spink2gt/gt mice is likely due to the production of a WT transcript by alternative splicing, resulting in hypomorphic mutation, a situation that often occurs in gene trap mutagenesis. It should be noted that the degree of reduction was variable among Spink2gt/gt mice (Fig. 5D). This could be because there is variable penetrance of the effect of the gene trap mutation on the expression of the Spink2 gene among mice. In subsequent phenotypic investigations of Spink2gt/gt mice, only mutant mice with >50% reduction in SPINK2 levels were examined.
FIGURE 5.
Generation of Spink2 mutant mice. A, schematic diagram of the Spink2 gene and gene trap vector insertion. The numbers in the boxes indicate exons, and filled boxes show coding regions of SPINK2. The gene trap construct contains a splicing acceptor sequence (SA), internal ribosome entry site (IRES), enhanced GFP gene (EGFP), polyadenylation signal (pA), neomycin resistance gene (neo), splicing donor sequence (SD), and β-galactosidase-neomycin resistance fusion gene. Arrows refer to primers (a, b, c, and d) used in B. WT, PCR from wild-type allele; GT, PCR from allele with gene trap mutation. B, PCR genotyping analysis using the allele-specific primers shown in A. Amplification with primers a and b (WT allele) yielded a 741-bp PCR product, and amplification with primers c and d (GT allele) yielded a 293-bp PCR product. C, SPINK2 expression levels in Spink2 mutant testes. Testis samples from WT (Spink2+/+), and Spink2 mutant mice (Spink2gt/gt) were immunoblotted with an antibody against SPINK2. An anti-α-tubulin antibody was used as a control for sample loading. Immunoblot from the analysis of Spink2gt/gt mouse showing the average value of reduction (∼75%) among the mutant mice is shown. D, expression level of SPINK2 in Spink2gt/gt mice subjected to phenotypic analysis.
Phenotypic Analyses of Spink2gt/gt Mice
To determine whether the reduction in SPINK2 levels affected reproductive functions, we performed a fertility test. Adult WT and Spink2gt/gt males were mated with WT females. The average litter size of pups produced over a breeding period was significantly reduced in Spink2gt/gt males compared with WT males, despite frequent observations of vaginal plugs in females. Overall, the in vivo fertility rate of Spink2gt/gt males was reduced by about 40% relative to WT males (Table 1). Adult Spink2gt/gt female mice exhibited normal fertility (data not shown). Next, we examined testes from adult Spink2gt/gt mice. Testes from mutant males were smaller than those from WT littermates (Fig. 6A). In fact, the testis-to-body-weight ratio was significantly lower in Spink2gt/gt mice than in WT mice (Fig. 6B), suggesting an abnormality in testicular function and/or spermatogenesis. To determine whether the observed testicular changes led to altered sperm production, we evaluated mature sperm collected from the cauda epididymis and vas deferens of 2-month-old Spink2gt/gt mice and WT littermates. It should be noted that the appearance and weight of epididymis from Spink2gt/gt mice were normal compared with those from WT mice (Fig. 6, C and D). An analysis of sperm revealed a significant reduction in sperm counts (48% of WT) (Fig. 6E). In addition, we found that Spink2gt/gt mice produced more morphologically abnormal sperm than did WT mice (Fig. 6F). The most frequently observed abnormalities were sperm in which the flagellum was bent at a region between the principal piece and the mid-piece (Fig. 6G). These results demonstrate that a decrease in the amount of SPINK2 disrupts testicular integrity and normal sperm production, leading to reduced fertility.
TABLE 1.
Effect of SPINK2 deficiency on male fertility
| Parameter | Genotype of males |
|
|---|---|---|
| Spink2+/+ | Spink2gt/gt | |
| Number of males mated | 7 | 7 |
| Number of females mated | 14 | 14 |
| Males producing plugs (%) | 100 | 100 |
| Pregnant females (%) | 100 | 92.86 |
| Average litter size | 8.56 | 5.19 |
| Fertility ratea (%) | 100 | 60.63b |
a Defined as the percentage of the average WT litter size.
b p < 0.01.
FIGURE 6.
Phenotypic analyses of Spink2 mutant mice. A, macroscopic appearance of adult testes from an 8-week-old Spink2gt/gt mouse and a WT littermate (Spink2+/+). Scale bar, 2 mm. B, comparison of testis weight in Spink2+/+ and Spink2gt/gt mice. The organs were trimmed of fat and weighed. Values are means ± S.E. (error bars). Statistical significance (*, p < 0.05; n = 8) was assessed using Student's t test. The average testis weight:body weight ratios for WT and Spink2gt/gt males were 0.0071 ± 0.0004 and 0.0055 ± 0.0005, respectively. C, macroscopic appearance of adult epididymis from Spink2gt/gt and Spink2+/+ mice. Scale bar, 4 mm. D, epididymis weight in Spink2gt/gt mouse and a WT littermate (n = 5). Values are means ± S.E. The average epididymis weight:body weight ratios for WT and Spink2gt/gt males were 0.0025 ± 0.0001 and 0.0025 ± 0.0001, respectively. E, number of mature sperm from Spink2+/+ and Spink2gt/gt mice. Sperm were collected from the cauda epididymis and vas deferens. Values are means ± S.E. Statistical significance (**, p < 0.01; n = 8) was assessed using Student's t test. The average numbers of mature sperm in WT and Spink2gt/gt males were 2.89 ± 0.40 × 107 and 1.29 ± 0.27 × 107, respectively. F, percentage of sperm from Spink2+/+ and Spink2gt/gt mice with morphological abnormalities. Sperm were collected from the cauda epididymis and vas deferens. Values are means ± S.E. Statistical significance (*, p < 0.05; n = 6) was assessed using Student's t test. The percentage of abnormal sperm in WT and Spink2gt/gt males was 3.24 ± 0.86% and 17.89 ± 5.78%, respectively. G, morphological abnormalities of Spink2gt/gt sperm. Sperm were collected from the cauda epididymis and vas deferens and observed under a light microscope. The principal morphological abnormality observed was bending at the principal and middle piece junction. Scale bar, 10 μm.
Abnormalities and Apoptosis in Spink2gt/gt Germ Cells
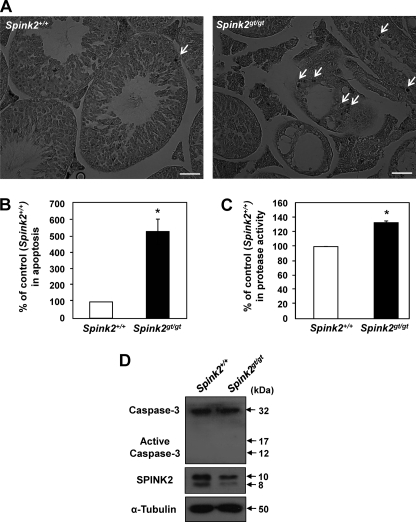
To identify the underlying cause of abnormalities in testicular size and sperm production in Spink2gt/gt mice, we analyzed the histological appearance of seminiferous tubules in the adult testis. Spink2gt/gt testes contained seminiferous tubules that were significantly reduced in size compared with those of WT testes (Fig. 7, A and B) and exhibited vacuolization and germ cell loss; in contrast, spermatogenesis was robust in testes from WT adult males (Fig. 7C). In severe cases, most germ cells were degenerated, and only a few spermatogenic cells were detected in the seminiferous tubules of Spink2gt/gt mice (Fig. 7A). To determine whether the loss of germ cells in Spink2 mutant testes was related to apoptosis, we performed TUNEL assays. TUNEL staining of histological sections of WT and Spink2gt/gt testes revealed a significant increase in the number of apoptotic cells in Spink2gt/gt mice compared with WT mice (Fig. 8, A and B). These results indicate that SPINK2 is important for the survival and development of male germ cells and may be involved in regulating apoptosis in these cells.
FIGURE 7.
Histological analyses of testes from adult Spink2 mutant mice. A, histological sections of seminiferous tubules prepared from testes of Spink2+/+ and Spink2gt/gt mice stained with hematoxylin and eosin. The seminiferous tubules in Spink2gt/gt mice were markedly degenerated compared with those in Spink2+/+ testes. Arrows indicate abnormal vacuoles. Some tubules contained only a few spermatogenic cells around basal compartments (asterisks). Scale bar, 100 μm (upper) and 50 μm (lower). B, comparison of the size of seminiferous tubules in Spink2+/+ and Spink2gt/gt mice. The size was the shortest diameter in each tubule. Values are means ± S.E. (error bars). Statistical significance (*, p < 0.05; n = 5) was assessed using Student's t test. The average size of seminiferous tubules in WT and Spink2gt/gt males was 283.82 ± 12.00 nm and 192.98 ± 12.10 nm, respectively. C, abnormal seminiferous tubules in Spink2+/+ and Spink2gt/gt mice. Values are means ± S.E. Statistical significance (*, p < 0.05; n = 3) was assessed using Student's t test. The average percentage of abnormal seminiferous tubules in WT and Spink2gt/gt males was 2.19% ± 0.28% and 33.03% ± 6.62%, respectively.
FIGURE 8.
Apoptosis and increased serine protease activity in Spink2 mutant testes. A, germ cell apoptosis in Spink2+/+ and Spink2gt/gt testes. Sections of testes were TUNEL-stained; positive cells are indicated by arrows. Scale bar, 50 μm. B, comparison of TUNEL-positive germ cells in Spink2+/+ and Spink2gt/gt testes. The percentage of apoptotic germ cells per seminiferous tubule in Spink2+/+ testes is shown in the y axis. Values are means ± S.E. (error bar). Statistical significance (*, p < 0.05; n = 4) was assessed using Student's t test. The average percentage of apoptotic germ cells in Spink2gt/gt testes was 529.88% ± 77.28% of that in WT testes. C, increased serine protease activity in Spink2gt/gt testes. Testis extracts from Spink2+/+ and Spink2gt/gt males were incubated with the rhodamine-conjugated substrate, and serine protease activity was measured. Values are means ± S.D. (n = 3). Statistical significance (*, p < 0.01) was assessed using Student's t test. Each experiment was repeated at least three times. Increased activity in Spink2gt/gt testes was 133.36 ± 2.09% of that in WT testes. D, caspase-3 activation in the testes. Immunoblot of whole cell lysates of Spink2+/+ and Spink2gt/gt testes was performed with anti-caspase-3 antibody. An anti-α-tubulin antibody was used as a control for sample loading.
Increased Serine Protease Activity in Spink2gt/gt Testes
Germ cell apoptosis in Spink2gt/gt testes might be directly related to excessive activity of serine proteases. We measured protease activity in the testis lysates of WT and Spink2gt/gt mice using the synthetic substrate (CBZ-Ile-Pro-Arg)2-Rhodamine110. This analysis revealed that serine protease activity is significantly higher in Spink2gt/gt testes compared with WT testes (Fig. 8C), indicating dysregulated protease activity in the mutant testes. Finally, we investigated whether caspase-3, an executioner of caspase-dependent apoptosis, is activated during apoptosis caused by SPINK2 deficiency. In general, upon caspase-dependent apoptotic stimulation, caspase-3 is formed from a 32-kDa pro-caspase-3 that is cleaved into 17- and 12-kDa subunits. Our immunoblot analysis showed that 17- and 12-kDa subunits of caspase-3 were absent from both WT and Spink2gt/gt testes samples (Fig. 8D). Thus, our results indicate that SPINK2 indeed functions as a serine protease inhibitor and the deficiency of SPINK2 causes apoptosis independent on caspase.
DISCUSSION
Genes expressed specifically or predominantly in male germ cells are critical for spermatogenesis. The present study provides new information on the Kazal-type serine protease inhibitor, Spink2, at transcriptional, protein, and functional levels in mice. We found that the Spink2 gene is transcribed predominantly in the testis, where its transcription is limited to germ cells. Protein analyses demonstrated the presence of SPINK2 in spermatogenic cells and mature sperm. Importantly, we generated mutant mice expressing SPINK2 at reduced levels and found that fertility was significantly decreased in mutant male mice. Further phenotypic analyses revealed that testicular integrity was disrupted, leading to significant changes in sperm number and morphology. Finally, we found that testes from mutant mice exhibited abnormal spermatogenesis and germ cell apoptosis.
Cell death occurs through several processes, namely necrosis, autophagy, and apoptosis. Apoptosis is a type of cell death that does not cause cell lysis and therefore does not initiate immune responses. Among the distinctive features of apoptotic cells are nuclear and cytoplasmic condensation, membrane blebbing, and internucleosomal DNA fragmentation, which produces a characteristic laddering pattern on agarose gels (24). Caspases have traditionally been presumed to play a dominant role as the primary mediator of apoptosis. However, several lines of evidence indicate that serine proteases also play crucial roles in mediating and promoting apoptosis through both caspase-independent (25) and -dependent mechanisms (26). The involvement of serine proteases in programmed cell death was first suggested in a study showing that inhibitors of serine proteases prevent apoptosis in SK-MEL-109 melanoma cells (27). To date, several serine proteases, including EOS, Omi/HtrA2, granzyme A and B, thrombin, and AP24, have been reported to regulate apoptosis. Consistent with the involvement of serine proteases in apoptosis, serine protease inhibitors, such as serpins, cytokine response modifier A (CrmA), serine protease inhibitor 1 (SPI-1), proteinase inhibitor 9 (PI-9), plasminogen activator inhibitor 2 (PAI-2), protease nexin 1 (PN-1), and serine protease inhibitor 2 (SPI-2), have also been reported to regulate apoptosis (28, 29). Collectively, these observations indicate that serine proteases are integrally involved in the induction of apoptosis.
Given the relationship between serine protease activity and apoptosis, serine protease inhibitors such as SPINKs deserve special attention. SPINK1, SPINK3, and SPINK5 have been found to have trypsin-inhibitory activities (13, 22, 30). Dysregulation of SPINK proteins is known to cause severe diseases. Spink5 knock-out mice exhibit premature proteolysis of corneodesmosin, causing Nertherton syndrome (30, 31). Mutations in human SPINK1 are associated with chronic pancreatitis (32). Mice lacking Spink3, an ortholog of human SPINK1, exhibit autophagic cell death and impaired regeneration of pancreatic acinar cell because of increased trypsin activity (22, 33). Importantly, recent studies have demonstrated that up-regulation of SPINK1 causes cellular resistance to serine protease-dependent apoptosis (34, 35). Our present study demonstrated that recombinant SPINK2 also possesses trypsin-inhibitory activity, and Spink2gt/gt testes exhibit an increase in serine protease activity. Further, we provided evidence that SPINK2 deficiency leads to apoptosis in germ cells without the activation of caspase-3. Thus, we propose that SPINK2 is directly involved in serine protease-dependent germ cell apoptosis. Whether SPINK2 regulation of serine proteases involved in programmed cell death takes place in intracellular or extracellular compartments is not yet clear. However, SPINK2 contains a signal peptide that was found to be processed during spermatogenesis, suggesting that the protein could be secreted; as such, it could regulate extracellular proteases that activate apoptosis-triggering cell surface receptors. Alternatively, SPINK2 may control intracellular proteolytic events involved in apoptosis.
Apoptosis of germ cells appears to be a continuous feature of the adult testis. The number of germ cells in seminiferous tubules is determined by a dynamic balance between proliferation and apoptotic death of germ cells. Germ cells undergoing both mitosis and meiosis tend to have a high probability of genetic errors; thus, germ cells depend on the capacity to induce cell death to eliminate cells with genetic defects (36). On the other hand, apoptosis should be regulated in postmeiotic germ cells to maintain the population of spermatozoa. Therefore, regulation of apoptosis is important for maintaining normal spermatogenesis. SPINK2 may act as a major regulator of apoptosis during the postmeiotic phase.
In conclusion, we comprehensively investigated Spink2 in mice, providing key information on testicular distribution, expression patterns in germ cells, and reproductive defects in Spink2 mutant mice. Notably, our study suggests that SPINK2 is required for maintaining normal spermatogenesis, potentially acting as a regulator of proteolytic events and apoptosis in germ cells. This is the first study highlighting the role of a Kazal-type protease inhibitor in reproduction. Our discovery provides a framework for future studies designed to address the detailed mechanisms during male germ cell development.
This work was supported by Korea Science and Engineering Foundation Grant 2010-0028776, a Korea Research Foundation Grant KRF-2008-313-C00736, and a Gwangju Institute of Science and Technology Systems Biology Infrastructure Establishment Grant.
- Spink2
- serine protease inhibitor Kazal-type 2.
REFERENCES
- 1. Eddy E. M. (1995) Reprod. Fertil. Dev. 7, 695–704 [DOI] [PubMed] [Google Scholar]
- 2. Eddy E. M. (1998) Semin. Cell Dev. Biol. 9, 451–457 [DOI] [PubMed] [Google Scholar]
- 3. Eddy E. M. (2002) Recent Prog. Horm. Res. 57, 103–128 [DOI] [PubMed] [Google Scholar]
- 4. Hooper N. M. (2002) Proteases in Biology and Medicine, Portland Press, London [Google Scholar]
- 5. Egeblad M., Werb Z. (2002) Nat. Rev. Cancer 2, 161–174 [DOI] [PubMed] [Google Scholar]
- 6. Krane S. M. (2003) Arthritis Res. Ther. 5, 2–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esler W. P., Wolfe M. S. (2001) Science 293, 1449–1454 [DOI] [PubMed] [Google Scholar]
- 8. Luttun A., Dewerchin M., Collen D., Carmeliet P. (2000) Curr. Atheroscler. Rep. 2, 407–416 [DOI] [PubMed] [Google Scholar]
- 9. Rawlings N. D. (2010) Biochimie 92, 1463–1483 [DOI] [PubMed] [Google Scholar]
- 10. Rockett J. C., Patrizio P., Schmid J. E., Hecht N. B., Dix D. J. (2004) Mutat. Res. 549, 225–240 [DOI] [PubMed] [Google Scholar]
- 11. Fink E., Hehlein-Fink C., Eulitz M. (1990) FEBS Lett. 270, 222–224 [DOI] [PubMed] [Google Scholar]
- 12. Kazal L. A., Spicer D. S., Brahinsky R. A. (1948) J. Am. Chem. Soc. 70, 3034–3040 [DOI] [PubMed] [Google Scholar]
- 13. Marchbank T., Freeman T. C., Playford R. J. (1998) Digestion 59, 167–174 [DOI] [PubMed] [Google Scholar]
- 14. Krause R., Hemberger M., Messerschmid M., Mayer W., Kothary R., Dixkens C., Fundele R. (1998) Differentiation 63, 285–294 [DOI] [PubMed] [Google Scholar]
- 15. Raghunath M., Tontsidou L., Oji V., Aufenvenne K., Schürmeyer-Horst F., Jayakumar A., Ständer H., Smolle J., Clayman G. L., Traupe H. (2004) J. Invest. Dermatol. 123, 474–483 [DOI] [PubMed] [Google Scholar]
- 16. Oh J., Lee J., Woo J. M., Choi E., Park I., Han C., Baek N., Lee H., Kim do H., Cho C. (2006) BMC Genomics 7, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jalkanen J., Kotimäki M., Huhtaniemi I., Poutanen M. (2006) Biochem. Biophys. Res. Commun. 349, 245–254 [DOI] [PubMed] [Google Scholar]
- 18. Lin M. H., Lee R. K., Hwu Y. M., Lu C. H., Chu S. L., Chen Y. J., Chang W. C., Li S. H. (2008) Reproduction 136, 559–571 [DOI] [PubMed] [Google Scholar]
- 19. Möritz A., Lilja H., Fink E. (1991) FEBS Lett. 278, 127–130 [DOI] [PubMed] [Google Scholar]
- 20. Phelps B. M., Koppel D. E., Primakoff P., Myles D. G. (1990) J. Cell Biol. 111, 1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leytus S. P., Patterson W. L., Mangel W. F. (1983) Biochem. J. 215, 253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohmuraya M., Hirota M., Araki K., Baba H., Yamamura K. (2006) Pancreas 33, 104–106 [DOI] [PubMed] [Google Scholar]
- 23. Araki M., Araki K., Yamamura K. (2009) Curr. Pharm. Biotechnol. 10, 221–229 [DOI] [PubMed] [Google Scholar]
- 24. Kerr J. F., Wyllie A. H., Currie A. R. (1972) Br. J. Cancer 26, 239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egger L., Schneider J., Rhême C., Tapernoux M., Häcki J., Borner C. (2003) Cell Death Differ. 10, 1188–1203 [DOI] [PubMed] [Google Scholar]
- 26. Gong B., Chen Q., Endlich B., Mazumder S., Almasan A. (1999) Cell Growth Differ. 10, 491–502 [PubMed] [Google Scholar]
- 27. Ruggiero V., Johnson S. E., Baglioni C. (1987) Cell. Immunol. 107, 317–325 [DOI] [PubMed] [Google Scholar]
- 28. Bird P. I. (1998) Results Probl. Cell Differ. 24, 63–89 [DOI] [PubMed] [Google Scholar]
- 29. Thiemmara V., Pays L., Danty E., Jourdan F., Moyse E., Mehlen P. (2002) Cell Death Differ. 9, 1343–1351 [DOI] [PubMed] [Google Scholar]
- 30. Yang T., Liang D., Koch P. J., Hohl D., Kheradmand F., Overbeek P. A. (2004) Genes Dev. 18, 2354–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Descargues P., Deraison C., Bonnart C., Kreft M., Kishibe M., Ishida-Yamamoto A., Elias P., Barrandon Y., Zambruno G., Sonnenberg A., Hovnanian A. (2005) Nat. Genet. 37, 56–65 [DOI] [PubMed] [Google Scholar]
- 32. Witt H., Luck W., Hennies H. C., Classen M., Kage A., Lass U., Landt O., Becker M. (2000) Nat. Genet. 25, 213–216 [DOI] [PubMed] [Google Scholar]
- 33. Ohmuraya M., Hirota M., Araki M., Mizushima N., Matsui M., Mizumoto T., Haruna K., Kume S., Takeya M., Ogawa M., Araki K., Yamamura K. (2005) Gastroenterology 129, 696–705 [DOI] [PubMed] [Google Scholar]
- 34. Lu X., Lamontagne J., Lu F., Block T. M. (2008) Apoptosis 13, 483–494 [DOI] [PubMed] [Google Scholar]
- 35. Lamontagne J., Pinkerton M., Block T. M., Lu X. (2010) J. Virol. 84, 907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shaha C., Tripathi R., Mishra D. P. (2010) Philos. Trans. R Soc. Lond. B Biol. Sci. 365, 1501–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]