Background: ASC mediates apoptosis and necrosis of tumor cells and necrosis of microbe-infected macrophages.
Results: ASC mediates necrosis only when cells express caspase-1; however, inhibition of caspase-1 proteolytic activity did not suppress the necrosis.
Conclusion: Caspase-1 but not its proteolytic activity is essential for ASC-mediated necrosis.
Significance: This study explains why ASC induces apoptosis or necrosis depending on the cell type.
Keywords: Apoptosis, Caspase, Macrophages, Necrosis (Necrotic Death), Nod-like receptors (NLR), Tumor Cell Biology
Abstract
The adaptor protein, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), connects pathogen/danger sensors such as NLRP3 and NLRC4 with caspases and is involved in inflammation and cell death. We have found that ASC activation induced caspase-8-dependent apoptosis or CA-074Me (cathepsin B inhibitor)-inhibitable necrosis depending on the cell type. Unlike necroptosis, another necrotic cell death, ASC-mediated necrosis, was neither RIP3-dependent nor necrostatin-1-inhibitable. Although acetyl–YVAD–chloromethylketone (Ac-YVAD-CMK) (caspase-1 inhibitor) did not inhibit ASC-mediated necrosis, comprehensive gene expression analyses indicated that caspase-1 expression coincided with the necrosis type. Furthermore, caspase-1 knockdown converted necrosis-type cells to apoptosis-type cells, whereas exogenous expression of either wild-type or catalytically inactive caspase-1 did the opposite. Knockdown of caspase-1, but not Ac-YVAD-CMK, suppressed the monocyte necrosis induced by Staphylococcus and Pseudomonas infection. Thus, the catalytic activity of caspase-1 is dispensable for necrosis induction. Intriguingly, a short period of caspase-1 knockdown inhibited IL-1β production but not necrosis, although longer knockdown suppressed both responses. Possible explanations of this phenomenon are discussed.
Introduction
ASC2 is an adaptor protein that connects several pattern recognition receptors (e.g. NLRP3 and NLRC4) with caspase-1 (1, 2). These pattern recognition receptors directly or indirectly recognize microbial molecular patterns (e.g. microbial RNA and flagellin) and/or danger signals from injured cells (e.g. ATP and uric acid), and then, together with ASC, they serve as platforms for the proteolytic maturation of caspase-1, which in turn catalyzes the proteolytic maturation of proinflammatory cytokines such as IL-1β and IL-18. Accordingly, ASC-deficient macrophages are severely defective in secreting these cytokines in response to microbial infection (3–5). Gain-of-function NLRP3 mutations cause auto-inflammatory syndromes, now collectively called cryopyrin-associated periodic syndromes (6). Importantly, IL-1 receptor antagonist has been shown to be effective in treating these syndromes. Thus, much attention has been paid to the role of ASC in caspase-1-mediated IL-1β maturation.
ASC also plays an important role in the monocyte and macrophage cell death induced by microbial infection. For instance, infection by an intracellular bacterium such as Salmonella typhimurium, Pseudomonas aeruginosa, or Listeria monocytogenes induces caspase-1-mediated cell death called “pyroptosis” (7–9). It has been described that pyroptosis has characteristics of both necrosis (e.g. cell swelling and plasma membrane rupture) and apoptosis (nuclear shrinkage) (10). As expected from the fact that ASC plays an important role in caspase-1 activation, recent studies have demonstrated that ASC also plays an important role in pyroptosis (11, 12). More recently, it was demonstrated that the expression of cryopyrin-associated periodic syndrome-associated NLRP3 mutants or the activation of endogenous NLRP3 by Shigella flexneri infection induces necrotic cell death in the THP-1 human monocytic cell line and/or mouse macrophages (13, 14). This necrotic cell death is also mediated by ASC; however, it is inhibited by a cathepsin B inhibitor, CA-074Me, but not by a caspase-1 inhibitor. Thus, this type of necrotic cell death has been considered to be distinct from pyroptosis, and it was named “pyronecrosis” (14).
Another line of studies demonstrated that ASC has the potential to induce apoptosis. ASC was originally identified as a protein that forms large aggregates in apoptotic human leukemia cells treated with chemotherapeutic agents (15) and as the product of a gene that is silenced in human cancer tissues by DNA methylation (16). In addition, it was demonstrated that ASC expression is induced by the p53 tumor suppressor and is involved in etoposide-induced apoptosis (17). Thus, ASC-mediated apoptosis seems to be important for tumor suppression and for cancer cell chemosensitivity. Furthermore, we recently demonstrated that transplanted human tumors in nude mice were completely eradicated by ASC activation in the tumor cells (18). Thus, ASC is a promising molecular target for cancer therapy. We previously showed that oligomerization of ASC using an NLRC4 mimicry system induced caspase-8-mediated apoptosis in five human cancer cell lines, including the NUGC-4 gastric cancer cell line (19). More recently, we found that ASC activation using the NLRC4 mimicry system or a cryopyrin-associated periodic syndrome-associated mutant of NLRP3 induced necrotic cell death in the COLO205 human colon adenocarcinoma cell line (18).
In this study, we investigated the mode of cell death induced by NLRC4 mimicry in six other ASC-expressing tumor cell lines and found that they were separated into apoptosis and necrosis type. We next sought to identify the molecular determinant of the mode of ASC-mediated cell death. We found that ASC activation induced necrosis in cells expressing caspase-1, but it induced caspase-8-dependent apoptosis in cells lacking caspase-1. Intriguingly, caspase-1, but not its catalytic activity, was essential for the ASC-mediated necrosis. The same was true for the ASC-dependent necrosis of the NOMO-1 human monocytic cell line induced by bacterial infection.
EXPERIMENTAL PROCEDURES
Reagents
An anti-human ASC mAb was prepared as described previously (20). Anti-caspase-1 polyclonal antibody (Cell Signaling, Beverly, MA), anti-cleaved caspase-1 mAb (clone D57A2, Cell Signaling), anti-β-actin mAb (clone AC-15, Sigma), anti-GAPDH mAb (clone MAB374, Millipore, Billerica, MA), muramyl dipeptide (MDP) (Sigma), Z-IETD-FMK (R&D Systems, Minneapolis, MN), Ac-YVAD-CMK (Bachem, Torrance, CA), and Z-DEVD-FMK and CA-074Me (Merck Japan, Tokyo, Japan) were purchased.
Plasmids
The expression plasmids for FLAG-C12N2, caspase-1, the caspase-1-C285S mutant, and pro-IL-1β were described previously (19, 21–23). Lentiviral vectors expressing NLRP3 and NLRP3-Y570C were described previously (18). To generate lentiviral vectors expressing GFP, FLAG-C12N2, caspase-1, and the caspase-1-C285S mutant, these cDNAs were inserted into pLenti6/V5-DEST (Invitrogen). To generate a plasmid expressing the GFP-caspase-1 fusion protein (pEGFP-Casp1), the caspase-1 cDNA was cloned into a pEGFP-C1 vector (Clontech). To generate lentiviral vectors expressing control and caspsae-1-targeting shRNA, oligonucleotide DNAs, including the corresponding sequences (5′-CTAAGGTTAAGTCGCCCTCGCTCTAGCGAGGGCGACTTAACCTTAG-3′ and 5′-ACACGTCTTGCTCTCATTATCTCGAGATAATGAGAGCAAGACGTGT-3′, respectively) were cloned into the pLKO.1-TRC vector (Addgene, Cambridge, MA).
Human Cell Lines
The NUC12N2 and CLC12N2 cell lines were described previously (18, 19). The NUGC-4, COLO205, KLM-1, and MIAPaca2 cell lines were obtained from the Cell Resource Center for Biomedical Research, Tohoku University (Sendai, Miyagi, Japan). The C32TG, G-361, HMV-II, SK-MEL-28, and KU812 cell lines were obtained from the RIKEN BioResource Center (Ibaraki, Tsukuba, Japan). The NOMO-1 cell line was obtained from the Health Science Research Resources Bank (Osaka, Japan). The THP-1 cell line was obtained from the American Type Culture Collection (Manassas, VA). To generate NOMO1-shCont and NOMO1-shCasp1 stable cell lines, NOMO-1 cells were transduced with a pLKO.1 lentiviral vector expressing control shRNA or caspase-1-targeting shRNA. The transduced cells were selected under puromycin treatment. The NU-GFP-Casp1 cell line was generated as follows. NUC12N2 cells were transfected with pEGFP-Casp1 using the Neon transfection system (Invitrogen), and GFP-expressing cells were sorted by flow cytometry. To generate the NU-Casp1WT and NU-Casp1C285S cell lines, NUC12N2 cells were transduced with pLenti6/V5-DEST expressing the wild type or C285S mutant of caspase-1. To generate the SKC12N2 cell line, SK-MEL-28 cells were transduced with pLenti6/V5-DEST expressing FLAG-C12N2. The transduced cells were selected by blasticidin.
Apoptosis and Necrosis Assay
The mode of cell death (apoptosis or necrosis) was determined primarily by the morphology of the dying cells. The proportions of apoptotic and necrotic cells were determined by flow cytometry after staining with propidium iodide (PI) and Cy5-annexin V as described previously (18). PI(+)Cy5(−) cells and PI(−)Cy5(+) cells were considered necrotic and early apoptotic cells, respectively. PI(+)Cy5(+) cells were considered necrotic when increased PI staining preceded or occurred simultaneously with increased Cy5-annexin V staining, whereas increased Cy5-annexin V staining that preceded PI staining was considered to be an indication of the secondary necrosis of apoptotic cells. Otherwise, the proportions of apoptotic and necrotic cells were determined in situ after Hoechst 33342 and PI staining, as described previously (18). A cell with a pyknotic nucleus without PI staining was considered apoptotic, whereas a PI(+) cell without profound nuclear condensation was considered necrotic. In some experiments, the proportion of necrosis was assessed by the release of cytoplasmic lactate dehydrogenase into culture supernatants using the CytoTox 96 kit (Promega), according to the manufacturer's protocol. In some experiments, cell death was assessed by WST1 assay, as described previously (24).
Microarray Analysis
Microarray analyses were performed using the Agilent whole human genome microarray kit (G4100F, Agilent, Santa Clara, CA), according to the manufacturer's protocol. Total RNA was purified from CLC12N2 subclones (CLC12N2-Nec and CLC12N2-Apo) or from COLO205 and NUGC-4 cells using the RNeasy Plus kit (Qiagen, Hilden, Germany). Cy3- and Cy5-labeled cRNAs were prepared using the Agilent Quick Amp labeling kit, two-color (Agilent). Hybridization and analysis were performed as described previously (25).
RT-PCR and Real-time PCR
Total RNA was purified using TRIzol reagent (Invitrogen), and cDNA was synthesized using the PrimeScript RT reagent kit (Takara Bio, Shiga, Japan). The following primers were used for PCR: ACTB, 5′-TCCCTGGAGAAGAGCTACGA-3′ and 5′-AAAGCCATGCCAATCTCATC-3′; RIPK3, 5′-TTTGGCCTGTCCACATTTCAG-3′ and 5′-GGTTGGCAACTCAACTTCTCT-3′; NLRP3, 5′-TCTCATGGATTGGTGAACAGC-3′ and 5′-GGTCCCCCAGAGAATTGTCA-3′; ASC (PYCARD), 5′-CTGGAGCCATGGGGCGCGCG-3′ and 5′-CGGAGTGTTGCTGGGAAGGAG-3′; NLRC4, 5′-CAGCAAGTTGAATAAGCAAG-3′ and 5′-ATCCTGTCGATCAGTTCATG-3′. Real-time PCR was performed using the THUNDERBIRD SYBR quantitative PCR mix (Toyobo, Osaka, Japan). The following primer sequences obtained from PrimerBank were used for real-time PCR: CASP1, 5′-TCCAATAATGGACAAGTCAAGCC-3′ and 5′-GCTGTACCCCAGATTTTGTAGCA-3′; ACTB, 5′-CATGTACGTTGCTATCCAGGC-3′ and 5′-CTCCTTAATGTCACGCACGAT-3′.
Transfection of Genes and siRNAs
Viral transduction was performed as described previously (18). CLC12N2 or NOMO-1 cells were transfected with siRNA (20 nm) using the Neon transfection system. The ASC-targeting (HSS147064), NLRP3-targeting (HSS132811), and NLRC4-targeting (HSS126850) siRNAs were purchased from Invitrogen. The caspase-1-targeting siRNA (L-004401-00) was purchased from Dharmacon (Chicago, IL).
Measurement of IL-1β
The amount of human IL-1β in culture supernatants was determined using the OptEIA ELISA kit (BD Pharmingen), according to the manufacturer's protocol.
Bacterial Infection
Staphylococcus aureus (strain Smith, kindly provided by Dr. Nakanishi, Kanazawa University, Kanazawa, Ishikawa, Japan) and P. aeruginosa (JCM14847, RIKEN BioResource Center, Wako, Saitama, Japan) in the log phase were used for infection. NOMO-1 and THP-1 cells were infected with these bacteria in antibiotic-free medium in 96-well plates. The plates were briefly centrifuged to improve the interaction between cells and bacteria. One hour after the infection, gentamycin (50 μg/ml) was added to kill the extracellular bacteria.
RESULTS
ASC Activation Induces Apoptosis or Necrosis in Tumor Cells Derived from Various Tissues
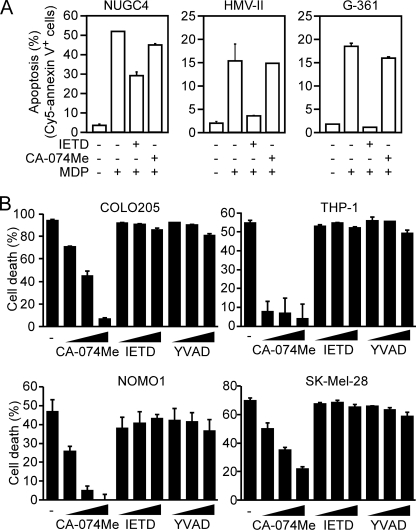
To investigate the functions of ASC, we previously established an experimental system in which a chimeric protein (C12N2) consisting of the caspase recruitment domain from NLRC4 and the nucleotide-binding oligomerization domain and leucine-rich repeats from NOD2 (also called NLRC2), a sensor for MDP, was expressed in cells (20). In this system, the simple addition of MDP to the culture medium induces ASC-dependent responses in the cells. Hereafter, this system is termed NLRC4 mimicry. Using this system, we previously showed that ASC activation induces apoptosis in the NUGC-4 human stomach cancer cell line but induces necrosis in the COLO205 human colon adenocarcinoma cell line (18). In the present study, we further investigated whether ASC activation induces apoptosis or necrosis in another 10 tumor cell lines, including leukemia cell lines, melanomas, and pancreatic cancers. Among them, six expressed substantial amounts of ASC (Fig. 1A). These 10 lines were transfected with C12N2 and treated with MDP. Among the six ASC-expressing lines, four (two monocytic leukemia cell lines, one melanoma, and one pancreatic cancer) exhibited necrosis, whereas two (melanomas) showed apoptosis (Fig. 1, B–E, and see supplemental Fig. S1). MDP treatment without C12N2 transfection induced neither necrosis nor apoptosis in these cell lines (data not shown). Consistent with our previous findings (18, 19), the NLRC4 mimicry-induced cell death of both apoptosis-type cells and necrosis-type cells was inhibited by ASC knockdown using siRNA (data not shown). The remaining cell lines, which expressed little or no ASC, were resistant to this procedure, confirming that the cell death induced by this procedure is ASC-dependent. These results indicate that ASC activation induces apoptosis or necrosis in ASC-expressing tumor cells of various tissue origins.
FIGURE 1.
ASC activation induces apoptosis or necrosis in various tumor cell lines. A, ASC and β-actin levels were examined by Western blotting. B–E, the indicated cell lines were transduced with a lentiviral vector expressing C12N2. The cells were then treated with or without MDP (100 ng/ml). B, cell morphology was examined under a microscope. Representative necrotic and apoptotic cells are shown by arrows and arrowheads, respectively. Scale bars, 20 μm. C, leukemia and melanoma cell lines were stained with PI and Cy5-annexin V, and the percentages of apoptotic (Apo) and necrotic (Nec) cells were determined by flow cytometry. The staining profiles are shown in supplemental Fig. S1. D, pancreatic cancer cell lines were stained with Hoechst 33342 and PI and examined under a fluorescence microscope. Necrotic cells (arrows) had morphologically normal nuclei that were stained with PI. Scale bar in the top left panel, 20 μm. E, apoptotic and necrotic cells were counted under a fluorescence microscope. At least 500 total cells were counted for each group. Error bars in C and E indicate S.D.
Effect of Protease Inhibitors on ASC-mediated Cell Death
We previously demonstrated that the ASC-mediated apoptosis of NUGC-4 cells was inhibited by a caspase-8 inhibitor, Z-IETD-FMK, whereas the ASC-mediated necrosis in COLO205 cells was inhibited by a cathepsin B inhibitor, CA-074Me (18). In addition, ASC has been implicated in pyroptosis that is inhibited by a caspase-1 inhibitor. Therefore, to further classify the type of cell death, we investigated the effect of these protease inhibitors. Consistent with our previous results, ASC-mediated cell death of all the apoptosis type was inhibited by Z-IETD-FMK but not by CA-074Me (Fig. 2A). In contrast, the ASC-mediated cell death of necrosis-type cells was inhibited by CA-074Me, whereas neither Ac-YVAD-CMK nor Z-IETD-FMK inhibited it (Fig. 2B). These characteristics of ASC-mediated necrosis are shared with pyronecrosis.
FIGURE 2.
Effect of protease inhibitors on ASC-mediated cell death. A, NUGC-4, HMV-II, and G-361 cells expressing C12N2 were left untreated or were treated with 40 μm of Z-IETD-FMK or CA-074Me for 1 h and were then cultured with or without MDP (100 ng/ml) for 12 h. The proportion of apoptotic cells was determined as described in the legend for Fig. 1. B, COLO205, THP-1, NOMO-1, and SK-MEL-28 cells expressing C12N2 were pretreated with the indicated inhibitors (20, 40 and 80 μm) for 1 h and then cultured with or without MDP (100 ng/ml) for 8 h. Cell viability was assessed by WST-1 assay. Cell death (percentage) = {(A450 of cell culture without MDP − A450 of cell culture with MDP)/A450 of cells cultured without MDP} × 100. Error bars in A and B indicate S.D.
The RIP1-RIP3 Axis Is Not Involved in the ASC-mediated Necrosis
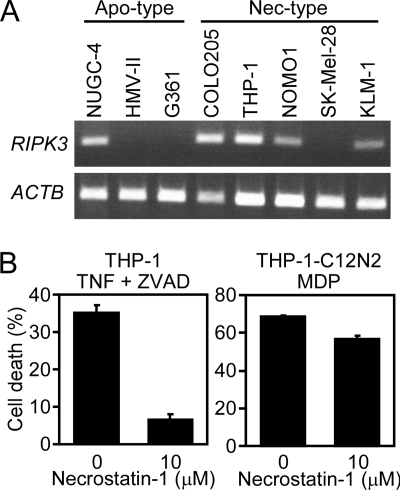
Death receptors that normally induce caspase-8-mediated apoptosis can elicit necrotic cell death (necroptosis), especially when caspases are inhibited. Necroptosis requires the expression of RIP1 and RIP3 (26–29) and is inhibited by a RIP1 inhibitor, necrostatin-1 (30). To investigate the involvement of RIP3 in ASC-mediated necrosis, we examined the RIP3 expression in tumor cell lines. RIP3 expression coincides with neither necrosis-type cells nor apoptosis-type cells (Fig. 3A). In addition, necrostatin-1 did not inhibit the NLRC4 mimicry-induced necrosis in THP-1 cells, whereas it inhibited the necroptosis induced by TNF-α plus Z-VAD-FMK (pan-caspase inhibitor) in the same cell line (Fig. 3B). These results indicate that ASC-mediated necrosis is distinct from necroptosis.
FIGURE 3.
The RIP1-RIP3 axis is not involved in ASC-mediated necrosis. A, the RIP3 (RIPK3) and β-actin (ACTB) mRNA levels in the indicated tumor cell lines were analyzed by RT-PCR. B, THP-1 cells (left) or C12N2-expressing THP-1 cells (right) were pretreated with necrostatin-1 (10 μm) for 1 h. Then, the THP-1 cells were treated with recombinant mouse TNF-α (30 ng/ml) and Z-VAD-FMK (40 μm) for 48 h, whereas the C12N2-expressing THP-1 cells were treated with MDP (100 ng/ml) for 10 h. Cell viability was assessed by WST-1 assay as described in the legend for Fig. 2. Error bars indicate S.D.
Caspase-1 Expression Correlates with the Necrotic Phenotype
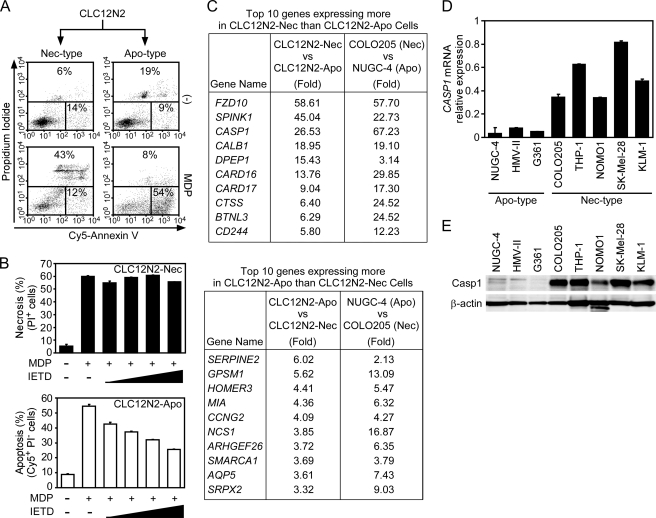
The stable transfectants of COLO205 cells expressing C12N2 (CLC12N2) exhibit necrosis in response to MDP stimulation (18). By limiting dilution, we obtained one subclone of CLC12N2 cells that exhibited necrosis (CLC12N2-Nec) just like the parental CLC12N2 cells and another subclone that exhibited apoptosis (CLC12N2-Apo) upon ASC activation (Fig. 4A and see supplemental Movie S1 and Movie S2). Consistent with our previous conclusion that ASC-mediated apoptosis is caspase-8-dependent (19), Z-IETD-FMK inhibited the cell death of CLC12N2-Apo but not CLC12N2-Nec cells (Fig. 4B).
FIGURE 4.
Caspase-1 expression correlates with the necrotic phenotype. A, CLC12N2-Apo and CLC12N2-Nec cells were treated with MDP (100 ng/ml) for 12 h and then analyzed as described in the legend for Fig. 1. Nec-type, necrosis-type cells; Apo-type, apoptosis-type cells. B, CLC12N2-Apo and CLC12N2-Nec cells were pretreated with Z-IETD-FMK (5, 10, 20, or 40 μm) for 1 h or left untreated and then were treated with MDP (100 ng/ml) for 12 h. The percentages of apoptotic and necrotic cells were determined by flow cytometry. C, gene expression profiles of CLC12N2-Nec and CLC12N2-Apo cells or COLO205 and NUGC-4 cells were compared by DNA microarray analyses. The top 10 genes with greater expression in CLC212N2-Nec cells than in CLC12N2-Apo cells are listed in the upper panel, whereas those expressed more in CLC12N2-Apo cells than in CLC12N2-Nec cells are shown in the lower panel. Numbers shown are the -fold difference in gene expression levels between the indicated cell lines. Original datasets of the microarray analyses are available at the Center for Information Biology Gene Expression (CIBEX) database (accession number CBX131). D, the caspase-1 mRNA levels relative to that of β-actin, as determined by quantitative RT-PCR, are shown for the indicated cell lines. E, caspase-1 and β-actin protein levels in the indicated cells were examined by Western blotting. Error bars in B and D indicate S.D.
To identify what determines the mode of ASC-mediated cell death, we compared the gene expression profiles between CLC12N2-Nec and CLC12N2-Apo cells and between the original COLO205 (necrosis type) and NUGC-4 cells (apoptosis type). We first selected those genes that expressed either more or less consistently in CLC12N2-Nec when compared with CLC12-Apo cells and in COLO205 cells when compared with NUGC-4 cells. Among them, the top 10 genes with the greatest difference in expression between the CLC12N2-Nec and CLC12-Apo cells are shown in Fig. 4C. Using real-time PCR, we examined the expression of these 20 genes in other tumor cell lines. To our surprise, CASP1 was the only gene that completely coincided with the necrosis-type cell lines (Fig. 4D and see supplemental Fig. S2; supplemental Table S1 shows primer sequences used in supplemental Figs. S2 and S4). Although CARD16 expression had a weak correlation with the necrosis type, CARD16 knockdown did not inhibit the MDP-induced necrosis of CLC12N2-Nec cells (see supplemental Fig. S3), suggesting that CARD16 is not required for ASC-mediated necrosis. No gene completely coincided with the apoptotic phenotype (see supplemental Fig. S4). Western blot analyses confirmed that substantial amounts of caspase-1 protein were expressed in the necrosis-type but not apoptosis-type cell lines (Fig. 4E). We previously reported that NLRC4 mimicry induces apoptosis in four gastrointestinal cancer cell lines, including NUGC-4, and one lung cancer cell line (19). Consistent with the above results, all of these cell lines expressed no caspase-1 (see supplemental Fig. S5). Therefore, caspase-1 is a novel cellular factor determining the mode of cell death.
Caspase-1 Is Required for ASC-mediated Necrosis
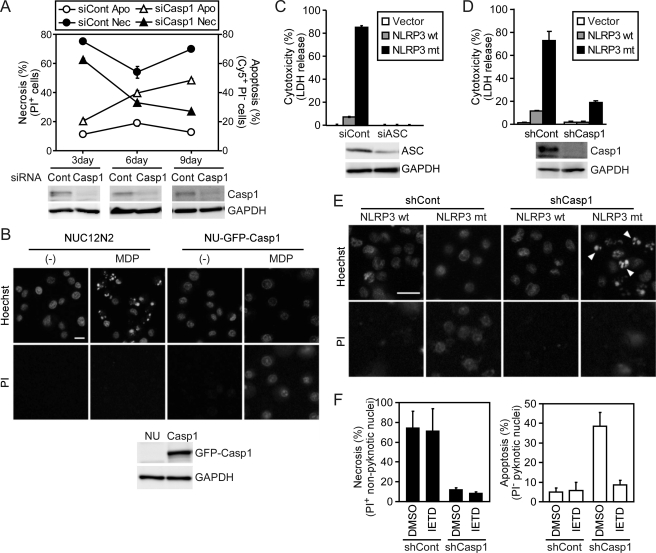
To investigate whether caspase-1 is required for ASC-mediated necrosis, caspase-1 expression in CLC12N2-Nec cells was down-regulated using siRNA. Although the one-time transfection of caspase-1-targeting siRNA but not control siRNA severely reduced the expression of caspase-1 protein, the NLRC4 mimicry-induced necrosis was only slightly suppressed by the caspase-1-targeting siRNA (Fig. 5A). However, after a 6- or 9-day culture with repetitive siRNA transfection, the necrosis was reduced and, interestingly, apoptosis gradually increased. These results suggest that a special caspase-1 with a long half-life is involved in ASC-mediated necrosis. To confirm the role of caspase-1 in ASC-mediated necrosis, we introduced a GFP-caspase-1 fusion gene into NUC12N2 cells (C12N2-expressing NUGC-4 cells) and thereby established NU-GFP-Casp1 cells. MDP stimulation induced apoptosis in NUC12N2 cells but induced necrosis in NU-GFP-Casp1 cells (Fig. 5B). Thus, exogenous caspase-1 expression converted apoptosis-type cells to necrosis-type cells.
FIGURE 5.
Caspase-1 is required for ASC-mediated necrosis. A, CLC12N2-Nec cells were transfected with control (siCont, circles) or caspase-1-targeting siRNA (siCasp1, triangles) 1–3 times at 3-day intervals. At 3, 6, or 9 days after the first transfection, the cells were treated with MDP (100 ng/ml) for 12 h, and the proportions of apoptotic (Apo, open symbols) and necrotic cells (Nec, closed symbols) were determined by flow cytometry as described in the legend for Fig. 1. Caspase-1 (Casp1) and GAPDH expression levels at the indicated times before MDP stimulation were examined by Western blotting (lower panels). B, NUC12N2 and NU-GFP-Casp1 cells (NU, Casp1) were treated with MDP (1000 ng/ml) or left untreated for 6 h and then stained with Hoechst 33342 and PI and examined under a fluorescence microscope. The upper and lower panels show Hoechst- and PI-stained images, respectively. Apoptotic cells had pyknotic nuclei that were brightly stained with Hoechst 33342 but not with PI, whereas necrotic cells had morphologically normal nuclei that were stained with PI. Scale bar, 20 μm. GFP-caspase-1 (GFP-Casp1) and GAPDH expression levels in these cells before MDP stimulation were examined by Western blotting (lower panels). C, NOMO-1 cells treated with control or ASC-targeting siRNA (siASC) for 72 h were transduced with empty lentiviral vector (Vector) or with the vector expressing wild-type (wt) NLRP3 or its Y570C mutant (mt). Twelve hours after transduction, cytotoxicity was assessed by lactate dehydrogenase (LDH) release (upper panel). ASC and GAPDH expression levels were examined by Western blotting 48 h after the siRNA transfection (lower panels). D and E, NOMO1-shCont (shCont) and NOMO1-shCasp1 (shCasp1) cells were transduced with an empty lentiviral vector or one expressing the wild type or Y570C mutant of NLRP3 and were cultured for 12 h. D, cytotoxicity was assessed as described in C. Caspase-1 and GAPDH levels were examined by Western blotting (lower panels). E, cells were examined as described in B. Scale bar, 20 μm. Arrowheads indicate apoptotic cells. F, NOMO1-shCont and NOMO1-shCasp1 cells were pretreated with Z-IETD-FMK (40 μm) or dimethyl sulfoxide (DMSO) (solvent control) for 1 h. Cells were then transduced with a lentiviral vector expressing the Y570C mutant of NLRP3 and were further cultured for 12 h. Apoptotic and necrotic cells were counted under a fluorescence microscope. Error bars in A, C, D, and F indicate S.D.
Cryopyrin-associated periodic syndrome-associated NLRP3 mutants induce necrotic cell death in the human monocytic leukemia cell line THP-1 (13, 14). Here we found that one such mutant, NLRP3-Y570C, but not the wild-type NLRP3, induced necrosis in another human monocytic cell line, NOMO-1. Consistent with the NLRC4 mimicry-induced necrosis of CLC12N2 cells, the NLRP3-Y570C-induced necrosis was suppressed by ASC-targeting siRNA (Fig. 5C) and stable expression of caspase-1-targeting shRNA (Fig. 5D), whose target sequence was completely different from that of the caspase-1-targeting siRNA used above. Furthermore, the NLRP3-Y570C-induced necrosis was converted to apoptosis by suppressing caspase-1 expression (Fig. 5, E and F). Z-IETD-FMK inhibited the apoptosis observed in caspase-1-deficient cells, but not the necrosis observed in the caspase-1-sufficient cells (Fig. 5F), indicating that ASC activation induces caspase-8-dependent apoptosis in cells lacking caspase-1.
Caspase-1 Catalytic Activity Is Not Required for ASC-mediated Necrosis
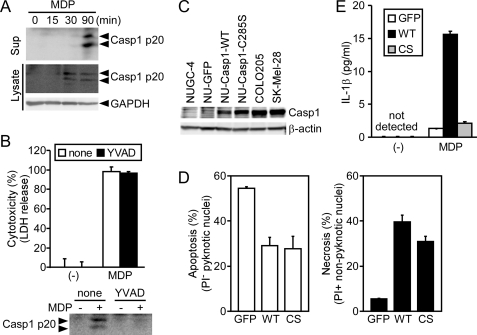
Because Ac-YVAD-CMK did not inhibit the NLRC4 mimicry-induced cell death of necrosis-type cell lines (Fig. 2B), we next investigated whether the catalytic activity of caspase-1 is involved in ASC-mediated necrosis. When SK-MEL-28 (SKC12N2) cells expressing C12N2 were treated with MDP, a fragment (p20) of mature caspase-1 was detected in the cell lysate and culture supernatant (Fig. 6A). Treatment with 40 μm Ac-YVAD-CMK completely inhibited the caspase-1 processing, but did not inhibit the ASC-mediated necrosis of SKC12N2 cells (Fig. 6B), supporting the notion that the caspase-1 catalytic activity is not required for ASC-mediated necrosis. To further confirm this notion, wild-type caspase-1, the catalytically inactive caspase-1-C285S mutant, or GFP was transduced into NUC12N2 cells using a lentiviral vector. The exogenous caspase-1 expression levels were comparable with those of endogenous caspase-1 in COLO205 and SK-MEL-28 cells (Fig. 6C and see supplemental Fig. S6A). Both the wild type and the C285S mutant of caspase-1 converted the mode of NLRC4 mimicry-induced cell death from apoptosis to necrosis, but GFP did not (Fig. 6D and see supplemental Fig. S6B). These cells were further transfected with an expression plasmid for pro-IL-1β and then stimulated with MDP. Importantly, cells expressing the wild-type caspase-1, but not the mutant caspase-1, secreted IL-1β (Fig. 6E), confirming that the C285S mutant was catalytically inactive. These results indicate that the catalytic activity of caspase-1 is not required for ASC-mediated necrosis.
FIGURE 6.
Caspase-1 catalytic activity is not required for ASC-mediated necrosis. A, SK-MEL-28 cells stably expressing C12N2 (SKC12N2) were treated with MDP (1000 ng/ml) for the indicated period. The large subunit of mature caspase-1 (p20) in cell lysates and culture supernatants (Sup) was detected by Western blotting. B, SKC12N2 cells were left untreated or were pretreated with Ac-YVAD-CMK (40 μm) and then stimulated with MDP (1000 ng/ml) for 4 h. Cytotoxicity was assessed by a lactate dehydrogenase (LDH) release assay, and caspase-1 p20 in culture supernatants was detected by Western blotting. C and D, NUC12N2 cells were transduced with a lentiviral vector expressing GFP, wild-type caspase-1, or the C285S mutant of caspase-1. C, caspase-1 and β-actin levels in the indicated cells were examined by Western blotting. D, NUC12N2 cells expressing GFP, wild-type caspase-1 (WT), or the C285S mutant of caspase-1 (CS) were cultured with MDP (1000 ng/ml) for 6 h and then stained with Hoechst 33342 and PI. Apoptotic and necrotic cells were counted under a fluorescence microscope. E, the cells described in D were further transfected with a pro-IL-1β-expressing plasmid. After 21 h, the cells were treated with MDP (1000 ng/ml) for 3 h, and IL-1β in the culture supernatant was measured by ELISA. Error bars in B, D, and E indicate S.D.
Caspase-1, but Not Its Catalytic Activity, Is Essential for the ASC-mediated Necrosis of S. aureus-infected Monocytes
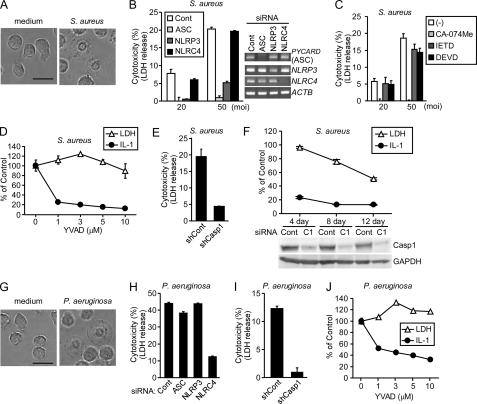
S. aureus α-hemolysin induces NLRP3- and ASC-dependent pyronecrosis in THP-1 cells (31). This necrotic cell death was reported to be caspase-1-independent because it was not inhibited by Z-VAD-FMK. Therefore, we next investigated the role of caspase-1 and its catalytic activity in the S. aureus-induced death of NOMO-1 cells. Consistent with the previous study (31), the S. aureus-induced cell death exhibited a necrotic morphology (Fig. 7A), and this necrosis was inhibited by siRNAs for ASC and NLRP3 but not for NLRC4 (Fig. 7B). Similar to NLRC4 mimicry-induced necrosis, the S. aureus-induced necrosis was inhibited by CA-074Me, but not by Z-IETD-FMK (Fig. 7C). In addition, a caspase-3 inhibitor, Z-DEVD-FMK, did not inhibit the S. aureus-induced necrosis, indicating that caspase-3, the executioner of apoptosis, was not involved in this cell death (Fig. 7C). Furthermore, Ac-YVAD-CMK that was sufficient to inhibit IL-1β production did not inhibit the S. aureus-induced necrosis (Fig. 7D). Similar results were obtained using THP-1 cells (see supplemental Fig. S7). Nonetheless, the S. aureus-induced necrosis was suppressed by caspase-1 knockdown (Fig. 7E). These results indicate that the S. aureus-induced necrosis requires caspase-1 but not its catalytic activity. Consistent with our observations in NLRC4 mimicry-induced necrosis, a short period of caspase-1 knockdown inhibited the S. aureus-induced IL-1β production but not necrosis, whereas longer knockdown suppressed both responses (Fig. 7F). However, S. aureus-induced necrosis was not inhibited even after a 7- or 12-day exposure of NOMO-1 cells to Ac-YVAD-CMK (see supplemental Fig. S8), further supporting that the catalytic activity of caspase-1 is not required for the ASC-mediated necrosis.
FIGURE 7.
Caspase-1, but not its catalytic activity, is required for the necrotic cell death of monocytes infected with bacteria. A and G, optical phase contrast images of NOMO-1 cells either uninfected (left panels) or infected with S. aureus (A, right panel) or P. aeruginosa (G, right panel) at an m.o.i. of 20 for 4 h. Scale bar, 20 μm. B and H, NOMO-1 cells were transfected with control (Cont), ASC-, NLRP3-, or NLRC4-targeting siRNA. After 72 h, the cells were infected with S. aureus (B, m.o.i. 20 or 50) for 2 h or with P. aeruginosa (H, m.o.i. 50) for 4 h. The knockdown efficiency was examined by RT-PCR (B, right panels). LDH, lactate dehydrogenase. C, NOMO-1 cells were pretreated with 20 μm of the indicated inhibitors for 1 h. Cells were then infected with S. aureus for 2 h. D and J, NOMO-1 cells were pretreated with the indicated concentrations of Ac-YVAD-CMK for 1 h. The cells were then infected with S. aureus (m.o.i. 20) for 2 h (D) or with P. aeruginosa (m.o.i. 50) for 4 h (J). E and I, NOMO1-shCont (shCont) and NOMO1-shCasp1 (shCasp1) cells were infected with S. aureus (m.o.i. 50) for 2 h (E) or P. aeruginosa (m.o.i. 50) for 4 h (I). F, NOMO-1 cells were transfected with control (Cont) or caspase-1-targeting siRNA (C1) 1–3 times at 4-day intervals. At 4, 8, or 12 days after the first transfection, the cells were infected with S. aureus (m.o.i. 20) for 2 h. Caspase-1 and GAPDH levels at the end of a 4-, 8- or 12-day culture before infection were examined by Western blotting. B–F and H–J, cytotoxicity was assessed by lactate dehydrogenase release assays. The amount of IL-1β in culture supernatants was examined by ELISA. Percentage of control = (value of cells treated with Ac-YVAD-CMK or caspase-1-targeting siRNA/value of cells treated with the solvent control or control siRNA) × 100. Error bars in B–F and H–J indicate S.D.
Caspase-1, but Not Its Catalytic Activity, Is Required for the NLRC4-dependent, ASC-independent Necrosis of P. aeruginosa-infected Monocytes
It was reported that P. aeruginosa infection induces NLRC4- and caspase-1-dependent pyroptosis in macrophages (32). Consistent with this study, we observed that P. aeruginosa induced necrosis in NOMO-1 cells (Fig. 7G), and this necrosis was inhibited by NLRC4-targeting siRNA but not by ASC- or NLRP3-targeting siRNA (Fig. 7H), although the necrosis observed after P. aeruginosa infection at a low multiplicity of infection (m.o.i.) was strongly suppressed by both NLRC4-targeting and ASC-targeting siRNAs (data not shown). Consistent with the above S. aureus infection model, the P. aeruginosa-induced necrosis was suppressed in NOMO-1 cells carrying the caspase-1-targeting shRNA (Fig. 7I). However, Ac-YVAD-CMK that was sufficient to inhibit IL-1β production did not inhibit the P. aeruginosa-induced necrosis (Fig. 7J). Thus, the caspase-1 catalytic activity is also dispensable for NLRC4-mediated pyroptosis.
DISCUSSION
In this study, we discovered that activation of ASC induces necrosis when cells express caspase-1; otherwise, the same conditions without caspase-1 expression induce apoptosis. Thus, caspase-1 is a novel molecular determinant of cell death modes. Intriguingly, we found that ASC-mediated necrosis does require caspase-1 expression, but not its catalytic activity. This conclusion was confirmed by experiments using a caspase-1 inhibitor and a catalytically inactive caspase-1 mutant. This is the first report of a catalytic activity-independent function of caspase-1. A caveat is that our results do not exclude the possibility that there are caspase-1 catalytic activity-dependent mechanisms of pyroptosis in addition to the catalytic activity-independent one. This concern is analogous to the fact that caspase-dependent DNase, which causes DNA degradation during apoptosis and hence would cause cell death, is not essential for the cell death itself (33).
In contrast to our conclusion described above, it has been thought that pyroptosis of human monocytes requires caspase-1 catalytic activity. This conclusion has been drawn from experiments using high concentrations (50–200 μm) of Z-VAD-FMK or Ac-YVAD-CMK (11, 34). Such high concentrations of these inhibitors could non-specifically inhibit other proteases including cathepsin B (35). Thus, the requirement of caspase-1 catalytic activity in pyroptosis of human macrophages should be re-evaluated using a lower dose of Ac-YVAD-CMK. Recently, Broz et al. (36) demonstrated that autoproteolysis of caspase-1 is required for IL-1β processing but not for pyroptosis of mouse macrophages infected by Salmonella or Legionella, although the proteolytic activity of caspase-1 was required for both responses. In addition, they demonstrated that formation of a large focus of ASC was required for IL-1β production, whereas formation of NLRC4-caspase1 complexes was sufficient and ASC was not required for the cell death. The results of Broz et al. (36) and our results are similar in that both indicate that the use of caspase-1 in IL-1β processing and cell death is somewhat different. However, they were different from each other in the requirement of the proteolytic activity of caspase-1 for cell death. This difference may be species-specific. Actually, our preliminary experiments using mouse peritoneal macrophages showed that low concentrations (2–10 μm) of Ac-YVAD-CMK significantly inhibited S. typhimurium- and P. aeruginosa-induced cell death, albeit less efficiently than they inhibited IL-1β release under the same conditions (see supplemental Fig. S9). In the experimental system used by Broz et al. (36), macrophages were killed in an ASC-independent manner. In contrast, we mainly investigated ASC-dependent necrosis in this study. However, the difference in ASC dependence is unlikely to be a reason for the different requirement in terms of the caspase-1 proteolytic activity for necrosis because ASC-independent necrosis of P. aeruginosa-infected NOMO-1 cells was not inhibited by Ac-YVAD-CMK (Fig. 7J).
On the other hand, pyronecrosis has been mainly characterized using THP-1 cells infected with S. flexneri, Neisseria gonorrhoeae, or adenovirus or treated with S. aureus α-hemolysin (14, 31, 37, 38). Pyronecrosis has been concluded to be caspase-1-independent, mainly because these cases of cell death were not inhibited by Z-VAD-FMK or Ac-YVAD-CMK at relatively low concentrations sufficient to inhibit caspase-1-mediated IL-1β secretion. Whether macrophages from caspase-1-deficient mice are resistant to Shigella-induced cell death has been controversial (14, 39). In other experimental systems of pyronecrosis, the requirement for caspase-1 expression has not been examined. Thus, the requirement for caspase-1 in the pyronecrosis observed in the above experimental systems needs to be re-examined.
Both pyroptosis and pyronecrosis are mediated by NLRs and ASC and are accompanied by IL-1β production. Morphologically, both modes of cell death are characterized by rapid cell swelling followed by plasma membrane rupture. Nuclear shrinkage has been observed for pyroptosis. However, it has not been clearly described whether pyronecrosis is also accompanied by nuclear shrinkage. Therefore, one cannot distinguish pyroptosis and pyronecrosis solely by the morphology of the dying cells. We observed nuclear shrinkage in some cells undergoing ASC-mediated necrosis. We found that all the instances of ASC-mediated necrosis examined in our study were inhibited by CA-074Me. This is a characteristic of pyronecrosis. However, it was recently reported that pyroptosis is also inhibited by CA-074Me (40). The sole factor that clearly discriminated the two modes of cell death was that caspase-1 was essential for pyroptosis, but not pyronecrosis. However, this distinction is now questionable as described above. Based on our results presented here, it is likely that pyroptosis and pyronecrosis are the same mode of cell death. The molecular mechanisms of pyroptosis and pyronecrosis have not been well understood. Our finding that caspase-1 is capable of inducing necrosis independently of its catalytic activity may provide important insight into the molecular mechanism of these necrotic cell death modes.
ASC-mediated necrosis induced by NLRC4 mimicry in CLC12N2 cells and by the NLRP3-Y570C mutant in NOMO-1 cells was converted to apoptosis when caspase-1 expression was suppressed by RNA interference. However, the ASC-mediated necrosis induced by S. aureus infection in NOMO-1 cells was merely suppressed, not converted to apoptosis, by reduced caspase-1 expression. One possible explanation for these distinctive outcomes by different stimuli in the same cell line is that the host cells or microbes in S. aureus-infected NOMO-1 cells might produce something that inhibits caspase-8 activation. Further study is required to clarify this point.
Although CA-074Me, the well known cathepsin B inhibitor, abrogated ASC-mediated necrosis, there was no correlation between cathepsin B expression and the mode of ASC-mediated cell death (see supplemental Fig. S10A). It should be noted that whether cathepsin B plays an essential role in this type of cell death is not clear because we previously found that knockdown of cathepsin B expression using siRNA failed to inhibit the MDP-induced cell death of CLC12N2 cells (18). Consistently, knockdown of cathepsin B in NOMO1-C12N2 cells also failed to inhibit the MDP-induced cell death (see supplemental Fig. S10B). Thus, further experiments are required to determine the target of CA-074Me in the inhibition of ASC-mediated necrosis.
Here we found that a short term knockdown of caspase-1 abrogated IL-1β production but not ASC-mediated necrosis, whereas its long term knockdown diminished both responses. Thus, the caspase-1 involved in necrosis might have a longer half-life when compared with that involved in IL-1β secretion. Alternatively, the absence of caspase-1 might result in gradual decrease of the amount and/or activity of another molecule that was essential for the ASC-mediated necrosis. Further study aiming to explain this interesting phenomenon may provide a clue to the molecular mechanism of caspase-1-meidated necrosis.
Supplementary Material
This work was supported in part by grants-in-aid for scientific research on priority areas (cancer) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Movies S1 and S2, Figs. S1–S10, and Table S1.
- ASC
- apoptosis-associated speck-like protein containing a caspase recruitment domain
- NLR
- Nod-like receptor
- MDP
- muramyl dipeptide
- m.o.i.
- multiplicity of infection
- PI
- propidium iodide
- Ac
- acetyl
- CMK
- chloromethylketone
- Z
- benzyloxycarbonyl
- FMK
- fluoromethyl ketone.
REFERENCES
- 1. Schroder K., Tschopp J. (2010) Cell. 140, 821–832 [DOI] [PubMed] [Google Scholar]
- 2. Franchi L., Eigenbrod T., Muñoz-Planillo R., Nuñez G. (2009) Nat. Immunol. 10, 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mariathasan S., Newton K., Monack D. M., Vucic D., French D. M., Lee W. P., Roose-Girma M., Erickson S., Dixit V. M. (2004) Nature 430, 213–218 [DOI] [PubMed] [Google Scholar]
- 4. Ozören N., Masumoto J., Franchi L., Kanneganti T. D., Body-Malapel M., Ertürk I., Jagirdar R., Zhu L., Inohara N., Bertin J., Coyle A., Grant E. P., Núñez G. (2006) J. Immunol. 176, 4337–4342 [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto M., Yaginuma K., Tsutsui H., Sagara J., Guan X., Seki E., Yasuda K., Yamamoto M., Akira S., Nakanishi K., Noda T., Taniguchi S. (2004) Genes Cells 9, 1055–1067 [DOI] [PubMed] [Google Scholar]
- 6. Hoffman H. M., Wanderer A. A. (2010) Curr. Allergy Asthma Rep. 10, 229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brennan M. A., Cookson B. T. (2000) Mol. Microbiol. 38, 31–40 [DOI] [PubMed] [Google Scholar]
- 8. Franchi L., Stoolman J., Kanneganti T. D., Verma A., Ramphal R., Núñez G. (2007) Eur. J. Immunol. 37, 3030–3039 [DOI] [PubMed] [Google Scholar]
- 9. Cervantes J., Nagata T., Uchijima M., Shibata K., Koide Y. (2008) Cell. Microbiol. 10, 41–52 [DOI] [PubMed] [Google Scholar]
- 10. Bergsbaken T., Fink S. L., Cookson B. T. (2009) Nat. Rev. Microbiol. 7, 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernandes-Alnemri T., Wu J., Yu J. W., Datta P., Miller B., Jankowski W., Rosenberg S., Zhang J., Alnemri E. S. (2007) Cell Death Differ. 14, 1590–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sauer J. D., Witte C. E., Zemansky J., Hanson B., Lauer P., Portnoy D. A. (2010) Cell Host Microbe 7, 412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujisawa A., Kambe N., Saito M., Nishikomori R., Tanizaki H., Kanazawa N., Adachi S., Heike T., Sagara J., Suda T., Nakahata T., Miyachi Y. (2007) Blood 109, 2903–2911 [DOI] [PubMed] [Google Scholar]
- 14. Willingham S. B., Bergstralh D. T., O'Connor W., Morrison A. C., Taxman D. J., Duncan J. A., Barnoy S., Venkatesan M. M., Flavell R. A., Deshmukh M., Hoffman H. M., Ting J. P. (2007) Cell Host Microbe 2, 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masumoto J., Taniguchi S., Ayukawa K., Sarvotham H., Kishino T., Niikawa N., Hidaka E., Katsuyama T., Higuchi T., Sagara J. (1999) J. Biol. Chem. 274, 33835–33838 [DOI] [PubMed] [Google Scholar]
- 16. Conway K. E., McConnell B. B., Bowring C. E., Donald C. D., Warren S. T., Vertino P. M. (2000) Cancer Res. 60, 6236–6242 [PubMed] [Google Scholar]
- 17. Ohtsuka T., Ryu H., Minamishima Y. A., Macip S., Sagara J., Nakayama K. I., Aaronson S. A., Lee S. W. (2004) Nat. Cell Biol. 6, 121–128 [DOI] [PubMed] [Google Scholar]
- 18. Motani K., Kawase K., Imamura R., Kinoshita T., Kushiyama H., Suda T. (2010) Cancer Sci. 101, 1822–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hasegawa M., Kawase K., Inohara N., Imamura R., Yeh W. C., Kinoshita T., Suda T. (2007) Oncogene 26, 1748–1756 [DOI] [PubMed] [Google Scholar]
- 20. Imamura R., Wang Y., Kinoshita T., Suzuki M., Noda T., Sagara J., Taniguchi S., Okamoto H., Suda T. (2010) J. Immunol. 184, 5874–5884 [DOI] [PubMed] [Google Scholar]
- 21. Hasegawa M., Imamura R., Kinoshita T., Matsumoto N., Masumoto J., Inohara N., Suda T. (2005) J. Biol. Chem. 280, 15122–15130 [DOI] [PubMed] [Google Scholar]
- 22. Wang Y., Hasegawa M., Imamura R., Kinoshita T., Kondo C., Konaka K., Suda T. (2004) Int. Immunol. 16, 777–786 [DOI] [PubMed] [Google Scholar]
- 23. Kinoshita T., Wang Y., Hasegawa M., Imamura R., Suda T. (2005) J. Biol. Chem. 280, 21720–21725 [DOI] [PubMed] [Google Scholar]
- 24. Fukui M., Imamura R., Umemura M., Kawabe T., Suda T. (2003) J. Immunol. 171, 1868–1874 [DOI] [PubMed] [Google Scholar]
- 25. Hasegawa M., Imamura R., Motani K., Nishiuchi T., Matsumoto N., Kinoshita T., Suda T. (2009) J. Immunol. 182, 7655–7662 [DOI] [PubMed] [Google Scholar]
- 26. Hitomi J., Christofferson D. E., Ng A., Yao J., Degterev A., Xavier R. J., Yuan J. (2008) Cell 135, 1311–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He S., Wang L., Miao L., Wang T., Du F., Zhao L., Wang X. (2009) Cell 137, 1100–1111 [DOI] [PubMed] [Google Scholar]
- 28. Cho Y. S., Challa S., Moquin D., Genga R., Ray T. D., Guildford M., Chan F. K. (2009) Cell 137, 1112–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang D. W., Shao J., Lin J., Zhang N., Lu B. J., Lin S. C., Dong M. Q., Han J. (2009) Science 325, 332–336 [DOI] [PubMed] [Google Scholar]
- 30. Degterev A., Hitomi J., Germscheid M., Ch'en I. L., Korkina O., Teng X., Abbott D., Cuny G. D., Yuan C., Wagner G., Hedrick S. M., Gerber S. A., Lugovskoy A., Yuan J. (2008) Nat. Chem. Biol. 4, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Craven R. R., Gao X., Allen I. C., Gris D., Bubeck Wardenburg J., McElvania-Tekippe E., Ting J. P., Duncan J. A. (2009) PLoS One 4, e7446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sutterwala F. S., Mijares L. A., Li L., Ogura Y., Kazmierczak B. I., Flavell R. A. (2007) J. Exp. Med. 204, 3235–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McIlroy D., Sakahira H., Talanian R. V., Nagata S. (1999) Oncogene 18, 4401–4408 [DOI] [PubMed] [Google Scholar]
- 34. Kelk P., Johansson A., Claesson R., Hänström L., Kalfas S. (2003) Infect. Immun. 71, 4448–4455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schotte P., Declercq W., Van Huffel S., Vandenabeele P., Beyaert R. (1999) FEBS Lett. 442, 117–121 [DOI] [PubMed] [Google Scholar]
- 36. Broz P., von Moltke J., Jones J. W., Vance R. E., Monack D. M. (2010) Cell Host Microbe 8, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duncan J. A., Gao X., Huang M. T., O'Connor B. P., Thomas C. E., Willingham S. B., Bergstralh D. T., Jarvis G. A., Sparling P. F., Ting J. P. (2009) J. Immunol. 182, 6460–6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barlan A. U., Griffin T. M., McGuire K. A., Wiethoff C. M. (2011) J. Virol. 85, 146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hilbi H., Moss J. E., Hersh D., Chen Y., Arondel J., Banerjee S., Flavell R. A., Yuan J., Sansonetti P. J., Zychlinsky A. (1998) J. Biol. Chem. 273, 32895–32900 [DOI] [PubMed] [Google Scholar]
- 40. Averette K. M., Pratt M. R., Yang Y., Bassilian S., Whitelegge J. P., Loo J. A., Muir T. W., Bradley K. A. (2009) PLoS One 4, e7913 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.