Abstract
AT1G78690, a gene found in Arabidopsis thaliana, has been reported to encode a N-acyltransferase that transfers an acyl chain from acyl-CoA to the headgroup of phosphatidylethanolamine (PE) to form N-acylphosphatidylethanolamine (N-acyl-PE). Our investigation suggests that At1g78690p is not a PE-dependent N-acyltransferase but is instead a lysoglycerophospholipid O-acyltransferase. We overexpressed AT1G78690 in Escherichia coli, extracted the cellular lipids, and identified the accumulating glycerophospholipid as acylphosphatidylglycerol (acyl-PG). Electrospray ionization quadrupole time-of-flight mass spectrometry (ESI-MS) analysis yielded [M − H]− ions, corresponding by exact mass to acyl-PG rather than N-acyl-PE. Collision-induced dissociation mass spectrometry (MS/MS) yielded product ions consistent with acyl-PG. In addition, in vitro enzyme assays using both 32P- and 14C-radiolabeled substrates showed that AT1G78690 acylates 1-acyllysophosphatidylethanolamine (1-acyllyso-PE) and 1-acyllysophosphatidylglycerol (1-acyllyso-PG), but not PE or phosphatidylglycerol (PG), to form a diacylated product that co-migrates with PE and PG, respectively. We analyzed the diacylated product formed by AT1G78690 using a combination of base hydrolysis, phospholipase D treatment, ESI-MS, and MS/MS to show that AT1G78690 acylates the sn-2-position of 1-acyllyso-PE and 1-acyllyso-PG.
Keywords: Arabidopsis, Escherichia coli, Lipid Metabolism, Lysophospholipid, Mass Spectrometry (MS), N-Acylphosphatidylethanolamine, Acylphosphatidylglycerol
Introduction
N-Acylphosphatidylethanolamine (N-acyl-PE)2 is an anionic minor membrane glycerophospholipid (GPL) that is abundant in animals, higher plants, and certain microorganisms, such as yeast and Escherichia coli (1–7). This GPL is of particular interest because of its well documented role as the precursor to N-acylethanolamines (NAEs), a class of bioactive lipids that are involved in a variety of physiological processes, such as responses to pathogens, plant development, and germination in plants (8–10) as well as control of appetite, inflammation, and apoptosis (11–13) in animals.
In animals, N-acyl-PE synthesis is catalyzed by two distinct biochemical activities. A Ca2+-dependent membrane-associated protein has been characterized in brain, testis, and heart (14). This enzyme has been shown to catalyze the transfer of an acyl chain from the sn-1-position of a diacylated GPL, functions most efficiently at alkaline pH, and requires divalent cations (13, 15, 16). The gene encoding this enzyme has yet to be identified.
A lecithin retinol acyltransferase-like protein from rats, RLP-1, has been shown to encode a Ca2+-independent N-acyltransferase (NAT) (17). It is predominantly expressed in testis and is, therefore, thought to be distinct from the Ca2+-dependent NAT. Ca2+-independent NAT activity is detected in both the soluble and membrane fractions when overexpressed in COS-7 cells and does not show preference for transfer of the sn-1 or sn-2 acyl chain to the amine of PE (17). Human and mouse homologs have been cloned and shown to possess similar NAT activity (18).
In plants, N-acyl-PE is proposed to be synthesized from PE and unesterified fatty acids by a membrane-bound N-acyl-PE synthase (3, 9, 19). Recently, Faure et al. (20) identified a gene from Arabidopsis thaliana, AT1G78690, as encoding an NAT that catalyzes the transfer of an acyl chain from acyl-CoA to the amine of PE. They reported that overexpression of AT1G78690 in E. coli led to the accumulation of N-acyl-PE in cells and the presence of acyl-CoA-dependent N-acyl-PE synthase activity in membranes. AT1G78690 has homology to lysoglycerophospholipid (lyso-GPL) acyltransferases such as the lysophosphatidic acid acyltransferase Slc1 from A. thaliana and the lysophosphatidylcholine acyltransferase YPR140wp from Saccharomyces cerevisiae (20, 21).
Multiple attempts to detect N-acyl-PE synthase activity from cell-free extracts prepared from E. coli induced to overexpress AT1G78690 were unsuccessful. Therefore, we reinvestigated the biochemical activity of AT1G78690 in E. coli. In this work, we show that this enzyme is a lyso-GPL acyltransferase and that its overexpression in E. coli leads to the accumulation of acylphosphatidylglycerol (acyl-PG), not N-acyl-PE.
EXPERIMENTAL PROCEDURES
Materials
Tryptone and yeast extract were from Fisher. Glass-backed silica gel 60 thin layer chromatography plates (0.25 mm) and high performance thin layer chromatography (HPTLC) plates were from Merck. Solvents were reagent grade from Mallinckrodt. Other chemicals were purchased from VWR or Sigma-Aldrich. GPLs, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (16:0, 18:1 PE), 1-oleoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (1-acyllyso-PE), 1-oleoyl-2-hydroxy-sn-glycero-3-phospho-rac-(1-glycerol) (1-acyllyso-PG), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-arachidonyl (N-acyl-PE), sn-(3-myristoyl-2-hydroxyl)-glycerol-1-phospho-sn-3′-(1′,2′-myristoyl)-glycerol (acyl-PG), 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (16:0, 18:1 PG), and sn-(3-oleoyl-2-hydroxy)-glycerol-1-phospho-sn-3′-(1′-oleoyl-2′-hydroxy)-glycerol (3,1′-BMP), were from Avanti Polar Lipids (Alabaster, AL). [32P]PO43− (8500 Ci/mmol, 10 mCi/ml) and palmitoyl-[1-14C]CoA (60 mCi/mmol, 0.02 mCi/ml) were from PerkinElmer Life Sciences. Palmitoyl-CoA and arachidonyl-CoA, porcine pancreas phospholipase A2, and Streptomyces chromofuscus phospholipase D were from Sigma.
Growth of E. coli
pET 15b and pAt1g78690p (a kind gift of D. Coulon, Laboratoire de Biogenèse Membranaire, Université Victor Segalen, Bordeaux, France) (20) were transformed into chemically competent E. coli BLR(DE3)pLysS. Transformants were selected on LB agar containing 10 g of NaCl, 5 g of yeast extract, 10 g of Tryptone, and 15 g of agar per liter (22) and 100 μg/ml ampicillin (LB-amp) and grown overnight at 37 °C. Single colonies were restreaked on LB-amp and grown at 37 °C overnight. Overnight cultures were inoculated from a single colony into liquid LB-amp containing 100 μg/ml ampicillin, grown shaking at 225 rpm at 37 °C overnight, and then diluted into LB-amp medium to an A600 of 0.01. The culture was grown at 37 °C, shaking at 225 rpm until the A600 was about 0.4–0.5. Isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to a final concentration of 0.5 mm, and cells were cultured an additional 3 h. Cells were harvested by centrifugation for 20 min at 2600 × g and washed with phosphate-buffered saline (PBS; 137 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, 1.8 mm KH2PO4) when cells were used for lipid extraction or with 15 mm Tris, pH 7.4, when cells were used for preparation of protein extracts. Cell pellets were frozen at −80 °C until further use.
Extraction of E. coli Total Lipids
The final cell pellet from a 50-ml growth of E. coli was resuspended in 0.8 ml of PBS and transferred to a disposable glass centrifuge tube equipped with Teflon-lined lids. The cellular lipids were extracted using the method of Bligh and Dyer (23), as described previously (24). Briefly, 1 ml of CHCl3 and 2 ml of CH3OH were added to the cell suspension to generate a single phase extraction mixture of CHCl3/CH3OH/PBS (1:2:0.8, v/v/v). After incubation at room temperature for 20 min, the mixture was centrifuged at 2600 × g for 15 min. The supernatant was transferred to a clean tube and converted to a two-phase Bligh-Dyer extraction mixture (CHCl3/CH3OH/PBS, 2:2:1.8, v/v/v) by the addition of 1 ml of chloroform and 1 ml of PBS. The extraction mixture was centrifuged as above to resolve the phases. The upper phase was removed, and the lower phase was washed with 2 ml of pre-equilibrated neutral upper phase. The resulting two-phase extraction mixture was centrifuged as described above. The upper phase was discarded, and the resulting lower phase was dried under N2 gas. Dried lipid films were stored at −20 °C.
Thin Layer Chromatography
Two solvent systems were used to display lipids on silica gel 60 thin layer chromatography (TLC) plates. Solvent system A was CHCl3/CH3OH/H2O (65:25:4, v/v/v) and was used to display mono-, di-, and triacylated lipids. Solvent system B was C6H12/CH3CH2OCH2CH3/(CH3)2CO:CH3COOH (50:40:10:1, v/v/v/v) and was used to distinguish monoacylglycerol from N-acylethanolamine (9). Lipids were visualized by exposure to iodine vapors, by charring with 10% H2SO4 in CH3CH2OH, or by phosphorimaging using a Bio-Rad Personal Molecular Imager.
Prep-TLC of Lipids
Total lipid extracts were redissolved in CHCl3/CH3OH, (2:1, v/v) and spotted continuously along the bottom of a 10 × 10-cm HPTLC plate. The plate was developed in solvent A, and lipids were visualized by exposure to iodine vapors. The region of the TLC plate containing the lipid of interest was wetted with water, and the silica chips were scraped from the plate and placed in a glass centrifuge tube. 2 ml of CHCl3/CH3OH (2:1, v/v) were added to the chips, and the silica chips were dispersed using a bath sonicator (Laboratory Supplies Co., Hicksville, NY). The dispersed silica chips were pelleted by centrifugation for 10 min in a clinical centrifuge. The supernatant was filtered through glass wool and transferred to a glass tube. The pelleted silica chips were re-extracted two additional times to maximize yield of the lipid of interest. All three supernatants were combined and dried under N2. Dried lipid films were stored at −20 °C.
Phospholipase D Treatment of in Vivo Accumulating Lipid
S. chromofuscus phospholipase D was used following the methods of Imamura and Horiuti (25). Each reaction contained 0.08 units/μl S. chromofuscus phospholipase D resuspended in 0.05% BSA and 10 mm Tris-HCl, pH 8.0, 4 mm CaCl2, 50 mm Tris-HCl, pH 8.0, 2.5 mg/ml Triton X-100 detergent, and 12.5 μg/μl lipid substrate. Reactions were incubated for 45 min at 37 °C and terminated by plating onto a TLC plate.
Mass Spectrometry of Total Lipid Extracts and Purified Accumulating Lipid
The dried lipid film was redissolved in 100 μl of CHCl3/CH3OH (2:1, v/v) to a concentration of ∼5 mg/ml. This solution was directly infused into the turbo electrospray ionization source of a QSTAR XL quadrupole time-of-flight tandem mass spectrometer (ABI/MDS-Sciex, Toronto, Canada) at 6 μl/min. Mass spectra were obtained scanning from 200 to 2000 Da in negative ion mode with the electrospray ionization source operating at the following settings: nebulizer gas, 21 kilopascals; curtain gas, 27 kilopascals; ion spray voltage, −4500 V; declustering potential, −55 V; focusing potential, −265 V; declustering potential 2, −15 V. The instrument was calibrated using polypropylene glycol (Applied Biosystems). Under normal operating conditions, the resolution of the instrument was 10,000–15,000. The mass accuracy of the instrument was between 5 and 20 ppm, and therefore measured masses are given to three decimal places. Collision-induced dissociation mass spectrometry (MS/MS) was performed with a collision energy of −50.0 V (laboratory frame of reference) and N2 as the collision gas. Data acquisition and analysis were performed using the Analyst QS 1.1 software. Exact masses of lipid species and product ions were obtained using CS Chem Draw Pro, version 11.0.
In Vivo 32P-Labeling of Lipids
A culture of BLR(DE3)pLysS/pET15b and BLR(DE3)pLysS/pAt1g78690p was grown overnight at 37 °C in LB-amp. This overnight was used to start a 10-ml culture in LB-amp containing 100 μCi of [32P]PO4. This culture, as well as an unlabeled culture for monitoring growth, was incubated at 37 °C. When the A600 reached 0.4–0.5, IPTG was added to a final concentration of 1 mm IPTG. The culture was grown for 3 h, and cells were harvested, extracted, and dried as described above.
Base Deacylation of 32P-Labeled Lipids
In vivo labeled lipids from BLR(DE3)pLysS/pET15b and BLR(DE3)pLysS/pAt1g78690p (1000 dpm) were redissolved in CHCl3/CH3OH (2:1, v/v), treated with NaOH (final concentration 0.2 m), and incubated at room temperature for 60 min. The reaction was spotted to TLC with an equivalent amount of untreated samples, developed in solvent system A, and phosphorimaged as described above.
Preparation of Cell-free Extracts and Membranes
E. coli BLR(DE3)pLysS/pET15b and BLR(DE3)pLysS/pAt1g78690p were grown and induced as described above. The cell pellet from a 50-ml culture was resuspended in 2 ml of 15 mm Tris, pH 7.4, and cells were lysed in a French pressure cell at 18,000 p.s.i. The lysate was centrifuged at 2600 × g to pellet unlysed cells. The resulting cell-free extract was transferred to a fresh tube and stored at −80 °C.
Washed membranes were prepared by centrifuging the cell-free extract at 4 °C, 100,000 × g for 1 h. The membrane pellet was resuspended by homogenization in 2 ml of 15 mm Tris, pH 7.4, and centrifuged again as described above. The final washed membrane pellet was resuspended by homogenization in 2.0 ml of 15 mm Tris, pH 7.4.
For membrane solubilization, Triton X-100, Tris-HCl, and double-distilled H2O were added to washed membranes to yield a final concentration of 1% Triton X-100, 15 mm Tris, pH 7.4, and 1 mg/ml protein. The samples were incubated on ice with periodic inversion for 30 min and then spun at 100,000 × g, 4 °C for 20 min. The supernatant was transferred to a fresh tube to yield solubilized membranes.
The concentration of all protein samples was determined using the bicinchoninic acid reagent (Thermo Scientific) with bovine serum albumin as the standard. All protein samples were stored at −80 °C.
Generation of 32P-GPL Substrates
E. coli MG1655 cells were cultured as described above. A 10-ml culture was inoculated to A600 of 0.01, and 1 mCi of [32P]PO43− was added to the culture. Cells were grown to an A600 of ∼1.0 and harvested by centrifugation for 15 min in a clinical centrifuge. The resulting cell pellet was resuspended in 0.4 ml of PBS and extracted as described above. All extraction volumes were adjusted to maintain the proper Bligh-Dyer extraction mixture ratios (23). The total lipid extract, which contains primarily [32P]PE and [32P]PG, was spotted along the bottom of a 20 × 20-cm TLC plate and developed in solvent A. [32P]PE and [32P]PG were identified by phosphorimaging. The silica chips were scraped and extracted as described above. The dried lipid films were resuspended by sonication in 15 mm Tris, pH 7.4, for use in aqueous reactions. Following this procedure, the [32P]PE and [32P]PG were ∼95% pure as evaluated by PhosphorImager analysis.
1-Acyllyso-[32P]PG and 1-acyllyso-[32P]PE were generated enzymatically using porcine pancreas phospholipase A2 (PLA2) (26). The reaction mixture (100 μl) containing [32P]PE or [32P]PG, 15 mm Tris, pH 8.0, 20 mm CaCl2, 150 mm NaCl, and 0.1 mg/ml of PLA2 was incubated at 37 °C for 1 h. The reaction mixture was converted to an acidic two-phase Bligh Dyer by the addition of 3 μl of 6 n HCl, 200 μl of CHCl3, and 200 μl of CH3OH (final proportions of CHCl3, CH3OH, and 0.1 n HCl were 2:2:1.8 (v/v/v)). The phases were resolved by centrifugation in a microcentrifuge for 1 min. The lower phase was removed to a fresh tube, and the upper phase was washed two times with 300 μl of pre-equilibrated acidic lower phase. The lower phases were combined and dried under N2. The dried lipids were stored at −20 °C.
In Vitro Enzyme Reactions
32P-GPLs or lyso-32P-GPLs were tested as substrates for the AT1G78690 enzyme. Reactions contained 100 μm acyl acceptor (GPL or lyso-GPL), ∼1000 cpm/μl 32P-GPL or lyso-32P-GPL, 100 μm palmitoyl-CoA, 15 mm Tris, pH 7.4, 0.05% Triton X-100, and 0.001–1 mg/ml protein. Reactions were incubated at 37 °C for the times indicated, and the reaction was stopped by spotting a 5-μl portion onto a silica gel 60 TLC plate. TLC plates were developed in solvent A and imaged using a PhosphorImager.
Reactions were also carried out with [14C]palmitoyl-CoA (60.0 mCi/mmol) as the labeled substrate. Reactions contained 100 μm acceptor GPL, 100 μm palmitoyl-CoA, ∼2000 dpm/μl [14C]palmitoyl-CoA, 15 mm Tris, pH 7.4, 0.05% Triton X-100, and 0.001–1 mg/ml protein. Reactions were incubated at 37 °C for the times indicated, and the reaction was stopped by spotting a 5-μl portion onto a TLC plate. TLC plates were developed in solvent A and imaged using a PhosphorImager.
Preparation of in Vitro Enzyme Products
In vitro products were generated in a 2-ml reaction that contained 117 μm arachidonyl-CoA, 2 mm 1-oleoyllyso-PE or 1-oleoyllyso-PG, 15 mm Tris, pH 7.4, 0.05% Triton X-100, and 0.46 mg/ml solubilized membranes from BLR(DE3)pLysS/pET15b or BLR(DE3)pLysS/pAt1g78690p. The reaction was incubated for 160 min at 37 °C and terminated by Bligh-Dyer extraction as described above. The lower phase was dried under N2 gas and stored at −20 °C until further analysis.
Mass Spectrometry of in Vitro Products
The products of the in vitro reaction were resuspended in 100 μl of CHCl3/CH3OH (2:1, v/v) and analyzed using a normal phase liquid chromatography electrospray ionization quadrupole time of flight mass spectrometer. The normal phase chromatography was performed using a Zorbax Rx-SIL (5 μm, 4.6 × 250 mm) column on an Agilent 1100 HPLC system as described previously (24). The HPLC effluent (0.5 ml/min) was analyzed using an Agilent 6520 quadrupole time-of-flight mass spectrometer. Samples were ionized by electrospray ionization in the negative or positive mode as indicated. Mass spectra were obtained scanning from 100 to 2000 at 1 spectrum/s with the following instrument parameters: fragmentor voltage, 175 V; drying gas temperature, 325 °C; drying gas flow, 11 liters/min; nebulizer pressure, 45 p.s.i.g.; capillary voltage, 4000 V. Data were collected with the instrument set to 3200 mass range under high resolution conditions at a 4-GHz data acquisition rate. Data were collected in profile mode. For MS/MS analysis, spectra were obtained scanning from 100 to 2000 at 1 spectrum/s with an isolation width of ∼4 m/z. The collision energy was −40.0 V (laboratory frame of reference), and N2 was the collision gas. The instrument was calibrated using Agilent ESI-L low concentration tuning mix, and under normal operating conditions the resolution of the instrument was ∼15,000. The mass accuracy of the instrument was between 1 and 5 ppm, and therefore measured masses are given to three decimal places. Data acquisition and analysis were performed using Agilent MassHunter Work station Acquisition software and Agilent MassHunter Workstation Qualitative Analysis software (Agilent Technologies, Santa Clara, CA), respectively.
RESULTS
Overexpression of AT1G78690 in E. coli Leads to Accumulation of Acylphosphatidylglycerol
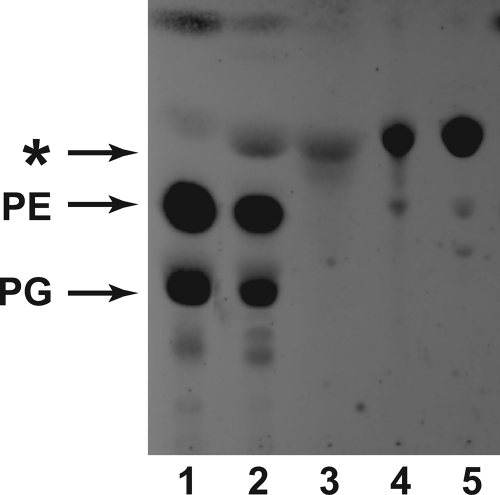
BLR(DE3)pLysS/pET and BLR(DE3)pLysS/pAt1g78690p were grown and induced with IPTG to express proteins under control of the T7 RNA polymerase promoter (27). Lipids were extracted and displayed on HPTLC plates (Fig. 1). A prominent band with an Rf higher than the major diacylated lipids, PE and PG, accumulated in cells induced to express AT1G78690 as compared with the lipid extract from the uninduced and vector control cells. This result is consistent with a previous report from Faure et al. (20), from which they concluded that this accumulating lipid was N-acyl-PE. As shown in Fig. 1 (lanes 2 and 3), the accumulating lipid nearly co-migrated with an authentic N-acyl-PE standard but also with an authentic acyl-PG standard (Fig. 1, lanes 4 and 5).
FIGURE 1.
Expression of AT1G78690 in E. coli leads to the accumulation of a lipid that co-migrates with a triacylated lipid. E. coli BLR(DE3)pLysS/pET15b and BLR(DE3)pLysS/pAt1g78690p were grown and induced with IPTG. Lipids were extracted, and the accumulating lipid was purified using preparative TLC as described under “Experimental Procedures.” All lipids were displayed on HPTLC by development in solvent A and visualized by charring with sulfuric acid. Lane 1, lipid extract from induced BLR(DE3)pLysS/pET15b; lane 2, lipid extract from induced BLR(DE3)pLysS/pAt1g78690p; lane 3, purified accumulating lipid; lane 4, N-acyl-PE standard; lane 5, acyl-PG standard. The migration of the major GPLs, PE and PG, are indicated. The asterisk indicates the lipid that accumulates in lipid extracts prepared from cells induced to overexpress At1g78690p.
Negative ion electrospray ionization mass spectrometry (ESI-MS) was performed on the total lipid extracts from BLR(DE3)pLysS/pET15b and BLR(DE3)pLysS/pAt1g78690p (Fig. 2, A and B). The predominant ions between m/z 650 and 800 represent doubly charged species [M − 2H]2− of cardiolipin and singly charged species [M − H]− of PE and PG by exact mass and MS/MS analysis (24, 29, 30, 48). In the 900–1200 atomic mass units range, the negative ions at m/z 915.702, 943.736, 953.743, 955.757, 971.768, 983.769, 985.771, 995.769, 997.780, 1011.780, 1013.799, 1023.786, and 1025.787 are enriched in total lipid extracts from cells overexpressing AT1G78690. These ions correspond by exact mass and MS/MS to acyl-PG (see Table 1). The accumulating lipid was purified from lipid extracts of BLR(DE3)pLysS/pAt1g78690p by prep-TLC and analyzed by negative ion ESI-MS (Fig. 2C). The predominant [M − H]− ions in the 870–1060 atomic mass units range correspond by exact mass to the m/z expected for acyl-PGs with acyl chains shown in Table 1. MS/MS verified that these ions corresponded to acyl-PG. Fig. 2D shows the results for the ion at m/z 983.5. The product ions are consistent with a GPL, as indicated by the product ion at m/z 152.996 that corresponds to C3H6O5P− (29, 31, 32). Acyl chains corresponding to palmitate (16:0, m/z 255.233) and 12-methylene-palmitoleate (17cp, m/z 267.234) are also seen (33). The product ion at m/z 733.501 corresponds to the loss of one of the 17cp acyl chains. The ions at m/z 391.225 and 403.228 correspond to the loss of the acylated headgroup and the 17cp acyl chain or the 16:0 acyl chain as a fatty acid (RCO2H), respectively. The MS/MS spectrum of a synthetic 54:3 (total number of carbons in the acyl chains/total number of unsaturations) acyl-PG standard yields analogous product ions (supplemental Fig. 1). In addition, the exact masses of the major [M − H]− ions do not match the exact mass predicted for N-acyl-PE molecular species, nor are the product ions observed consistent with those of N-acyl-PE (4).
FIGURE 2.
Negative ion ESI-MS of lipid extracts from E. coli BLR(DE3)pLysS/pET15b and BLR(DE3)pLysS/pAt1g78690p. A, negative ion ESI-MS from m/z 500 to 1100 of lipid extract prepared from BLR(DE3)pLysS/pET15b. B, negative ion ESI-MS from m/z 500 to 1100 of lipid extract prepared from BLR(DE3)pLysS/pAt1g78690p. In A and B, the major ions in m/z 650–800 correspond to [M − 2H]2− ions of CL and [M − H]− ions of PE and PG. C, negative ion ESI-MS from m/z 900 to 1050 of purified accumulating lipid. The major [M − H]− ions correspond to acyl-PG molecular species as shown in Table 1. D, negative ion MS/MS of m/z 983.7. The inset shows the major product ions from a predicted precursor ion. At the given molecular mass of 983.7, several distinct molecular species of acyl-PG are possible. The inset shows one possible molecular species consistent with the product ion spectra. The MS/MS technique we employed does not allow for definitive assignment of the acyl chains to the sn-1, sn-2, sn-2′, or sn-3′ position.
TABLE 1.
Acyl-PG species identified in BLR(DE3)pLysS/pAt1g78690p
| Acyl-PG molecular speciesa | Molecular formula [M − H]− | Exact mass m/z | Observed mass m/z |
|---|---|---|---|
| 42:0 | C48H92O11P− | 875.6383 | 875.636 |
| 42:1 | C48H90O11P− | 873.6226 | 873.623 |
| 43:1 | C49H92O11P− | 887.6383 | 887.637 |
| 43:2 | C49H90O11P− | 885.6226 | 885.629 |
| 44:1 | C50H94O11P− | 901.6539 | 901.661 |
| 44:2 | C50H92O11P− | 899.6383 | 889.660 |
| 45:1 | C51H96O11P− | 915.6696 | 915.670 |
| 45:2 | C51H94O11P− | 913.6539 | 913.680 |
| 46:1 | C52H98O11P− | 929.6852 | 929.692 |
| 46:2 | C52H96O11P− | 927.6696 | 927.698 |
| 47:1 | C53H100O11P− | 943.7009 | 943.701 |
| 47:2 | C53H98O11P− | 941.6852 | 941.715 |
| 48:1 | C54H102O11P− | 957.7165 | 957.717 |
| 48:2 | C54H100O11P− | 955.7009 | 955.714 |
| 49:1 | C55H104O11P− | 971.7322 | 971.730 |
| 49:2 | C55H102O11P− | 969.7165 | 969.719 |
| 50:2 | C56H104O11P− | 983.7322 | 983.731 |
| 50:3 | C56H102O11P− | 981.7165 | 981.737 |
| 51:2 | C57H106O11P− | 997.7478 | 997.747 |
| 51:3 | C57H104O11P− | 995.7322 | 995.736 |
| 52:2 | C58H108O11P− | 1011.7635 | 1011.761 |
| 52:3 | C58H110O11P− | 1009.7478 | 1009.748 |
| 53:2 | C59H110O11P− | 1025.7791 | 1025.773 |
| 53:3 | C58H108O11P− | 1023.7635 | 1023.763 |
| 54:3 | C60H110O11P− | 1037.7791 | 1037.779 |
| 55:3 | C61H112O11P− | 1051.7948 | 1051.786 |
a Acyl-PG species are denoted by the total number of carbons in the acyl chains:total number of unsaturations in the acyl chains.
To further verify that acyl-PG is the lipid accumulating in cells overexpressing AT1G78690, the product released by phospholipase D treatment of the isolated lipid was analyzed. If the lipid were predominantly N-acyl-PE, then NAE would be released, as shown by the treatment of the N-acyl-PE standard with PLD (Fig. 3, lane 4). If the lipid were acyl-PG, then monoacylglycerol (MAG) would be released by PLD treatment, as shown by treatment of the acyl-PG standard (Fig. 3, lane 3). Treatment of the isolated lipid yielded a product that corresponds by TLC to MAG (Fig. 3, lane 2), consistent with the identification of the accumulating lipid as acyl-PG. In TLC solvent B, there is a clear difference in the migration of MAG from NAE, unlike other solvents in which these fatty acyl derivatives migrate near the solvent front. This allowed us to establish that the accumulating lipid releases MAG as opposed to NAE, consistent with it being acyl-PG and not N-acyl-PE.
FIGURE 3.
Phospholipase D treatment of the lipid that accumulates upon overexpression of AT1G78690 in E. coli. Samples were treated with phospholipase D, displayed by TLC using solvent B, and visualized by exposure to iodine vapor. Lane 1, total lipid extracts from BLR(DE3)pLysS/pET15b; lane 2, purified accumulating lipid; lane 3, acyl-PG standard; lane 4, N-acyl-PE standard.
Interestingly, an [M − H]− ion at m/z 952.8 detected in the purified accumulating lipid corresponds to N-acyl-PE with 50:2 acyl chains (Fig. 2) (4). MS/MS analysis of this ion confirmed that it is indeed N-acyl-PE (4). Although this is probably N-acyl-PE that is endogenously produced in E. coli (4), the possibility remained that overexpression of AT1G78690 leads to the accumulation of both acyl-PG and N-acyl-PE. Because N-acyl-PE ions are nearly isobaric with acyl-PG, it is difficult to detect endogenous N-acyl-PE with MS without prior separation. To determine if N-acylated lipids were accumulating in cells overexpressing AT1G78690, we took advantage of the fact that ester-linked but not amide-linked acyl chains can be removed by treatment with base (34).
BLR(DE3)pLysS/pET15b and BLR(DE3)pLysS/pAt1g78690p were continuously labeled with [32P]PO43− and induced with IPTG to promote the accumulation of the lipid in vivo. Cells were extracted, and equal counts were subjected to base hydrolysis. As shown in Fig. 4, left panel, lipids from cells overexpressing AT1G78690 accumulated a lipid with an Rf consistent with N-acyl-PE/acyl-PG as compared with cells containing pET15b. When the total lipid extract was treated with base and subjected to TLC, only N-acylated lipids migrated off the origin. As can be seen in Fig. 4, right panel, the amount of N-acylated lipids was not altered when AT1G78690 was overexpressed. Quantification of the amount of N-acylated lipid in BLR(DE3)pLysS/pET15b and pAt1g78690p was 12.0 ± 0.8 and 12.2 ± 0.5%, respectively. The 32P-labeled in vivo accumulating lipid was purified by prep-TLC and similarly subjected to base hydrolysis. No product corresponding to N-acylglycerophosphoethanolamine was detected (supplemental Fig. 2). Taken together, this strongly suggests that AT1G78690 is not promoting the accumulation of N-acyl-PE (or other N-acylated GPLs, such as N-acyllyso-PE) in vivo and is consistent with our identification of the accumulating lipid as acyl-PG.
FIGURE 4.
Base hydrolysis of 32P-labeled lipids from BLR(DE3)pLysS/pET15b and BLR(DE3)pLysS/pAt1g78690p. GPLs were in vivo labeled by culturing in the presence of [32P]PO43− and extracted as described under “Experimental Procedures.” 1000 dpm of each sample was treated with base, spotted to TLC, and developed in solvent A. As indicated, lipid extracts from BLR(DE3)pLysS/pAt1g78690p or BLR(DE3)pLysS/pET15b were treated or not treated with 0.2 m NaOH. The predicted products and the migration of the base hydrolysis products of acyl-PG and N-acyl-PE are shown. Base treatment of the standards yields similar products (supplemental Fig. 3).
Identification of the Enzymatic Activity of AT1G78690
With the confirmation of acyl-PG accumulation in cells overexpressing AT1G78690, we set out to determine the enzymatic activity of this protein. Despite repeated attempts using 32P-labeled PE and PG as substrates, we were unable to detect the formation of a triacylated lipid product from either substrate in an AT1G78690-dependent manner (supplemental Fig. 4). Similar attempts to detect acylation of PE or PG using 14C-labeled acyl-CoA also were unsuccessful (supplemental Fig. 5).
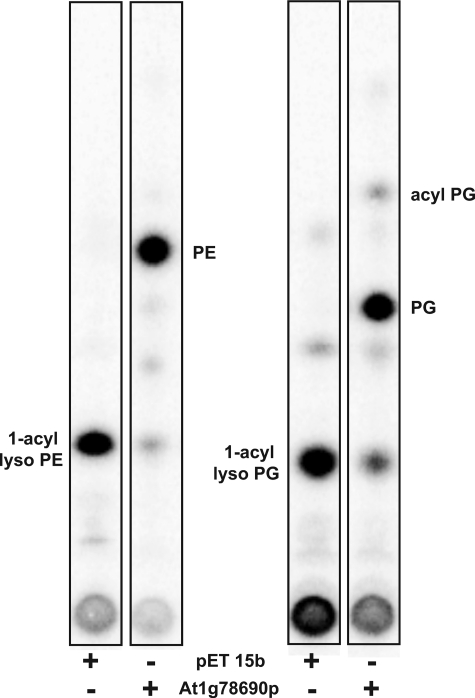
In the course of testing for the acylation of [32P]PE, we observed that low levels of lyso-[32P]PE, a contaminant in the [32P]PE substrate, decreased in the presence of cell-free extracts prepared from induced BLR(DE3)pLysS/pAt1g78690p (data not shown). This suggested that AT1G78690 might acylate lyso-GPLs. To test this, 1-acyllyso-[32P]PE was generated by PLA2 treatment of [32P]PE and tested as a substrate for AT1G78690. When 1-acyllyso-[32P]PE was used as a substrate, the major product formed when cell-free extracts containing AT1G78690 were used as the protein source co-migrated with PE (Fig. 5). Very little product was formed when cell-free extracts prepared from BLR(DE3)pLysS/pET15b were utilized. No triacylated lipid, such as N-acyl-PE, was detected.
FIGURE 5.
Acylation of 32P-labeled 1-acyl-PE and 1-acyl-PG by At1g78690p. Crude extracts (0.05 mg/ml) prepared from induced BLR(DE3)pLysS/pET15b or BLR(DE3)pLysS/At1g78690p were tested for the ability to acylate 32P-labeled 1-acyllyso-PE, or 1-acyllyso-PG using palmitoyl-CoA as the acyl donor in a 10-min reaction at 37 °C. The E. coli lysophospholipase L2, PldB (35–37), is known to catalyze the synthesis of acyl-PG from PG and lyso-PE or lyso-PG and may be responsible for the acyl-PG formed in these assays.
1-Acyllyso[32P]PG was similarly generated by PLA2 treatment of [32P]PG and tested as a substrate for AT1G78690. When 1-acyllyso-[32P]PG was tested as a substrate, the major product formed co-migrated with PG (Fig. 5).
This in vitro assay result was confirmed using [14C]palmitoyl-CoA as the acyl donor with unlabeled 1-acyllyso-PE and 1-acyllyso-PG as acyl acceptors (Fig. 6). When no acyl acceptor was included in the assay, a small amount of product that co-migrated with PE was formed in the presence of AT1G78690, probably from endogenous lyso-PE present in the membranes. When 1-acyllyso-PE was used as the acyl acceptor, a product that co-migrated was formed only in the presence of AT1G78690. No product that co-migrated with N-acyl-PE was detected.
FIGURE 6.
Acylation of 1-acyl-PE and 1-acyl-PG using 14C-labeled palmitoyl-CoA. Solubilized membranes (0.05 mg/ml) prepared from induced BLR(DE3)pLysS/pET15b or BLR(DE3)pLysS/At1g78690p were tested for acyltransferase activity using unlabeled 1-acyl-PE, 1-acyl-PG, or no acyl acceptor with 14C-labeled palmitoyl-CoA as the acyl donor in a 10-min reaction at 37 °C. [14C]palmitate released during the reaction by the action of thioesterase (47) present in the solubilized membranes is detected near the solvent front. A small amount of PE is formed in the absence of acyl acceptor presumably from lyso-PE present in the solubilized membranes. The product migrating below the PG in the presence of 1-acyllyso-PG may be lyso-PG formed by transacylation activity of AT1G78690.
When 1-acyllyso-PG was used as the acyl acceptor, a product that co-migrated with PG was formed. Interestingly, a product that migrated lower than PG on TLC was also formed. This may be the formation of 14C-labeled 1-acyllyso-PG or 14C-labeled 2-acyllyso-PG from low levels of transacylase or phospholipase activity associated with AT1G78690. In addition, a low amount of product that co-migrated with acyl-PG was formed.
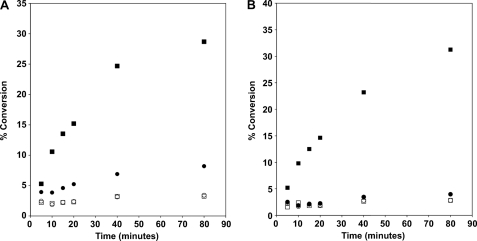
As shown in Fig. 7, the conversion of 1-acyllyso-PE to PE (A) or 1-acyllyso-PG to PG (B) was detected in solubilized membranes prepared from induced BLR(DE3)pLysS/pAt1g78690p (Fig. 7, solid circles and squares) but not BLR(DE3)pLysS/pET15b (Fig. 7, open circles and squares). The conversion was linear with time for approximately ∼30 min and was dependent on the inclusion of acyl acceptor (Fig. 7, squares versus circles). As mentioned above, low levels of PE and PG were formed in the absence of added 1-acyllyso-PE and 1-acyllyso-PG, presumably from low levels of lyso-GPLs present in the solubilized membranes. Under the in vitro assay conditions reported here, the specific activity of solubilized membranes for the formation of PE and PG from the respective 1-acyllyso-GPL was 2.3 ± 0.1 and 2.0 ± 0.1 nmol/min/mg, respectively.
FIGURE 7.
Time dependence of conversion of 1-acyllyso-PE and 1-acyllyso-PG to diacylated products. Solubilized membranes (0.05 mg/ml) prepared from induced BLR(DE3)pLysS/pET15b (open circles and squares) or BLR(DE3)pLysS/At1g78690p (closed circles and squares) were tested for acyltransferase activity using 1-acyllyso-PE (A), 1-acyllyso-PG (B) (closed circles and squares), or no acyl acceptor (open circles and squares) with 14C-labeled palmitoyl-CoA as the acyl donor.
Determining the Site of Acylation by AT1G78690
Having established that AT1G78690 acylates 1-acyllyso-PE and 1-acyllyso-PG, the site at which the acyl chain is placed was determined. 1-acyllyso-PE could be acylated at the sn-2 hydroxyl or the terminal amine of the headgroup. Despite the evidence presented above that N-acylated lipids do not accumulate in cells overexpressing AT1G78690, low levels of N-acylation may be possible in vitro. 1-Acyllyso-PG has three potential sites of acylation, the sn-2-, sn-2′-, and sn-3′-positions.
The site of acylation was determined using normal phase liquid chromatography-electrospray ionization quadrupole time-of-flight MS (LC/ESI-MS). An in vitro product was generated using 1-oleoyllyso-PE or 1-oleoyllyso-PG as the acyl acceptor, arachidonyl-CoA as the acyl-donor, and solubilized membranes generated from induced BLR(DE3)pLysS/pET15b or BLR(DE3)pLysS/At1g78690p. The use of arachidonyl-CoA as the acyl-donor allowed us to generate an in vitro product with a mass distinct from the endogenous PE and PG molecular species present in the solubilized membranes used as the enzyme source.
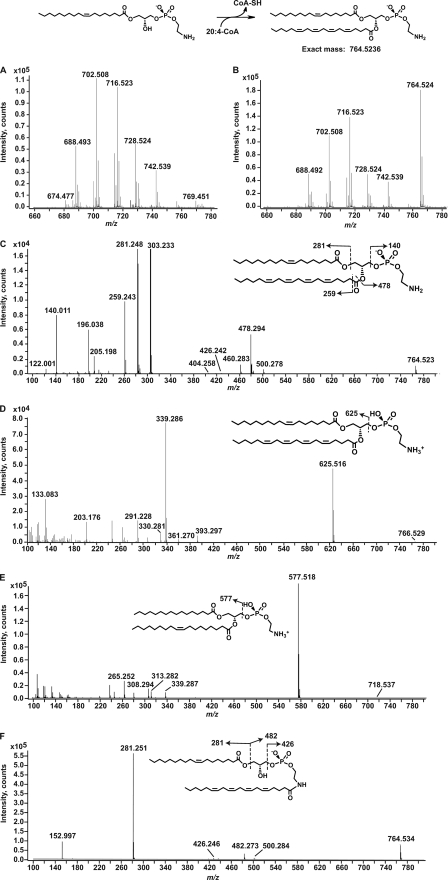
Fig. 8 shows the MS analysis of the in vitro product produced by AT1G78690 from 1-oleoyllyso-PE and arachidonoyl-CoA. Fig. 8, A and B, shows the negative ion LC/ESI-MS in the m/z 660–780 of the extracted lipids from the in vitro reaction mixture that utilized solubilized membranes from BLR(DE3)pLysS/pET15b (A) or BLR(DE3)pLysS/pAt1g78690p (B). The predicted product (m/z 764.5236) was generated only when solubilized membranes containing AT1G78690 were used as the enzyme source. The other major ions correspond to [M − H]− ions of endogenous PE molecular species that co-elute during normal phase chromatography. Ions corresponding to the transfer of arachidonate to endogenous molecular species of PE or PG (to generate N-acyl-PE or acyl-PG) were not detected, corroborating the results obtained using radiolabeled substrates.
FIGURE 8.
Determination of the site of acylation of 1-acyllyso-PE. Solubilized membranes prepared from induced BLR(DE3)pLysS/pET15b or BLR(DE3)pLysS/At1g78690p were incubated in an in vitro assay mixture using 1-oleoyllyso-PE as the acyl acceptor and arachidonyl-CoA as the acyl donor as described under “Experimental Procedures.” The lipids from the reaction mixture were extracted and analyzed using LC/ESI-MS and MS/MS. A, negative ion LC/ESI-MS from m/z 660 to 780 of lipids extracted from the reaction of solubilized membranes from BLR(DE3)pLysS/pET15b. B, negative ion ESI-MS from m/z 660 to 780 of lipids extracted from the reaction of solubilized membranes from BLR(DE3)pLysS/pAt1g78690p. The mass spectra in A and B are of the material eluting between minutes 22.0 and 25.0 of the normal phase chromatography. C, negative ion MS/MS of the in vitro product at m/z 764.5. D, positive ion MS/MS of the in vitro product at m/z 766.5. E, positive ion MS/MS of 16:0, 18:1 PE standard. F, positive ion MS/MS of N-arachidonyl 18:1 lyso-PE. The insets show the major product ions predicted from each precursor ion.
Negative ion MS/MS of the ion at m/z 764.5 is shown in Fig. 8C. The product ions generated are consistent with the acylation of the sn-2-position of 1-oleoyllyso-PE. The product ions at m/z 196.038 and 140.011 are characteristic of PE and correspond to glycerophosphoethanolamine and phosphoethanolamine, respectively (29, 30). The product ions corresponding to oleate (18:1, m/z 281.248) and arachidonate (20:4, m/z 303.233) are clearly observed. The presence of the arachidonate product ion strongly indicates that this fatty acyl chain was attached to the precursor ion via an ester linkage to the sn-2-position, the only available site of O-acylation. The arachidonate product ion is not detected in MS/MS analysis of N-arachidonyl lyso-PE (Fig. 8F), where the arachidonyl chain is attached via an amide linkage to the terminal amine. In Fig. 8C, the product ions at m/z 478.294 and 460.283 correspond to the loss of the arachidonate as a ketene (RCH=O) and a free acid (RCOOH), respectively (31). The product ion at m/z 500.278 corresponds to the loss of the 18:1 acyl chain as a ketene (RCH=O). The minor product ion at m/z 426.242 corresponds to N-arachidonylphosphoethanolamine (Fig. 8F, inset) (4). This may indicate that the in vitro product is N-acylated; however, negative ion MS/MS analysis of 16:0, 18:1 PE standard also yielded a similar minor product ion (supplemental Fig. 6, A and B), indicating that the formation of the product ion at m/z 426.242 is formed during the gas phase collision-induced dissociation of PE acylated at the sn-1- and sn-2-positions.
Positive ion MS/MS analysis of the in vitro product (m/z 766.5; Fig. 8D) shows the formation of a prominent product ion at m/z 625.516 that corresponds to the loss of phosphoethanolamine (Fig. 8D, inset). This product ion was consistent with the arachidonate added to the sn-2-position of 1-oleoyllyso-PE. The corresponding product ion (m/z 577.518) was generated from positive ion MS/MS analysis of standard 16:0, 18:1 PE (Fig. 8E). The corresponding product ion was not detected in positive ion MS/MS analysis of lyso-N-acyl-PE generated by PLA2 treatment of standard N-arachidonyldioleoyl-PE (supplemental Fig. 6C). The product ion at m/z 339.286 and 361.270 (Fig. 8D) corresponds to loss of arachidonate and oleate, respectively, as a fatty acid from the product ion at m/z 625. The product ions at m/z 330.281 and 308.291 correspond to [arachidonylethanolamine − H2O + H]+ and [oleoylethanolamine − H2O + H]+. The corresponding product ion, [oleoylethanolamine − H2O + H]+ at m/z 308.294, is also detected for the standard 16:0, 18:1 PE (Fig. 8E). This indicates that these N-acyl product ions are formed during gas phase collision-induced dissociation of sn-1-, sn-2-acylated PE as seen previously in the negative ion MS/MS analysis. Taken together, these data suggest that the 20:4 acyl chain is transferred to the sn-2-position and not the terminal amine of the headgroup.
A similar approach was used to determine the site AT1G78690 acylates when 1-oleoyllyso-PG is used as a substrate. Fig. 9, A and B, shows the negative ion ESI-MS in the m/z 700–800 of the extracted lipids from the in vitro reaction mixture that utilized 1-oleoyllyso-PG, arachidonoyl-CoA, and solubilized membranes from BLR(DE3)pLysS/pET15b (A) or BLR(DE3)pLysS/pAt1g78690p (B). The predicted product (m/z 795.5182) was generated only when solubilized membranes containing AT1G78690 were used as the enzyme source. The other major ions correspond to [M − H]− ions of endogenous PG molecular species (29, 31) that co-elute during normal phase chromatography.
FIGURE 9.
Determination of the site of acylation of 1-acyllyso-PG. Solubilized membranes prepared from induced BLR(DE3)pLysS/pET15b or BLR(DE3)pLysS/At1g78690p were incubated in an in vitro assay using 1-oleoyllyso-PG as the acyl acceptor and arachidonyl-CoA as the acyl donor as described under “Experimental Procedures.” The lipids from the reaction mixture were extracted and analyzed using ESI-MS. A, negative ion ESI-MS from m/z 700 to 800 of lipids extracted from the reaction of solubilized membranes from BLR(DE3)pLysS/pET15b. B, negative ion ESI-MS from m/z 700 to 800 of lipids extracted from the reaction of solubilized membranes from BLR(DE3)pLysS/pAt1g78690p. The mass spectra in A and B are of the material eluting between minutes 16.5 and 19.5 of the normal phase chromatography. C, negative ion MS/MS of the in vitro product at m/z 795.5. D, positive ion MS/MS of the in vitro product at m/z 797.5. E, positive ion MS/MS of 16:0, 18:1 PG standard. F, positive ion MS/MS of 3,1′-BMP. The insets show the major product ions predicted from each predicted precursor ion.
Negative ion MS/MS of the ion at m/z 795.518 is shown in Fig. 9C. The product ions generated are consistent with the acylation of the sn-2-position of oleoyllyso-PG. The product ion at m/z 721.482 corresponds to the loss of the glycerol headgroup and is highly suggestive of O-acylation of the sn-2-position and not acylation of the sn-2′ or sn-3′ positions. The product ions at m/z 509.289 and 491.278 correspond to the loss of the arachidonate as a ketene (RCH=O) and the free fatty acid (RCOOH), respectively. The product ion at m/z 531.273 corresponds to the loss of the 18:1 acyl chain. The product ions at m/z 417.241 and 439.226 correspond to loss of the glycerol headgroup and the 18:1 and 20:4, respectively, as free fatty acids.
Positive ion MS/MS analysis of the in vitro product (m/z 797.519, Fig. 9D) shows the formation of a prominent product ion at m/z 625.501 that corresponds to the loss of the glycerol headgroup. As with the PE in vitro product, this product ion is consistent with acylation of the sn-2-position by AT1G78690. Fig. 9E shows that the corresponding product ion (m/z 577.518) is the major product generated from positive ion MS/MS analysis of 16:0, 18:1 PG standard. The corresponding product ion was not detected in positive ion MS/MS analysis of 3,1′-BMP (Fig. 9F), strongly suggesting that AT1G78690 acylates the sn-2-position of 1-oleoyllyso-PG to form PG.
DISCUSSION
In this work, we investigated the biochemical function of the enzyme encoded by the A. thaliana gene AT1G78690 when it is ectopically expressed in E. coli. We have shown that the A. thaliana enzyme AT1G78690, when overexpressed in E. coli, promotes the accumulation of acyl-PG, not N-acyl-PE (20), in lipid extracts. Ions corresponding to acyl-PG clearly accumulate in lipid extracts prepared from cells overexpressing AT1G78690. The odd integer mass of those [M − H]− ions is inconsistent with their previous identification as [M − H]− ions of N-acyl-PE (20). In addition, the MS/MS of the major species does not yield acyl chains or product ions that are consistent with N-acyl-PE. MS and MS/MS analysis of the purified accumulating lipid corroborates this finding.
Previous work has suggested that N-acylated lipids accumulate when AT1G78690 is overexpressed. Faure et al. (20) suggested N-acyl-PE accumulation based on MS analysis and phospholipase D treatment of the accumulating lipid. In our study, the major product formed by the phospholipase D treatment of the isolated accumulating lipid co-migrates with MAG in a TLC system where MAG and NAE have different Rf values.
Base hydrolysis of in vivo 32P-labeled lipids strongly suggests that there is little to no accumulation of N-acylated lipids when AT1G78690 is overexpressed in E. coli in our hands. Recently, lipid extracts from E. coli overexpressing AT1G78690 were shown by Guo et al. (39) to have increased levels of N-acyl-PE as assessed by LC-MS-based detection of N-acylglycerophosphates following base deacylation with methyl amine. Triple quadrupole mass spectrometry in multiple reaction-monitoring mode was used to detect transitions specific for the N-acylglycerophosphate products of base hydrolysis of N-acyl-PE and thereby quantify N-acyl-PE in lipid extracts. Our base hydrolysis data of in vivo 32P-labeled cells indicates that N-acylated lipids do not accumulate in E. coli expressing AT1G78690; however, the multiple reaction-monitoring mass spectrometry system used by Guo et al. (39) is probably more sensitive. An ion consistent with N-acyl-PE (m/z 952.8) is detected in our ESI-MS analysis of the accumulating lipid. This may indicate that N-acyl-PE (or other N-acylated lipid) is somewhat increased in cells overexpressing AT1G78690 but may not be detected in our analyses. The method of Guo et al. (39) would not detect the increases in acyl-PG (40) that are seen in our data.
The growth conditions we used to induce expression of AT1G78690 differ from those used previously (20, 39). Guo et al. (39) used modified growth conditions to optimize the levels of N-acyl-PE (39). In our hands, a 3-h induction of BLR(DE3)pLysS/pAt1g78690p with 0.5 mm IPTG at 37 °C consistently yielded lipid extracts with increased levels of acyl-PG and protein extracts with overexpressed lyso-GPL transferase activity. Although not investigated, perhaps the extended induction used by Guo et al. (39) leads to increased levels of N-acyl-PE either as a long term consequence of increased levels of acyl-PG or the presence of this lyso-GPL acyltransferase activity in vivo.
We have not been able to detect AT1G78690-catalyzed acylation of PE using either 32P-labeled PE or [14C]palmitoyl-CoA as was previously reported (20). Careful following of the growth conditions, membrane preparation, and in vitro assay conditions reported by Faure et al. (20) did not yield any detectable N-acyl-PE synthase activity in our hands. To determine the enzymatic function of AT1G78690, we utilized growth conditions, protein extract preparation techniques, and in vitro assay conditions previously shown to successfully express and detect the activity of membrane-associated lipid metabolic enzymes (40, 41). In particular, the in vitro assay conditions that we used are based on inclusion of non-ionic detergents commonly used in assays for surface dilution kinetic analysis of enzymes that utilize mixed micellar substrates (42).
We have clear evidence that AT1G78690 efficiently acylates lyso-GPLs, such as 1-acyllyso-PE and 1-acyllyso-PG. The specific activity of AT1G78690 for the acylation of 1-acyllyso-PE and 1-acyllyso-PG in solubilized membranes is ∼1000-fold higher than that reported previously for the acylation of PE (20). This activity is clearly dependent on both substrates. The formation of diacylated product is formed when the labeled substrate is the lyso-GPL (acyl acceptor) or the acyl-CoA (acyl donor).
Preliminary data suggest that AT1G78690 does not have a stringent preference for the headgroup 1-acyllyso-GPL substrate. 1-Acyllyso-PE and 1-acyllyso-PG work equally well as substrates. 2-Acyllyso-GPLs are also potential substrates that need to be tested. In E. coli, 2-acyllyso-GPLs are generated when the sn-1 acyl chain of PE or PG is transferred to lipoproteins (43) or lipid A (44), making these relevant substrates for the AT1G78690 in E. coli.
AT1G78690 may utilize other acyl donors or acyl acceptors or possess additional enzymatic activities than defined in this work. For example, evidence of transacylation activity is suggested when 1-acyllyso-PG is used as a substrate. With higher concentrations of enzyme, a labeled product is generated that co-migrates with lyso-PG. AT1G78690 may be able to transfer the sn-2 acyl chain to sn-glycero-3-phospho-3′-glycerol. Alternatively, AT1G78690 might be able to hydrolyze the sn-1 acyl chain to generate 14C-labeled 2-acyllyso-PG. AT1G78690 may also hydrolyze acyl-CoA to yield a free fatty acid and CoA. Free fatty acids are generated in in vitro reactions, which we attribute to the two known thioesterases of E. coli, tesA and tesB, soluble enzymes present in E. coli protein extracts (45). Highly purified AT1G78690 will be required to identify its enzymatic activities and determine the substrate specificity.
Faure et al. (20) first investigated AT1G78690 because of its homology to YPR140wp, the yeast homolog of tafazzin (21). YPR140wp has been reported to be a membrane-associated acyl-CoA-independent lysophosphatidylcholine acyltransferase (21), consistent, in part, with our report of lyso-GPL acyltransferase activity for AT1G78690. Drosophila tafazzin has been shown to be GPL-lyso-GPL transacylase with specificity for transferring a linoleoyl residue from PC to monolysocardiolipin (46). Despite the fact that Drosophila tafazzin is ∼50% homologous to AT1G78690, tafazzin does not utilize acyl-CoA as an acyl donor. Future research may yield insights into the structural motifs that distinguish between acyl transfer from GPL (or lyso-GPL) acyl donors and phosphopantetheine-linked acyl donors (acyl-CoA or acyl-acyl carrier protein) in acyltransferases.
Although our data strongly suggest that the major site of acylation is the sn-2 hydroxyl of 1-acyllyso-PE, we cannot completely rule out the possibility that AT1G78690 possesses some low level of NAT activity. Negative ion MS/MS analysis of the in vitro product generated from 1-acyllyso-PE yields a low level of a product ion (m/z 426) that is consistent with N-acylation. Although the corresponding product ion (m/z 404) is also observed in MS/MS analysis of the standard PE compound, the product ion at m/z 426 could also be the result of low levels of N-acylated 1-acyllyso-PE.
Our data also strongly suggest that AT1G78690 catalyzes the acylation of the sn-2 hydroxyl of 1-acyllyso-PG. The positive ion MS/MS analysis of the in vitro product generated from 1-acyllyso-PG yields a product ion possible only when the sn-2-position is acylated. However, as with 1-acyllyso-PE, with our current data, we cannot completely rule out acylation at the sn-2′- or sn-3′-positions at some level.
Lyso-GPL acyltransferases play an important role in Land's cycle of acyl chain remodeling (28, 38, 47). With the identification of AT1G78690 as a lyso-GPL acyltransferase, its role in relation to the other lyso-GPL acyltransferases of A. thaliana (38) needs to be determined.
Monoacylated GPLs are acylated by AT1G78690 to form diacylated products in vitro, yet a triacylated lipid, acyl-PG, accumulates in vivo in E. coli overexpressing AT1G78690. Careful determination of the substrate specificity of this acyltransferase may illuminate the mechanism by which acyl-PG accumulates in cells overexpressing AT1G78690 and shed light on the complex interplay between lyso-GPL metabolism and headgroup acylated GPL metabolism in E. coli.
Supplementary Material
Acknowledgments
We thank Denis Coulon of the Laboratoire de Biogenèse Membranaire, Université Victor Segalen Bordeaux, France for supplying the plasmid containing At1g78690p. Christian Raetz provided helpful discussions and use of the LIPID MAPS mass spectrometry facility at Duke University Medical Center.
This work was supported, in whole or in part, by National Institutes of Health LIPID MAPS Large Scale Collaborative Grant GM 069338 (to Christian R.H. Raetz). This work was also supported by National Science Foundation Major Research Instrumentation Award 1039659 and Research Corporation for Science Advancement Cottrell College Science Single Investigator Award 7914.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6.
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- ESI-MS
- electrospray ionization quadrupole time-of-flight mass spectrometry
- NAE
- N-acylethanolamine
- GPL
- glycerophospholipid
- NAT
- N-acyltransferase
- HPTLC
- high performance thin layer chromatography
- 3
- 1′-BMP, sn-(3-oleoyl-2-hydroxy)-glycerol-1-phospho-sn-3′-(1′-oleoyl-2′-hydroxy)-glycerol
- IPTG
- isopropyl β-d-1-thiogalactopyranoside
- PLA2
- phospholipase A2
- MAG
- monoacylglycerol.
REFERENCES
- 1. Merkel O., Schmid P. C., Paltauf F., Schmid H. H. (2005) Biochim. Biophys. Acta 1734, 215–219 [DOI] [PubMed] [Google Scholar]
- 2. Astarita G., Ahmed F., Piomelli D. (2008) J. Lipid Res. 49, 48–57 [DOI] [PubMed] [Google Scholar]
- 3. Chapman K. D., Moore T. S., Jr. (1993) Plant Physiol. 102, 761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mileykovskaya E., Ryan A. C., Mo X., Lin C. C., Khalaf K. I., Dowhan W., Garrett T. A. (2009) J. Biol. Chem. 284, 2990–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rawyler A. J., Braendle R. A. (2001) Plant Physiol. 127, 240–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holmbäck J., Karlsson A. A., Arnoldsson K. C. (2001) Lipids 36, 153–165 [DOI] [PubMed] [Google Scholar]
- 7. Coulon D., Faure L., Salmon M., Wattelet V., Bessoule J. J. (2011) J. Biochimie, in press [DOI] [PubMed] [Google Scholar]
- 8. Chapman K. D. (2000) Chem. Phys. Lipids. 108, 221–229 [DOI] [PubMed] [Google Scholar]
- 9. Chapman K. D., Moore T. S., Jr. (1993) Arch. Biochem. Biophys. 301, 21–33 [DOI] [PubMed] [Google Scholar]
- 10. Kilaru A., Blancaflor E. B., Venables B. J., Tripathy S., Mysore K. S., Chapman K. D. (2007) Chem. Biodivers. 4, 1933–1955 [DOI] [PubMed] [Google Scholar]
- 11. Rodríguez de Fonseca F., Navarro M., Gómez R., Escuredo L., Nava F., Fu J., Murillo-Rodríguez E., Giuffrida A., LoVerme J., Gaetani S., Kathuria S., Gall C., Piomelli D. (2001) Nature 414, 209–212 [DOI] [PubMed] [Google Scholar]
- 12. Petersen G., Sørensen C., Schmid P. C., Artmann A., Tang-Christensen M., Hansen S. H., Larsen P. J., Schmid H. H., Hansen H. S. (2006) Biochim. Biophys. Acta 1761, 143–150 [DOI] [PubMed] [Google Scholar]
- 13. Ueda N., Tsuboi K., Uyama T. (2010) Biochim. Biophys. Acta 1801, 1274–1285 [DOI] [PubMed] [Google Scholar]
- 14. Schmid H. H. (2000) Chem. Phys. Lipids 108, 71–87 [DOI] [PubMed] [Google Scholar]
- 15. Cadas H., di Tomaso E., Piomelli D. (1997) J. Neurosci. 17, 1226–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Natarajan V., Reddy P. V., Schmid P. C., Schmid H. H. (1982) Biochim. Biophys. Acta 712, 342–355 [DOI] [PubMed] [Google Scholar]
- 17. Jin X. H., Okamoto Y., Morishita J., Tsuboi K., Tonai T., Ueda N. (2007) J. Biol. Chem. 282, 3614–3623 [DOI] [PubMed] [Google Scholar]
- 18. Jin X. H., Uyama T., Wang J., Okamoto Y., Tonai T., Ueda N. (2009) Biochim. Biophys. Acta 1791, 32–38 [DOI] [PubMed] [Google Scholar]
- 19. Chapman K. D., Sriparameswaran A. (1997) Plant Cell Physiol. 38, 1359–1367 [Google Scholar]
- 20. Faure L., Coulon D., Laroche-Traineau J., Le Guedard M., Schmitter J. M., Testet E., Lessire R., Bessoule J. J. (2009) J. Biol. Chem. 284, 18734–18741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Testet E., Laroche-Traineau J., Noubhani A., Coulon D., Bunoust O., Camougrand N., Manon S., Lessire R., Bessoule J. J. (2005) Biochem. J. 387, 617–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller J. R. (1972) Experiments in Molecular Genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 23. Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 24. Garrett T. A., Raetz C. R., Richardson T., Kordestani R., Son J. D., Rose R. L. (2009) J. Lipid Res. 50, 1589–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Imamura S., Horiuti Y. (1978) J. Biochem. 83, 677–680 [DOI] [PubMed] [Google Scholar]
- 26. Janssen M. J., van de Wiel W. A., Beiboer S. H., van Kampen M. D., Verheij H. M., Slotboom A. J., Egmond M. R. (1999) Protein Eng. 12, 497–503 [DOI] [PubMed] [Google Scholar]
- 27. Studier F. W., Moffatt B. A. (1986) J. Mol. Biol. 189, 113–130 [DOI] [PubMed] [Google Scholar]
- 28. Lands W. E. (2000) Biochim. Biophys. Acta 1483, 1–14 [DOI] [PubMed] [Google Scholar]
- 29. Pulfer M., Murphy R. C. (2003) Mass Spectrom. Rev. 22, 332–364 [DOI] [PubMed] [Google Scholar]
- 30. Hsu F. F., Turk J. (2000) J. Am. Soc. Mass Spectrom. 11, 892–899 [DOI] [PubMed] [Google Scholar]
- 31. Hsu F. F., Turk J. (2009) J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877, 2673–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsu F. F., Turk J., Shi Y., Groisman E. A. (2004) J. Am. Soc. Mass Spectrom. 15, 1–11 [DOI] [PubMed] [Google Scholar]
- 33. Wang A. Y., Cronan J. E., Jr. (1994) Mol. Microbiol. 11, 1009–1017 [DOI] [PubMed] [Google Scholar]
- 34. Garrett T. A., Que N. L., Raetz C. R. (1998) J. Biol. Chem. 273, 12457–12465 [DOI] [PubMed] [Google Scholar]
- 35. Karasawa K., Kudo I., Kobayashi T., Sa-Eki T., Inoue K., Nojima S. (1985) J. Biochem. 98, 1117–1125 [DOI] [PubMed] [Google Scholar]
- 36. Kobayashi T., Kudo I., Karasawa K., Mizushima H., Inoue K., Nojima S. (1985) J. Biochem. 98, 1017–1025 [DOI] [PubMed] [Google Scholar]
- 37. Kobayashi T., Homma H., Natori Y., Kudo I., Inoue K., Nojima S. (1984) J. Biochem. 96, 137–145 [DOI] [PubMed] [Google Scholar]
- 38. Stålberg K., Ståhl U., Stymne S., Ohlrogge J. (2009) BMC Plant Biol. 9, 60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo L., Amarnath V., Davies S. S. (2010) Anal. Biochem. 405, 236–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garrett T. A., Kadrmas J. L., Raetz C. R. (1997) J. Biol. Chem. 272, 21855–21864 [DOI] [PubMed] [Google Scholar]
- 41. Metzger L. E., 4th, Raetz C. R. (2009) Biochemistry 48, 11559–11571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carman G. M., Deems R. A., Dennis E. A. (1995) J. Biol. Chem. 270, 18711–18714 [DOI] [PubMed] [Google Scholar]
- 43. Gupta S. D., Gan K., Schmid M. B., Wu H. C. (1993) J. Biol. Chem. 268, 16551–16556 [PubMed] [Google Scholar]
- 44. Bishop R. E. (2005) Mol. Microbiol. 57, 900–912 [DOI] [PubMed] [Google Scholar]
- 45. Naggert J., Narasimhan M. L., DeVeaux L., Cho H., Randhawa Z. I., Cronan J. E., Jr., Green B. N., Smith S. (1991) J. Biol. Chem. 266, 11044–11050 [PubMed] [Google Scholar]
- 46. Xu Y., Malhotra A., Ren M., Schlame M. (2006) J. Biol. Chem. 281, 39217–39224 [DOI] [PubMed] [Google Scholar]
- 47. Shindou H., Shimizu T. (2009) J. Biol. Chem. 284, 1–5 [DOI] [PubMed] [Google Scholar]
- 48. Garrett T. A., Raetz C. R., Son J. D., Richardson T. D., Bartling C., Guan Z. (2011) Biochim. Biophys. Acta, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.