Abstract
Natriuretic peptides and ATP activate and Gö6976 inhibits guanylyl cyclase (GC)-A and GC-B. Here, the mechanism of inhibition was determined. Gö6976 progressively increased the Michaelis-Menten constant and decreased the Hill coefficient without reducing the maximal velocity of GC-A and GC-B. In the presence of 1 mm ATP, the Ki was 1 μm for both enzymes. Inhibition of GC-B was minimal in the absence of ATP, and 1 mm ATP increased the inhibition 4-fold. In a reciprocal manner, 10 μm Gö6976 increased the potency of ATP for GC-B 4-fold. In contrast to a recent study (Duda, T., Yadav, P., and Sharma, R. K. (2010) FEBS J. 277, 2550–2553), neither staurosporine nor Gö6976 activated GC-A or GC-B. This is the first study to show that Gö6976 reduces GTP binding and the first demonstration of a competitive inhibitor of a receptor guanylyl cyclase. We conclude that Gö6976 reduces GTP binding to the catalytic site of GC-A and GC-B and that ATP increases the magnitude of the inhibition.
Keywords: ATP, Cyclic GMP (cGMP), Cyclic Nucleotides, Enzyme Inhibitors, Guanylate Cyclase (Guanylyl Cyclase), Natriuretic Peptides
Introduction
Receptor guanylyl cyclases (GCs)2 control a myriad of functions in organisms ranging from bacteria to humans by catalyzing the conversion of GTP to cGMP (1). Guanylyl cyclase-A (GC-A) is the archetypical particulate GC that is activated by atrial natriuretic peptide (ANP) and B-type natriuretic peptide. The intracellular domain of guanylyl cyclase-B (GC-B) is 78% identical to GC-A, but GC-B is activated by C-type natriuretic peptide (CNP) (2). Both GC-A and GC-B are composed of a single polypeptide chain that contains a large extracellular domain, a single membrane span, and intracellular kinase homology, dimerization, and C-terminal guanylyl cyclase domains. ANP binds GC-A at a stoichiometry of 1:2 and induces a rotation of the juxtamembrane region (3, 4). Structural modeling studies suggest that CNP binds GC-B similarly (5, 6). Natriuretic peptide binding is hypothesized to activate the receptors by relieving basal repression exerted by the kinase homology domains on the guanylyl cyclase domains (7, 8).
GC-A and GC-B are phosphorylated on multiple serines and threonines located slightly before and expanding into the N-terminal portion of the kinase homology domain (9). Phosphorylation is required for peptide activation, and prolonged natriuretic peptide exposure or acute exposure to phorbol esters or calcium-elevating agents causes receptor dephosphorylation and inactivation (10).
ATP increases the activity of both receptors in broken cell preparations by serving as a phosphate donor (11–13). ATP is also an allosteric activator that reduces the Michaelis-Menten constant without affecting maximal velocities (14). In a reciprocal manner, GTP decreases the EC50 for ATP. Thus, ATP increases GTP binding, and GTP increases ATP binding.
We recently reported that the staurosporine derivative, Gö6976, inhibits basal and natriuretic peptide-stimulated guanylyl cyclase activity of GC-A and GC-B but does not inhibit activity measured in the presence of detergent with Mn2+-GTP as substrate (15). Because Gö6976 inhibits ATP binding to protein kinases, we investigated whether Gö6976 inhibition requires changes in GC-B phosphorylation. However, Gö6976 inhibited a constitutively pseudo-phosphorylated form of GC-B similarly to the wild type receptor.
Here, we determined the mechanism of the inhibition. Gö6976 reduces the binding of GTP to the catalytic sites of GC-A and GC-B, and ATP increases the magnitude of the inhibition by increasing affinity of the catalytic site for Gö6976.
EXPERIMENTAL PROCEDURES
Reagents
125I-cGMP radioimmunoassay kits were from PerkinElmer Life Sciences. Gö6976, GF-109203X (Gö6850), and staurosporine were from EMD Chemicals (Gibbstown, NJ).
Cells and Culture
293T-GC-A and 293T-GC-B cells were maintained as described (16).
Guanylyl Cyclase Assays
Crude membranes were prepared in phosphatase inhibitor buffer from 293T-GC-A or 293T-GC-B cells as described (17). All assays were performed at 37 °C in a mixture containing 25 mm Hepes, pH 7.4, 50 mm NaCl, 0.1% BSA, 0.5 mm isobutylmethylxanthine, 1 mm EDTA, 0.5 mm microcystin, and 5 mm MgCl2. Free magnesium (that not bound to ATP, GTP, or EDTA) was maintained at 1 mm or less. The time each reaction was incubated at 37 °C is shown in the legends for Figs. 1–3, 5, and 6. Unless otherwise indicated, 1 mm ATP and 1 mm GTP were included in the mixture. Reactions were initiated by adding 20 μl of crude membranes containing 7–11 μg of protein suspended in phosphatase inhibitor buffer to 80 μl of prewarmed reaction mixture. Reactions were stopped with 0.4 ml of ice-cold 50 mm sodium acetate buffer containing 5 mm EDTA. Cyclic GMP concentrations were determined by radioimmunoassay as described (18). Concentration-response assays were with the following Mg2+ GTP concentrations: 3000, 1500, 750, 375, 187.5, 93.8, 46.9, 23.4, 11.7, and 5.8 μm. Because enzymatic activity was not completely linear with time, we qualify the kinetic parameters obtained under these conditions as “apparent.”
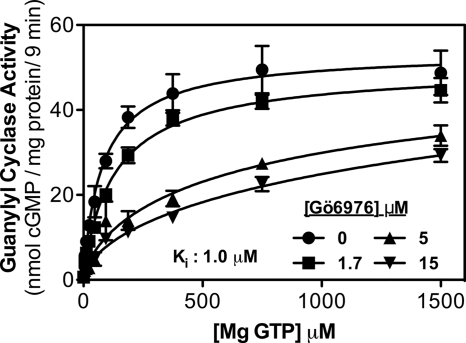
FIGURE 1.
Gö6976 increases the Km of GC-A. Guanylyl cyclase activity was determined for 9 min in 293T-GC-A cell membranes containing 1 μm ANP, 1 mm ATP, and the indicated concentrations of Gö6976 and GTP where n = 4 from two experiments. The Ki for Gö6976 was 1.0 μm.
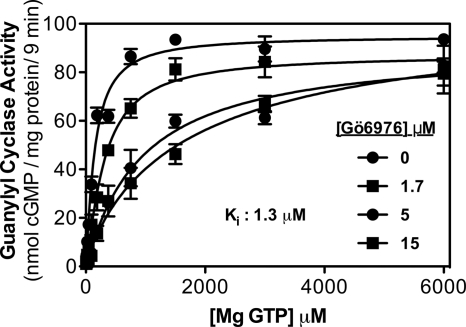
FIGURE 2.
Gö6976 increases the Km of GC-B. Guanylyl cyclase activity was determined for 9 min in 293T-GC-B cell membranes containing 1 μm CNP, 1 mm ATP, and the indicated concentrations of Gö6976 and GTP where n = 4 from two experiments. The Ki for Gö6976 was 1.3 μm.
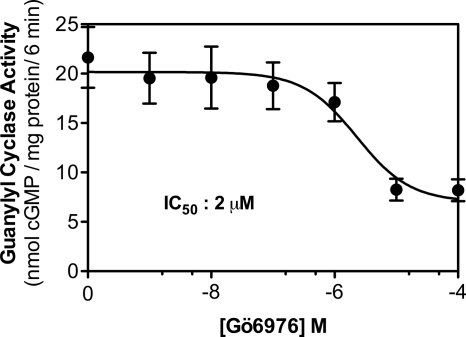
FIGURE 3.
Gö6976 is a potent inhibitor of GC-B. Guanylyl cyclase activity was determined in 293T-GC-B membranes incubated with 1 μm CNP, 1 mm ATP, and 1 mm GTP and the indicated concentrations of Gö697 for 6 min where n = 12 from three experiments.
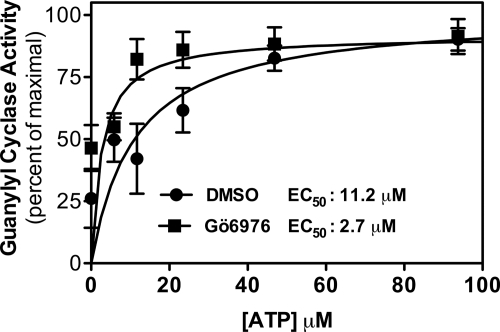
FIGURE 5.
Gö6976 increases the potency of ATP for GC-B. Guanylyl cyclase activity was determined for 9 min in the presence of 10 μm Gö6976 or dimethyl sulfoxide (DMSO) in 293T-GC-B membranes containing with 1 μm CNP, 1 mm GTP, and the indicated ATP concentrations and plotted as the percentage of the maximum response where n = 6.
FIGURE 6.
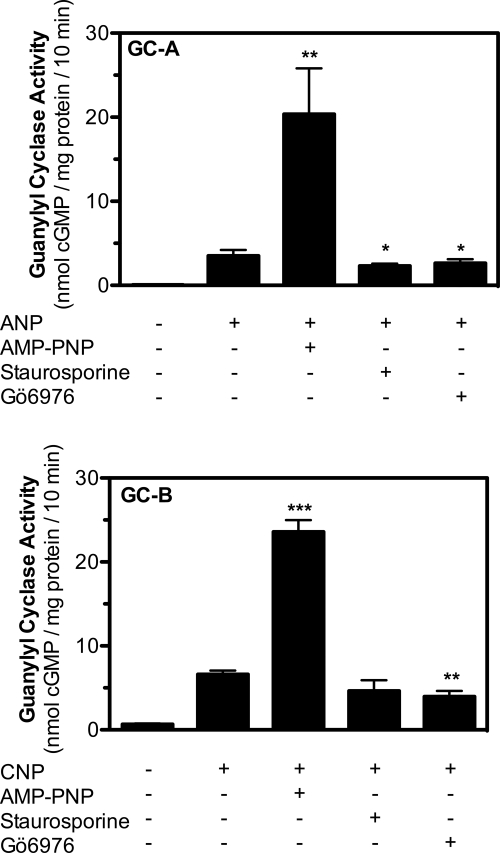
Staurosporine and related derivatives do not activate GC-A or GC-B. Guanylyl cyclase activity was measured in membranes from 293T-GC-A (top) or 293T-GC-B (bottom) cells for 10 min with 1 mm GTP in the absence or presence of 100 nm ANP or 100 nm CNP, 0.5 mm AMP-PNP or 10 μm staurosporine, or 10 μm Gö6976. *, **, and *** indicate significance at p values of <0.05, p < 0.01, and p < 0.005, respectively, where n = 8 from four experiments.
Statistical Analysis
Statistics and graphs were generated with the Prism 5 software. p values were obtained using the Student's paired t test where p ≤ 0.05 was considered significant. The vertical bars within the symbols in Figs. 1–5 and the vertical bars in Fig. 6 represent the S.E. IC50 values were calculated using following equation, where LogIC50 equals the middle value of Y based on the nonlinear regression curve fitting: Y = Bottom + (Top − Bottom)/(1 + 10(X − LogIC50)). EC50 values were calculated based on the nonlinear curve fitting equation Y = Top × X/(EC50 + X). Substrate-velocity curves were analyzed using a Michaelis-Menten model on samples lacking Gö6976 and using an allosteric sigmoidal model in samples containing Gö6976 to generate Hill coefficients.
FIGURE 4.
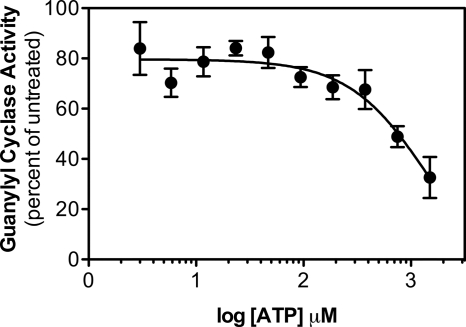
ATP increases the magnitude of Gö6976-dependent inhibition of GC-B. Guanylyl cyclase activity determined in 293T-GC-B membranes containing 1 μm CNP, 1 mm GTP, 10 μm Gö6976, and the indicated ATP concentrations was divided by activities obtained under identical conditions except that the samples lacked Gö6976, and the quotient was multiplied by 100. The resulting data were plotted as a function of the log of the ATP concentration where n = 6.
RESULTS
Gö6976 Is a Competitive Inhibitor or GC-A and GC-B
Substrate-velocity curves were generated using 293T-GC-A or 293T-GC-B membranes as the enzyme source in a reaction mixture containing in 1 μm natriuretic peptide, 1 mm ATP, and increasing concentrations of Gö6976 and Mg2+ GTP to determine whether the inhibition was competitive, uncompetitive, or mixed. Increased Km and unchanged Vmax values indicate competitive inhibition. Reduced Km and Vmax values characterize uncompetitive inhibition, and reduced Vmax and increased Km values are reflective of mixed inhibition. Assays conducted with multiple concentrations of GTP and Gö6976 allowed the determination of the Ki of inhibition.
Gö6976 markedly increased the Km of GC-A but did not significantly reduce the Vmax (Fig. 1). Similarly, Gö6976 increased the Km of GC-B without reducing the Vmax (Fig. 2). The Ki was 1.0 and 1.3 μm for GC-A and GC-B, respectively. Consistent with these values, when GC-B activity was determined in the presence of 1 mm ATP and 1 mm GTP and multiple concentrations of Gö6976, the IC50 was 2 μm (Fig. 3).
Unexpectedly, Gö6976 decreased the Hill coefficient for both enzymes. For GC-A, the Hill coefficient was 0.93, 0.91, 0.69, and 0.60 in the presence of 0, 1.7, 5, and 15 mm concentrations of Gö6976, respectively. For GC-B, the Hill coefficient was 1.19, 1.25, 1.02, and 0.87 in the presence of 0, 1.7, 5, and 15 mm concentrations of Gö6976, respectively. These data suggest that at the higher concentrations, Gö6976 may be inhibiting the allosteric activation of GC-A and GC-B.
ATP Increases the Magnitude of the Gö6976-dependent Inhibition
Because Gö6976 competitively inhibits ATP binding to protein kinases (19), we hypothesized that Gö6976 would compete for ATP binding to GC-B and that ATP would reduce the inhibitory effect of Gö6976. However, the opposite result was observed; ATP increased the magnitude of the inhibition in a concentration-dependent manner (Fig. 4). In the absence of ATP, 10 μm Gö6976 only reduced CNP-dependent GC-B activity 16%, but in the presence of 1.5 mm ATP, activity was reduced 68%, a more than 4-fold difference. The half-maximal concentration of ATP required to elicit the maximal inhibitory response was between 0.5 and 1 mm, which is below the cellular concentration of ATP.
Gö6976 Increases the Potency of ATP for GC-B
To determine whether Gö6976 reciprocally increased ATP binding to GC-B, guanylyl cyclase activity was determined in the presence or absence of Gö6976 as a function of increasing ATP concentrations (Fig. 5). The EC50 for ATP activation of GC-B in the absence of Gö6976 was 11.2 μm, whereas the EC50 in the presence of Gö6976 was 2.7 μm. Thus, 10 μm Gö6976 reduced the EC50 for ATP more than 4-fold. Hence, like GTP, Gö6976 increases the potency of ATP for GC-B.
Staurosporine Derivatives Do Not Activate GC-A or GC-B
Two recent studies suggest that staurosporine effectively substitutes for ATP in the activation of GC-A (20, 21). Therefore, we tested the ability of Gö6976 and the related compound, staurosporine, to activate GC-A and GC-B (Fig. 6). ATP markedly increased the activity of both enzymes when assayed in the presence of their respective natriuretic peptide, but neither of the indolocarbazoles increased activity. In fact, both staurosporine and Gö6976 inhibited GC-A, and Gö6976 inhibited GC-B. The diminished inhibitory effect of Gö6976 was expected because ATP was not included in these assays. Thus, staurosporine and Gö6976 are inhibitors, not activators, of GC-A and GC-B.
DISCUSSION
As a result of these studies, the mechanism of Gö6976-mediated inhibition of GC-A and GC-B was determined. Substrate-velocity experiments indicated that Gö6976 is a competitive inhibitor of GTP binding to the catalytic site of both receptors. To our knowledge, this is the first study to show that a staurosporine derivative reduces GTP binding and the first study to describe a competitive inhibitor of a transmembrane guanylyl cyclase.
Surprisingly, although Gö6976 blocks ATP binding to protein kinases, it did not block ATP-dependent activation of GC-A and GC-B. Instead, ATP potentiated the inhibitory effect by increasing the affinity of the catalytic domain for Gö6976, which ultimately increased the magnitude of the inhibition. Gö6976 also reduced the Hill coefficient of the receptors and increased the potency of ATP activation of GC-B, which is consistent with Gö6976 interacting with a second noncatalytic binding site. The ability of ATP to increase the potency of Gö6976 is also consistent with reduced IC50 values in whole cell experiments where intracellular ATP concentrations are higher and GTP concentrations are lower than those used in our assays (15). The reciprocal regulation between the ATP and GTP sites in GC-A and GC-B is consistent with previous data showing that ATP decreases the Km for GTP and that GTP decreases the EC50 for ATP (14).
We do not know why our results are the opposite of those recently published by Duda et al. (20, 21). However, one major difference is that GTP concentrations were 1 mm in our assay but 0.1 mm in their assay. Other differences are that we studied rat receptors stably expressed in human 293T cells, whereas they studied rat GC-A transiently expressed in monkey COS cells and that they used theophylline as a phosphodiesterase inhibitor, whereas we used isobutylmethylxanthine.
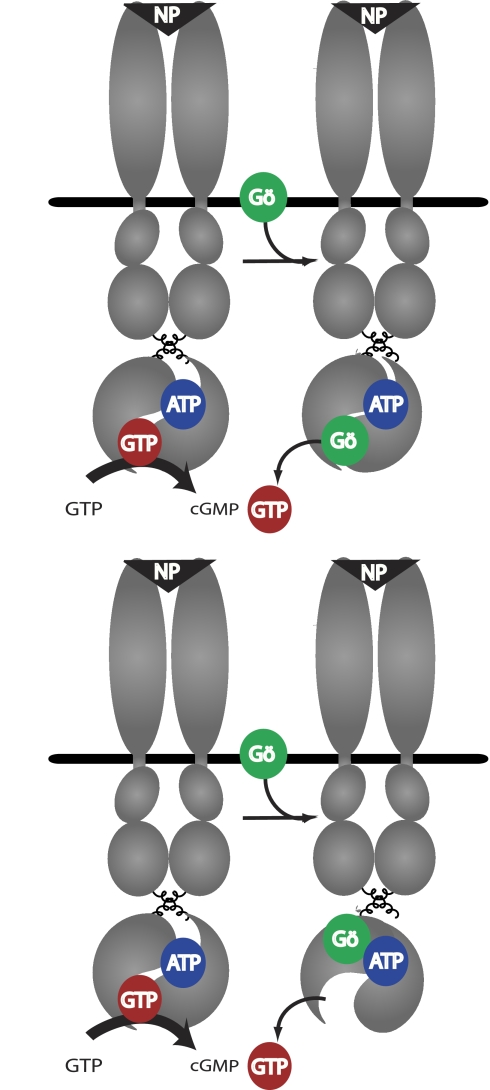
Based on the data reported here, we propose two possible inhibition models. In the direct competition model (Fig. 7, top), Gö6976 directly competes with GTP for binding to the catalytic site. If we make the assumption that the Km (100–200 μm) and Ki (1–2 μm) values for GTP and Gö6976, respectively, are reflective of the binding constants, then Gö6976 binds to the catalytic site about 100-fold tighter than GTP. Because the inhibitory effect of Gö6976 is increased in the presence of ATP, we conclude that ATP increases the affinity of the catalytic site for Gö6976 more than it increases the affinity for GTP. Because of the increased affinity for Gö6976 when compared with GTP, the major effect of ATP binding is to accentuate the inhibitory effect of Gö6976 on the catalytic site. However, at higher concentrations, Gö6976 may bind a second site, which reduces the allosteric activation of the receptors as suggested by the decreased Hill coefficient.
FIGURE 7.
Two Models of Gö6976-dependent inhibition of GC-A and GC-B. Upper panel, direct competition. In this model, Gö6976 (Gö) directly competes with GTP for binding to the catalytic site. Lower panel, allosteric competition. In this model, Gö6976 binds to a separate site that allosterically reduces the affinity of the catalytic site for GTP. NP, natriuretic peptide.
In the allosteric competition model (Fig. 7, bottom), Gö6976 binds a separate, noncatalytic, site that allosterically reduces the affinity of the catalytic site for GTP. High concentrations of Gö6976 in this model may ultimately lead to a conformational change that not only inhibits GTP binding but completely abolishes it, which would be congruent with the negative Hill slope observed at high Gö6976 concentrations. Future experiments will investigate the exact location of the Gö6976 binding site in these important signaling enzymes.
Acknowledgments
We are grateful to the anonymous reviewers for insightful suggestions.
This work was supported by Grant-in Aid 21,922 from the University of Minnesota Graduate School (to L. R. P.) and National Institutes of Health Training Grant T32AR050938 (to J. W. R.) from the NIAMS.
- GC
- guanylyl cyclase
- ANP
- atrial natriuretic peptide
- CNP
- C-type natriuretic peptide
- AMP-PNP
- adenosine 5′-(β,γ-imino)triphosphate.
REFERENCES
- 1. Potter L. R. (2011) Pharmacol. Ther. 130, 71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schulz S., Singh S., Bellet R. A., Singh G., Tubb D. J., Chin H., Garbers D. L. (1989) Cell 58, 1155–1162 [DOI] [PubMed] [Google Scholar]
- 3. Ogawa H., Qiu Y., Ogata C. M., Misono K. S. (2004) J. Biol. Chem. 279, 28625–28631 [DOI] [PubMed] [Google Scholar]
- 4. Rondeau J. J., McNicoll N., Gagnon J., Bouchard N., Ong H., De Léan A. (1995) Biochemistry 34, 2130–2136 [DOI] [PubMed] [Google Scholar]
- 5. He X. L., Dukkipati A., Garcia K. C. (2006) J. Mol. Biol. 361, 698–714 [DOI] [PubMed] [Google Scholar]
- 6. Yoder A. R., Kruse A. C., Earhart C. A., Ohlendorf D. H., Potter L. R. (2008) Peptides 29, 1575–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chinkers M., Garbers D. L. (1989) Science 245, 1392–1394 [DOI] [PubMed] [Google Scholar]
- 8. Koller K. J., de Sauvage F. J., Lowe D. G., Goeddel D. V. (1992) Mol. Cell. Biol. 12, 2581–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoder A. R., Stone M. D., Griffin T. J., Potter L. R. (2010) Biochemistry 49, 10137–10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dickey D. M., Barbieri K. A., McGuirk C. M., Potter L. R. (2010) Mol. Pharmacol. 78, 431–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abbey-Hosch S. E., Smirnov D., Potter L. R. (2005) Biochem. Pharmacol. 70, 686–694 [DOI] [PubMed] [Google Scholar]
- 12. Foster D. C., Garbers D. L. (1998) J. Biol. Chem. 273, 16311–16318 [DOI] [PubMed] [Google Scholar]
- 13. Joubert S., Labrecque J., De Léan A. (2001) Biochemistry 40, 11096–11105 [DOI] [PubMed] [Google Scholar]
- 14. Antos L. K., Potter L. R. (2007) Am. J. Physiol. Endocrinol. Metab. 293, E1756–1763 [DOI] [PubMed] [Google Scholar]
- 15. Robinson J. W., Lou X., Potter L. R. (March 3, 2011) Br. J. Pharmacol. 10.1111/j.1476-5381.2011.01291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan D., Bryan P. M., Antos L. K., Potthast R. J., Potter L. R. (2005) Mol. Pharmacol. 67, 174–183 [DOI] [PubMed] [Google Scholar]
- 17. Flora D. R., Potter L. R. (2010) Endocrinology 151, 2769–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abbey S. E., Potter L. R. (2002) J. Biol. Chem. 277, 42423–42430 [DOI] [PubMed] [Google Scholar]
- 19. Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marmé D., Schächtele C. (1993) J. Biol. Chem. 268, 9194–9197 [PubMed] [Google Scholar]
- 20. Duda T., Yadav P., Sharma R. K. (2010) FEBS J. 277, 2550–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duda T., Yadav P., Sharma R. K. (2011) Biochemistry 50, 1213–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]