Abstract
The innate ability to detect pathogens is achieved by pattern recognition receptors, which recognize non-self-components such as β1,3-glucan. β1,3-Glucans form a triple-helical structure stabilized by interchain hydrogen bonds. β1,3-Glucan recognition protein (βGRP)/Gram-negative bacteria-binding protein 3 (GNBP3), one of the pattern recognition receptors, binds to long, structured β1,3-glucan to initiate innate immune response. However, binding details and how specificity is achieved in such receptors remain important unresolved issues. We solved the crystal structures of the N-terminal β1,3-glucan recognition domain of βGRP/GNBP3 (βGRP-N) in complex with the β1,3-linked glucose hexamer, laminarihexaose. In the crystals, three structured laminarihexaoses simultaneously interact through six glucose residues (two from each chain) with one βGRP-N. The spatial arrangement of the laminarihexaoses bound to βGRP-N is almost identical to that of a β1,3-glucan triple-helical structure. Therefore, our crystallographic structures together with site-directed mutagenesis data provide a structural basis for the unique recognition by such receptors of the triple-helical structure of β1,3-glucan.
Keywords: Carbohydrate Binding Protein, Carbohydrate Structure, Crystal Structure, Innate Immunity, Pattern Recognition Receptor, GNBP3, Beta-glucan, BetaGRP, Triple Helix
Introduction
Carbohydrate polymers form a wonderful variety of shapes and architecture with their different glycosidic linkages and many hydrogen bonding possibilities, as exemplified by the glucose polymers, cellulose, amylose, and β-glucan (1). β-Glucan, mostly found in fungi and plant cell walls, is a polymer consisting of a backbone of β1,3-linked glucose units and side chains of β1,6-linked units of varying lengths. β1,3-Glucan assumes a triple-helical structure according to x-ray fiber diffraction (2), solid state 13C nuclear magnetic resonance spectroscopy (3), multiangle laser light scattering (4), fluorescence resonance energy transfer spectroscopy (5), and molecular dynamics simulation (6). In a widely accepted hydrogen bonding model, one β1,3-glucan chain forms intramolecular hydrogen bonds with the other two strands, perpendicular to the axis of the triple helix (7).
The β-glucan of pathogens is recognized as non-self by pattern recognition receptors in the host defense system (8–11). Accumulating evidence suggests that the long triple-helical conformation of β-glucan is important for binding to certain β-glucan-binding receptors (7, 12). β1,3-Glucan recognition protein/Gram-negative bacteria-binding protein 3 (βGRP/GNBP3)3 (13–15), a pattern recognition receptor of the innate immune system, binds triple-helical β-glucan strongly but has little affinity for denatured β-glucan or shorter β1,3-linked glucose oligomers (16, 17). Furthermore, injection into the fly, Drosophila, of the alkali-insoluble fraction of the Aspergillus fumigatus cell wall, which includes long β1,3-glucans, strongly activates the antifungal Toll pathway (14), whereas short laminarioligosaccharides do not (16). To date, three-dimensional structures of the ligand-free βGRP/GNBP3 N-terminal domain were reported revealed by x-ray crystallography (16) and solution NMR spectroscopy (16, 17). However, details of the structural basis of the biological activity of β-glucan have not been elucidated. Here, we report how the triplex β-glucan is recognized by βGRP/GNBP3.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
N-terminal β1,3-glucan recognition domain of the Plodia interpunctella βGRP gene was amplified by PCR from the original gene. The PCR product (Tyr1–Thr104) was cloned into a pCold-MBP-TRX vector with hexahistidine, maltose-binding protein (MBP), and thioredoxin (TRX) tags. This vector is capable of expressing a target protein at low temperature (15 °C) using a cold shock promoter cspA (18). It was constructed by insertion of MBP, TRX genes, and a tobacco etch virus protease cleavage sequence into NdeI and BamHI sites of a cold shock vector pCold-I (TaKaRa Bio, Inc.). The construct was composed of the His6-MBP-TRX tag and βGRP-N with a tobacco etch virus protease cleavage site between them. For the expression of Bombyx mori βGRP-N, a DNA fragment encoding Tyr1–Ala102 was cloned into pCold-MBP-TRX vector. Each expression plasmid was transformed into the Escherichia coli strain Rosetta2(DE3)pLysS (Novagen). The transformed cells were grown in LB medium at 37 °C and induced with 0.1 mm isopropyl β-d-thiogalactoside (Wako) for 24 h at 15 °C. The harvested cells were suspended in a buffer containing 50 mm Tris-HCl (pH 8.0), 50 mm NaCl, and Bugbuster (Novagen) and sonicated. After centrifugation, the supernatants were collected and applied to a Ni Sepharose column (GE Healthcare) equilibrated with PBS (8 mm Na2HPO4, 1 mm KH2PO4, 137 mm NaCl, and 3 mm KCl, pH 7.4). After washing the column with PBS, the proteins were eluted with PBS containing 500 mm imidazole. After dialysis against PBS containing 3 mm reduced glutathione and 0.3 mm oxidized glutathione, the fused proteins were digested by His6-tagged tobacco etch virus protease at 4 °C for 12 h. The digested proteins were passed through a nickel-Sepharose column, and applied to HiLoad 16/60 Superdex 75 prep grade column (GE Healthcare) equilibrated with PBS. The purified protein fractions were collected and replaced with a 10 mm Tris-HCl buffer (pH 8.0) and used for the crystallization.
Crystallization
Crystals of the N-terminal domain of βGRP were obtained by the sitting drop vapor diffusion method, in which 0.5 μl protein solution was mixed with an equal volume of reservoir solution. Plodia βGRP-N crystals complexed with laminarihexaose (Seikagaku Corp.) were obtained using the following reservoir solution: 0.2 m lithium chloride, 20% (v/v) PEG 3350 and 5 mm laminarihexaose. The ligand-free crystals were grown in a reservoir solution containing 1.6 m ammonium sulfate, 0.1 m MES monohydrate (pH 6.5), and 10% (v/v) 1,4-dioxane. Bombyx βGRP-N crystals complexed with laminarihexaose were obtained in a buffer of 0.1 m ammonium sulfate, 0.1 m HEPES sodium buffer (pH 7.5), 10% (w/v) PEG 4000, and 5 mm laminarihexaose.
X-ray Data Collection and Structure Determination
The diffraction data were collected at the synchrotron radiation source at AR-NE3A, AR-NW12A, and BL5A in the Photon Factory (High Energy Accelerator Research Organization (KEK), Japan). The crystals were cryoprotected with a reservoir solution containing 25% glycerol (Bombyx βGRP-N) or 25% ethylene glycol (Plodia βGRP-N). The diffraction data were processed using HKL2000 (19). The crystal parameters are shown in Table 1. The structures of Bombyx and Plodia βGRP-N·laminarihexaose complexes and ligand-free Plodia βGRP-N were solved by the molecular replacement method using the program Molrep (20) with Drosophila βGRP-N (Protein Data Bank code 3IE4), the solved ligand-free Plodia βGRP-N and liganded Bombyx βGRP-N as search models, respectively. Model building was manually performed using programs XtalView/Xfit (21) and COOT (22). Refinement was carried out using the programs CNS1.1 (23) and REFMAC5 (24). The stereochemical quality of the final models was assessed by PROCHECK (25). The refinement statistics are summarized in Table 1.
TABLE 1.
Data collection and refinement statistics of Plodia and Bombyx βGRP-Ns
| Plodia βGRP-N | Plodia βGRP-N·Glc6 | Bombyx βGRP-N·Glc6 | |
|---|---|---|---|
| Data collection | |||
| Beamline | PF BL-5A | PF-AR NW-12A | PF-AR NE-3A |
| Wavelength (Å) | 1.0000 | 1.0000 | 1.0000 |
| Space group | C2 | P212121 | P41 |
| Cell dimensions | |||
| a, b, c (Å) | 106.4, 38.0, 55.6 | 47.0, 69.3, 81.0 | 74.2, 74.2, 47.6 |
| α, β, γ | 90, 117.3, and 90° | 90, 90, and 90° | 90, 90, and 90° |
| Resolution (Å) | 50-1.57 (1.60-1.57) | 50-2.20 (2.28-2.20) | 50-2.05 (2.09-2.05) |
| Rmerge | 4.6 (13.0) | 13.7 (47.1) | 17.2 (46.1) |
| I/σI | 25.6 (9.5) | 11.1 (3.3) | 9.1 (2.0) |
| Completeness (%) | 97.5 (85.3) | 97.9 (97.0) | 98.3 (87.9) |
| Redundancy | 3.6 (3.0) | 4.6 (4.6) | 6.2 (3.3) |
| Refinement | |||
| Resolution (Å) | 20.00-1.58 | 20.00-2.20 | 20.00-2.05 |
| No. of reflections | 25,461 | 12,944 | 15,273 |
| Rwork/Rfree | 20.7/23.8 | 22.2/26.9 | 17.4/21.6 |
| No. of atoms | |||
| Protein | 1629 | 1661 | 1630 |
| Ligand/ion | 134 | 140 | |
| Water | 173 | 113 | 209 |
| B-factors (Å2) | |||
| Protein | 19.5 | 24.2 | 14.4 |
| Ligand/ion | 21.9 | 12.6 (8.7a) | |
| Water | 29.1 | 26.4 | 23.5 |
| Root mean square deviations | |||
| Bond lengths (Å) | 0.013 | 0.014 | 0.013 |
| Bond angles | 1.47° | 1.58° | 1.51° |
| Ramachandran plot (%) | |||
| Most favored | 92.8 | 92.4 | 91.5 |
| Additionally allowed | 7.2 | 7.6 | 8.5 |
| Generously allowed | 0 | 0 | 0 |
| Disallowed | 0 | 0 | 0 |
a Alternative conformation.
Construction of Complex Model
A model of a β-glucan·βGRP-N complex was constructed by fitting the coordinates of Glc-5(A), Glc-6(A), Glc-3(B), Glc-4(B), Glc-1(C), and Glc-2(C) of the laminarihexaoses to those of the corresponding residues in β1,3-glucan.
Preparation of Mutant Proteins
Single or double amino acid substitutions of Plodia βGRP-N were performed by a PCR-based site-directed mutagenesis technique. The mutant proteins were expressed and purified using the same methods as for wild-type βGRP-N. CD spectra of these mutant proteins showed that their overall structure was similar to wild-type (data not shown).
Isothermal Titration Calorimetry
Laminarin binding experiments of wild-type and mutant Plodia βGRPs were conducted on an Auto-ITC instrument (MicroCal, Inc.) at 25 °C. Laminarin from Laminaria digitata was purchased from Sigma. In a typical isothermal titration calorimetry (ITC) experiment, the cell was filled with 200 μl of 50 μm protein solution dissolved in 10 mm HEPES, 150 mm NaCl (pH 7.4). The protein sample was titrated with successive injections (2 μl) of laminarin solution (0.67 mm as a triplex) in the same buffer. Reference experiments were performed by injecting laminarin solution into buffer. The experimental data were fitted using the program Origin (version 7.0, OriginLab).
RESULTS
Overall Structure of βGRP-N
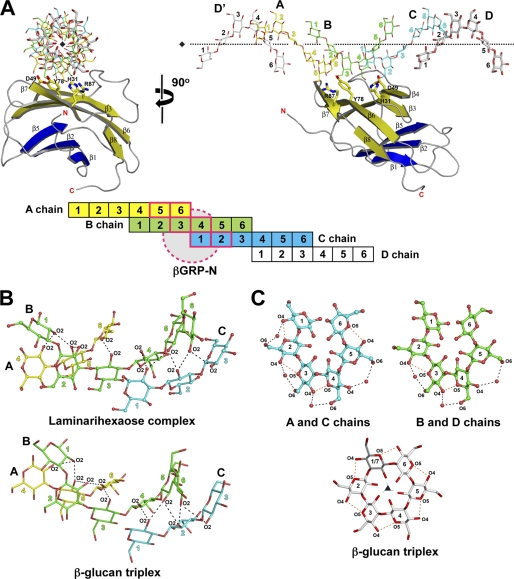
After extensive screening, we were able to crystallize the N-terminal β-glucan-binding domains (βGRP-N) of the moths P. interpunctella and B. mori in the presence of laminarihexaose and then solved the crystal structures at 2.20 and 2.05 Å resolution, respectively (Fig. 1, supplemental Fig. S1, and Table 1). We also obtained crystals of the ligand-free βGRP-N of Plodia and solved its structure at 1.58 Å resolution (Table 1). The Plodia and Bombyx βGRP-N structures each have an immunoglobulin-like β-sandwich fold composed of two antiparallel β-sheets containing three and five β-strands (concave β-sheet, β1–2 and β5; convex β-sheet, β3–4 and β6–8). There is little structural difference between liganded and unliganded forms of Plodia βGRP-Ns (root mean square deviation = 0.49 Å for superimposed 98 Cα atoms). In addition, the overall structure of Plodia βGRP-N is almost identical to the liganded Bombyx βGRP-N (root mean square deviation = 0.35 Å for superimposed 99 Cα atoms) and unliganded crystal (Drosophila) or NMR (Bombyx) structures (root mean square deviation = 0.60 and 1.25 Å for superimposed 96 and 85 Cα atoms, respectively) (16, 17). Taken together, these results indicate that βGRP-Ns assume a common structural fold irrespective of the binding of ligand.
FIGURE 1.
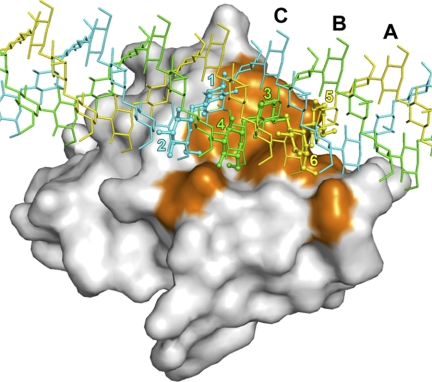
Overview of the crystal structure of Plodia βGRP-N. Ribbon models of Plodia βGRP-N are shown on the left and right. The two structures are related by a rotation of 90° around a vertical crystallographic 21 screw axis. Secondary structures are highlighted (β-strands belonging to the convex β-sheet, yellow; β-strands belonging to the concave β-sheet, blue), and the loops are colored in gray. Three laminarihexaoses bound to βGRP-N are shown as stick models with the 2Fo − Fc electron density map contoured at 1.3σ; chain A, yellow; chain B, green; chain C, cyan. Each laminarihexaose chain is numbered from the reducing end.
Structure of Laminarihexaoses
The crystal of laminarihexaose-bound Plodia βGRP-N belongs to space group P212121 with two proteins and two laminarihexaoses per asymmetric unit. Two laminarihexaose helices are related by noncrystallographic 4-fold screw symmetry around crystallographic 21 screw axis, thus forming a pseudoquadruplex structure (supplemental Fig. S2A). The crystal of laminarihexaose-bound Bombyx βGRP-N belongs to space group P41 with two proteins and two laminarihexaoses per asymmetric unit. Two laminarihexaose helices are related by crystallographic 4-fold screw symmetry around the 41 screw axes respectively, forming pseudoquadruplexes structures (supplemental Fig. S2B). In both Plodia and Bombyx complexes, three (chains A, B, and C) of four laminarihexaoses interact with one βGRP-N molecule (structures of the Plodia complex shown in Fig. 2A and of Bombyx in supplemental Fig. S3A). The structure of the laminarihexaoses can be compared with a model of right-handed triple-helical β-glucan derived from x-ray fiber diffraction (2). In the case of the laminarihexaoses, interstrand hydrogen bonds are observed between O2 atoms (Fig. 2B, top, and supplemental Fig. S3B, top). The O2 atoms of Glc-1(C), Glc-2(C), and Glc-3(C) make hydrogen bonds with the O2 atoms of Glc-4(B), Glc-5(B), and Glc-6(B), whereas the O2 atoms of Glc-1(B), Glc-2(B), and Glc-3(B) bond with those of Glc-4(A), Glc-5(A), and Glc-6(A), respectively. These hydrogen-bonding patterns are very similar to those in the β-glucan structure, which is also stabilized via interchain hydrogen bonds between O2 atoms (2) (Fig. 2B, bottom, and supplemental Fig. S3B, bottom). In addition to the interstrand hydrogen bond interactions, the helical structure of laminarihexaoses is formed by intrastrand hydrogen bonds between their O4(i) and O5(i+1) atoms, as is also observed in the β-glucan structure, and by water molecule-mediated hydrogen bonds between O4(i) and O6(i+1) (Fig. 2C and supplemental Fig. S3C). The inter- and intrastrand hydrogen bonds of the laminarihexaoses contribute to stabilize their highly ordered helical structure, an arrangement almost certainly induced in association with βGRP-N to mimic the triplex structure of the β-glucan. In fact, the similar bonding patterns in the quadruplex and triplex structures unexpectedly derive from an almost identical spatial arrangement of six protein-binding residues in the two ligands (see below).
FIGURE 2.
Comparison of pseudoquadruplex laminarihexaoses and β-glucan triplex. A, laminarihexaoses (stick models) on Plodia βGRP-N are shown. Neighboring laminarihexaoses (chain D and D′) are indicated as gray stick models. The horizontal pseudo 41 screw axis is shown with dotted lines (see also supplemental Fig. S2A). The schematic drawing of the laminarihexaose-βGRP interaction is shown below the structures. The βGRP-N protein is indicated with dashed circles, and its interacting glucose residues from three laminarihexaoses (A chain, 5 and 6; B chain, 3 and 4; and C chain, 1 and 2) are shown with boldface pink lines. B, interchain hydrogen bonds between O2 atoms of laminarihexaoses (top) and β-glucan triplex (2) (bottom) are shown as ball-and-stick and stick models, respectively. C, intrachain hydrogen bonds between O4(i) and O5(i+1) and water-mediated hydrogen bonds between O4(i) and O6(i+1) are shown as orange and black dashed lines, respectively. The crystallographic vertical 31 screw axis of the β-glucan structure (2) is shown as a black triangle.
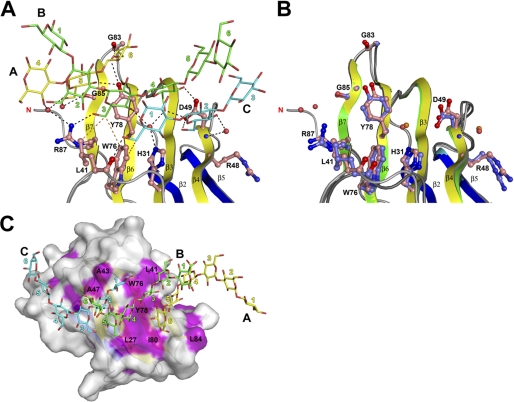
Laminarihexaose-βGRP-N Interaction
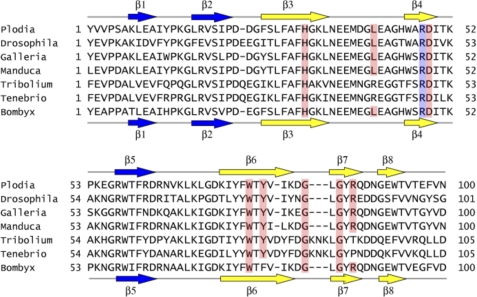
Ligand-binding of βGRP-N is attained through a convex β-sheet and a characteristic long loop between β3 and β4, and a short loop between β6 and β7 (Fig. 1 and supplemental Fig. S1). Binding interactions in the proteins from both insect species are almost identical, suggesting a common binding mode for this entire protein family. Six Glc residues from three laminarihexaose chains, Glc-5(A), Glc-6(A), Glc-3(B), Glc-4(B), Glc-1(C), and Glc-2(C), are involved in binding to βGRP-N (Fig. 2A), and eight amino acid residues including His31, Leu41, Asp49, Trp76, Tyr78 (Phe78 in Bombyx), Gly83, Gly85, and Arg87 show extensive polar and nonpolar interactions (Fig. 3A and supplemental Fig. S4A). The structure around the ligand-binding site is almost identical in liganded and unliganded forms (Fig. 3B), indicating that the site is rigid, and the surface is preformed. The side chain of Asp49 interacts simultaneously with laminarihexaoses B and C. The two carboxyl oxygens of the Asp49 side chain bind to the Glc-4(B) O4, O6, and Glc-2(C) O6 atoms via hydrogen bonds. There is a hydrophobic patch, including Trp76 and Tyr78 (Phe78 in Bombyx) alongside the ligand-binding site (Fig. 3C and supplemental Fig. S4B), and it possibly forms part of the binding site or plays a role in the initial encounter with a longer, structured β1,3-glucan. Most of the residues involved in binding to the laminarihexaoses are conserved among invertebrate βGRP-Ns (Fig. 4).
FIGURE 3.
Interaction of βGRP-N with three laminarihexaoses. A, close up view of the laminarihexaose-binding site of Plodia βGRP-N. Ligand-binding residues are shown as ball-and-stick models. Dashed lines indicate potential hydrogen bonds (black) and hydrophobic interactions (orange). Potential hydrophobic interactions between Tyr78 and ligands are omitted for clarity. Water molecules are shown as red spheres. B, comparison of ligand-free and ligand-bound βGRP-N structures. The residues and water molecules in the unliganded form are shown as ball-and-stick and orange spheres, respectively. C, surface model of βGRP-N. Hydrophobic residues are colored in magenta.
FIGURE 4.
Sequence alignment of insect βGRP-Ns. Residues within hydrogen-binding distances and involved in hydrophobic interaction with lamitrihexaoses on Plodia and Bombyx βGRP-Ns are highlighted in red. The corresponding residues of the insect orthologues are also highlighted. Arg48 that may be involved in binding of triplex β-glucan on βGRP-Ns are highlighted in blue. Secondary structures of Plodia and Bombyx βGRP-Ns are shown above and below the amino acid sequences, respectively. The βGRP sequence accession nos. are as follows: P. interpunctella (AAM95970), Drosophila melanogaster (AAF50349), Galleria mellonella (CAK22401), Manduca sexta (AAF44011), Tribolium castaneum (EFA01356), Tenebrio molitor (BAC99308), and Bombyx mori (BAA92243).
Verification of Interaction in Solution
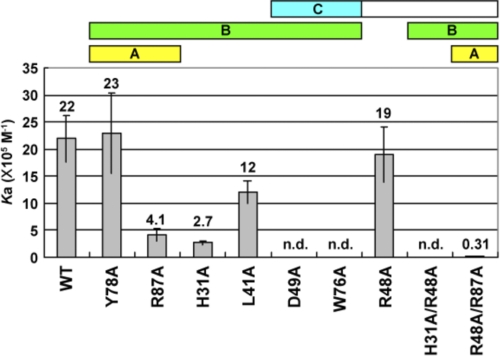
To verify that these intermolecular interactions occur in solution and with β-glucan, we performed a mutational analysis of Plodia βGRP-N. A series of single/double mutants was prepared (H31A, L41A, R48A, D49A, W76A, Y78A, R87A, H31A/R48A, and R48A/R87A), focusing on the residues located on the ligand-binding surface (Fig. 3). We examined the affinities of wild-type and mutant βGRP-Ns to laminarin, a soluble β1,3-glucan with a triple-helical structure (5), using isothermal titration calorimetry (supplemental Fig. S5 and summarized in Fig. 5). The concentration of laminarin solution is calculated as a triplex. The binding constant between wild-type Plodia βGRP-N and laminarin is 2.2 × 106 m−1, in agreement with the binding constant of Drosophila βGRP-N to laminarin (2.1 × 106 m−1) (16). The apparent stoichiometry (n = 1.5) observed in the ITC experiment may reflect the insufficient triple-helical structure of some part of laminarin due to its heterogeneity in length and linkage (26). Mutations D49A, W76A, and H31A/R48A abolished binding and H31A, L41A, R87A, and R48A/R87A decreased the affinity, indicating that His31, Leu41, Arg48, Asp49, Trp76, and Arg87 are important for the binding to triplex β-glucan in solution. The essential nature of Asp49 is seen in the extensive hydrogen bonds between two laminarihexaoses and the Asp49 side chain (Fig. 3A). The importance of Trp76 has been reported previously in Drosophila βGRP-N (Trp77 in Drosophila) (16). Trp76 directly interacts with two laminarihexaoses through hydrophobic interactions and builds the ligand-binding surface through extensive hydrophobic contacts with ligand-binding residues, including His31, Leu41, Tyr78, and Arg87 (Fig. 3A). Double mutations of Arg48 and His31 or Arg87 decrease the affinity for laminarin. Although Arg48 does not directly interact with laminarihexaoses, the potential rotamers of the side chain without crystal packing constraints are within hydrogen-bonding distances to the laminarihexaose(s). Taken together, we suggest that the Arg48 side chain does in fact interact with β-glucan in solution. We constructed a model of βGRP-N·triplex β1,3-glucan complex based on our crystal structure (Fig. 6 and supplemental Fig. S6). In this model, the relative spatial arrangement of six binding Glc residues from the βGRP-N·laminarihexaoses complex is almost identical with that of the corresponding Glc residues of the β1,3-glucan, and there are no significant steric clashes between βGRP-N and β1,3-glucan (2). From these observations, we conclude that the type of interaction between βGRP-N and laminarihexaoses in the crystal is applicable to that between βGRP-N and triplex β1,3-glucan in solution.
FIGURE 5.
Binding constants of laminarin for wild-type and mutant Plodia βGRP-Ns. The binding constants were determined by using isothermal titration calorimetry and shown with S.E. The thermograms are shown in supplemental Fig. S5.
FIGURE 6.
Structural model for binding between βGRP-N and triplex β1,3-glucan. Surface model of Plodia βGRP-N is shown and residues involved in binding to laminarihexaoses are colored orange. In the βGRP-N·laminarihexaose complex, interacting Glc residues are shown as ball-and-stick models. Triple-helical β1,3-glucan is shown as thin stick models: chain A, yellow; chain B, green; chain C, cyan.
DISCUSSION
The biological effect of β-glucan in relation to its tertiary structure is one of the fundamental immunological issues (7). Although many efforts have been made to reveal the structure-function relationship (4, 12), there is still no consensus on the basic requirements of the biological activity of β-glucan. This is in part due to insufficient characterization of the tertiary structure of β-glucan in association with its counter receptor. Here, we reveal the structural basis of triplex β-glucan recognition by βGRP. Three strands of the triplex β-glucan can simultaneously interact with particular amino acid residues on the convex β-sheet. It clearly explains why βGRP binds specifically to the β-glucan triplex structure and not to shorter glucose oligomers, which cannot assume a triple-helical conformation in solution.
In a typical carbohydrate-protein interaction, the actual region of contact between the carbohydrate and the proteins involves only one to three monosaccharide residues (27). As a consequence, carbohydrate-binding proteins tend to be of relatively low affinity. To mediate biologically relevant interaction, many carbohydrate-binding proteins are oligomeric and achieve high affinity binding by making multiple interactions with multivalent ligands (27, 28). Another possible strategy to gain the affinity is to have an extended carbohydrate-binding site. βGRP seems to adopt the latter strategy. A unique interaction mode is attained using an extensive binding surface of the βGRP-N protein. The unexpectedly large contact area of βGRP-N reaps a benefit of high affinity binding to triple-helical β-glycan using one carbohydrate recognition domain. Large binding platform of βGRP-N is constituted by hydrophobic and charged residues mostly on the convex β-sheet. A solvent-exposed hydrophobic patch is formed by Leu27, Leu41, Trp76, Tyr78 (Phe78 in Bombyx), and Ile80, thus contributing to the binding to β-glucan. Glucose rings are obliquely oriented onto the side chains of Trp76 and Tyr78 and seem to exhibit weak hydrophobic interactions. There are no parallel stacking interactions between glucose rings and aromatic side chains as often observed in protein-carbohydrate interactions (27, 29). This is well explained from the structure of triple-helical β-glucan, in which hydrophobic surface of the glucose ring is not exposed to solvent and losing an ability to have a parallel stacking interaction with aromatic ring. Polar and charged residues (His31, Arg48, Asp49, and Arg87) are surrounding this hydrophobic patch and involved in the interaction with O4 and O6 atoms of glucose residues. The residues, Asp49 (β4) and Trp76 (β6) are positioned to interact with more than one β-glucan strand, thus contributing to stabilize the β-glucan helix. The essential feature of these residues are supported the ITC experiment, in which mutation of each residue effectively eliminate the binding to laminarin. Takahasi et al. (17) reported the solution structure of the GNBP3 N-terminal domain and proposed the β-glucan binding surface as a concave β-sheet composed of β1–2 and β5 strands. On the other hand, our crystallographic and mutagenesis studies show the binding surface to be convex β-sheet composed of β3–4 and β6–8. The discrepancy may be due to the different sources of laminarin used in the studies. Little structural rearrangement of the side chains were observed upon binding to laminarihexaoses. In common with lectins, βGRPs have preformed a carbohydrate-recognition site, which can accommodate the β-glucan ligand. This feature seems to minimize the energetic penalty paid upon binding to carbohydrate ligands (29).
The structural basis of triplex β-glucan recognition by a β-glucan binding protein can now be understood. The interaction mode elucidated in this study will provide the basis for the understanding of structure/activity relationship of other β-glucan-binding proteins such as Dectin-1 found in vertebrates (10). Trp221 and His223 residues of mouse Dectin-1 are the key residues for the binding to β-glucan and for Toll-like receptor 2-mediated cellular activation (30). Although the topology of Dectin-1 carbohydrate recognition domain is different from that of βGRP-N (31), these residues may interact with triple-helical β-glucan by the non-stacking mode that is observed in the βGRP-N·laminarihexaose complex.
Full-length βGRP consists of a well conserved N-terminal domain and a C-terminal β1,3-glucanase-like domain (13, 15). The N-terminal domain of βGRP plays a critical role for the detection of pathogen. In contrast, the C-terminal glucanase-like domain does not have either glucanase activity or affinity with the β1,3-glucan (13, 15). The functional role of the C-terminal domain is still not established; however, recent evidence suggests that this domain is required for the activation of Toll signaling pathway by recruiting a downstream modular serine protease (32). Upon infection of fungi into hosts, βGRP recognizes the β1,3-glucan of the cell wall via βGRP-N. In this βGRP·β1,3-glucan complex, C-terminal domain of βGRP seems located apart from the bound β1,3-glucan because the C terminus of βGRP-N is opposite to the β1,3-glucan-binding surface. It is tempting to speculate that the accumulation and clumping of βGRP on the fungal cell wall will expose its C-terminal domains to recruit and active the downstream signaling molecule. The crystal structure of SPN48, one of the serine protease inhibitors (serpins) from Tenebrio molitor, has been determined recently (33). SPN48 has a putative basic heparin-binding site and heparin inhibit a serine protease, Spätzle-processing enzyme, which produces a Toll receptor ligand, Spätzle by bridging SPN48 and the serine protease. Roh et al. (34) demonstrated that all the components essential for the recognition of β-glucan and pro-Spätzle were bound to a heparin-immobilized column. In the βGRP-N structure, basic residues (Lys7, Lys52, Lys54, Arg57, Arg61, and Arg63 in Plodia) are clustered opposite to the β1,3-glucan-binding surface (supplemental Fig. S7). It is expected that the βGRP·β-glucan complex may recruit the heparin or signaling/inhibiting components through the basic patch. Further experimental evidences are necessary to understand the signal transduction and inhibition mechanism.
Polysaccharides such as β-glucan recently have been shown to act as potent immunomodulating agents (35). Because the anti-tumor and anti-infective activities of β1,3-glucan (35, 36) appear to be conformation-dependent, an analysis of β-glucan-protein interaction together with our structural information would be useful in the pharmaceutical field. From a diagnostic viewpoint, the presence of β-glucan in blood and sterile body fluid is a good marker because mammalian systems cannot produce it. High level of β-glucan was reported in the patients with infectious diseases such as invasive aspergillosis (37). Our findings may help the development of a β-glucan detection assay for diagnosis and therapeutic monitoring of certain infectious diseases.
Supplementary Material
Acknowledgments
We thank Drs. Akihiro Miyanoshita and Taro Imamura (National Food Research Institute) for kindly providing P. interpunctella and Masaki Ishii for isolation of the P. interpunctella βGRP gene. We also thank Drs. Yukishige Ito, Yoichi Takeda (RIKEN/ERATO), Shinya Hanashima (RIKEN), and Yasutoshi Sakoda and Yasushi Sakaguchi (DKSH Japan) for ITC measurements and for helpful discussions. We thank the beamline staff of Photon Factory (PF) High Energy Accelerator Research Organization (KEK) (Japan), SPring-8 (Japan), and National Synchroton Radiation Research Center (NSRRC) (Taiwan) for providing data collection facilities and support.
The atomic coordinates and structure factors (codes 3AQZ, 3AQX, and 3AQY) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7.
- βGRP/GNBP3
- β1,3-glucan recognition protein/gram-negative bacteria-binding protein 3
- TRX
- thioredoxin
- βGRP-N
- N-terminal β1,3-glucan recognition domain of βGRP/GNBP3
- ITC
- isothermal titration calorimetry
- MBP
- maltose-binding protein.
REFERENCES
- 1. Atkins E. (1986) Int. J. Biol. Macromol. 8, 323–329 [Google Scholar]
- 2. Chuah C. T., Sarko A., Deslandes Y., Marchessault R. H. (1983) Macromolecules 16, 1375–1382 [Google Scholar]
- 3. Yoshioka Y., Uehara N., Saitô H. (1992) Chem. Pharm. Bull. 40, 1221–1226 [DOI] [PubMed] [Google Scholar]
- 4. Kulicke W. M., Lettau A. I., Thielking H. (1997) Carbohydr. Res. 297, 135–143 [DOI] [PubMed] [Google Scholar]
- 5. Young S. H., Dong W. J., Jacobs R. R. (2000) J. Biol. Chem. 275, 11874–11879 [DOI] [PubMed] [Google Scholar]
- 6. Okobira T., Miyoshi K., Uezu K., Sakurai K., Shinkai S. (2008) Biomacromolecules 9, 783–788 [DOI] [PubMed] [Google Scholar]
- 7. Sletmoen M., Stokke B. T. (2008) Biopolymers 89, 310–321 [DOI] [PubMed] [Google Scholar]
- 8. Goodridge H. S., Wolf A. J., Underhill D. M. (2009) Immunol. Rev. 230, 38–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferrandon D., Imler J. L., Hetru C., Hoffmann J. A. (2007) Nat. Rev. Immunol. 7, 862–874 [DOI] [PubMed] [Google Scholar]
- 10. Brown G. D. (2006) Nat. Rev. Immunol. 6, 33–43 [DOI] [PubMed] [Google Scholar]
- 11. Brown G. D., Gordon S. (2005) Cell Microbiol. 7, 471–479 [DOI] [PubMed] [Google Scholar]
- 12. Mueller A., Raptis J., Rice P. J., Kalbfleisch J. H., Stout R. D., Ensley H. E., Browder W., Williams D. L. (2000) Glycobiology 10, 339–346 [DOI] [PubMed] [Google Scholar]
- 13. Ochiai M., Ashida M. (2000) J. Biol. Chem. 275, 4995–5002 [DOI] [PubMed] [Google Scholar]
- 14. Gottar M., Gobert V., Matskevich A. A., Reichhart J. M., Wang C., Butt T. M., Belvin M., Hoffmann J. A., Ferrandon D. (2006) Cell 127, 1425–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma C., Kanost M. R. (2000) J. Biol. Chem. 275, 7505–7514 [DOI] [PubMed] [Google Scholar]
- 16. Mishima Y., Quintin J., Aimanianda V., Kellenberger C., Coste F., Clavaud C., Hetru C., Hoffmann J. A., Latgé J. P., Ferrandon D., Roussel A. (2009) J. Biol. Chem. 284, 28687–28697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takahasi K., Ochiai M., Horiuchi M., Kumeta H., Ogura K., Ashida M., Inagaki F. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 11679–11684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qing G., Ma L. C., Khorchid A., Swapna G. V., Mal T. K., Takayama M. M., Xia B., Phadtare S., Ke H., Acton T., Montelione G. T., Ikura M., Inouye M. (2004) Nat. Biotechnol. 22, 877–882 [DOI] [PubMed] [Google Scholar]
- 19. Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 20. Vagin A., Teplyakov A. (1997) J. Appl. Crystallogr. 30, 1022–1025 [Google Scholar]
- 21. McRee D. E. (1999) J. Struct. Biol. 125, 156–165 [DOI] [PubMed] [Google Scholar]
- 22. Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 23. Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 24. Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 25. Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 26. Read S. M., Currie G., Bacic A. (1996) Carbohydr. Res. 281, 187–201 [DOI] [PubMed] [Google Scholar]
- 27. Weis W. I., Drickamer K. (1996) Annu. Rev. Biochem. 65, 441–473 [DOI] [PubMed] [Google Scholar]
- 28. Rini J. M. (1995) Annu. Rev. Biophys. Biomol. Struct. 24, 551–577 [DOI] [PubMed] [Google Scholar]
- 29. Boraston A. B., Bolam D. N., Gilbert H. J., Davies G. J. (2004) Biochem. J. 382, 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adachi Y., Ishii T., Ikeda Y., Hoshino A., Tamura H., Aketagawa J., Tanaka S., Ohno N. (2004) Infect. Immun. 72, 4159–4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown J., O'Callaghan C. A., Marshall A. S., Gilbert R. J., Siebold C., Gordon S., Brown G. D., Jones E. Y. (2007) Protein Sci. 16, 1042–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee H., Kwon H. M., Park J. W., Kurokawa K., Lee B. L. (2009) BMB Rep. 42, 506–510 [DOI] [PubMed] [Google Scholar]
- 33. Park S. H., Jiang R., Piao S., Zhang B., Kim E. H., Kwon H. M., Jin X. L., Lee B. L., Ha N. C. (2011) J. Biol. Chem. 286, 1567–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roh K. B., Kim C. H., Lee H., Kwon H. M., Park J. W., Ryu J. H., Kurokawa K., Ha N. C., Lee W. J., Lemaitre B., Söderhäll K., Lee B. L. (2009) J. Biol. Chem. 284, 19474–19481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tzianabos A. O. (2000) Clin. Microbiol. Rev. 13, 523–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Di Luzio N. R., Williams D. L., McNamee R. B., Edwards B. F., Kitahama A. (1979) Int. J. Cancer 24, 773–779 [DOI] [PubMed] [Google Scholar]
- 37. Pazos C., Pontón J., Del Palacio A. (2005) J. Clin. Microbiol. 43, 299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.