Abstract
Activation of G protein-coupled receptors at the cell surface leads to the activation or inhibition of intracellular effector enzymes, which include various Rho guanine nucleotide exchange factors (RhoGEFs). RhoGEFs activate small molecular weight GTPases at the plasma membrane (PM). Many of the known G protein-coupled receptor-regulated RhoGEFs are found in the cytoplasm of unstimulated cells, and PM recruitment is a critical aspect of their regulation. In contrast, p63RhoGEF, a Gαq-regulated RhoGEF, appears to be constitutively localized to the PM. The objective of this study was to determine the molecular basis for the localization of p63RhoGEF and the impact of its subcellular localization on its regulation by Gαq. Herein, we show that the pleckstrin homology domain of p63RhoGEF is not involved in its PM targeting. Instead, a conserved string of cysteines (Cys-23/25/26) at the N terminus of the enzyme is palmitoylated and required for membrane localization and full basal activity in cells. Conversion of these residues to serine relocates p63RhoGEF from the PM to the cytoplasm, diminishes its basal activity, and eliminates palmitoylation. The activity of palmitoylation-deficient p63RhoGEF can be rescued by targeting to the PM by fusion with tandem phospholipase C-δ1 pleckstrin homology domains or by co-expression with wild-type Gαq but not with palmitoylation-deficient Gαq. Our data suggest that p63RhoGEF is regulated chiefly through allosteric control by Gαq, as opposed to other known Gα-regulated RhoGEFs, which are instead sequestered in the cytoplasm, perhaps because of their high basal activity.
Keywords: G Protein-coupled Receptors (GPCR), Guanine Nucleotide Exchange Factor (GEF), Heterotrimeric G Proteins, Protein Palmitoylation, Rhoa, Galphaq, Plasma Membrane Recruitment, p63RhoGEF
Introduction
Rho GTPases act as molecular switches that mediate a wide variety of cellular processes, such as actin cytoskeletal organization, cell migration, cell cycle progression, and transcriptional control (1–3). They are activated by Rho guanine nucleotide exchange factors (RhoGEFs),3 which catalyze the exchange of GDP for GTP on the G protein (4, 5). The largest family of RhoGEFs is characterized by a Dbl homology (DH) domain that is almost always found immediately N-terminal to a pleckstrin homology (PH) domain. The DH domain forms the primary binding site for the GTPase, whereas the PH domain either modulates nucleotide exchange by the DH domain or has other functions.
Heterotrimeric G-protein α subunits are known to directly interact with and activate at least two classes of Dbl family RhoGEFs (6–8). Gα12/13 subunits bind to the regulator of G protein signaling (RGS) homology (RH) domain of RH domain-containing RhoGEFs (RH-RhoGEFs) such as leukemia-associated RhoGEF (LARG), PDZ-RhoGEF, and p115-RhoGEF (9–12). Gαq/11 subunits bind to the DH/PH core of another subfamily of RhoGEFs that includes p63RhoGEF and the C-terminal DH/PH domains of Trio and Duet (13, 14). Because small GTPases are tethered to the plasma membrane (PM) via posttranslational prenylation of their C-terminal CAAX motifs (15), RhoGEFs must also be targeted to the PM in order to efficiently activate their substrates in living cells. Because Gα subunits are also lipid-modified and localized to the PM (16–18), one mechanism by which they can activate RhoGEFs is to recruit them to specific locations within the cell. A second mechanism is to directly bind and allosterically activate the RhoGEFs. The relative contribution of these mechanisms to the regulation of Gα-regulated RhoGEFs in vivo is poorly understood.
In resting cells, RH-RhoGEFs are distributed throughout the cytoplasm of unstimulated cells and translocate to the PM upon activation of Gα12 or Gα13 subunits (19–21). Direct activation of these RhoGEFs has been difficult to demonstrate in vitro with the exception of p115RhoGEF (22), suggesting that the dominant mechanism by which Gα12/13 subunits activate RH-RhoGEFs is via membrane recruitment. This would be consistent with their high basal activity (23) and the fact that Gα12/13 subunits bind primarily to the RH domain, which is located ∼200 amino acids N-terminal to the catalytic DH/PH tandem domains (24). In contrast, p63RhoGEF seems to be constitutively localized to the PM (25), has relatively low basal activity, and is readily activated by Gαq in vitro through direct interactions with its catalytic core (13). Thus, it seems that p63RhoGEF and, by extension, the homologous domains in Trio and Duet are regulated by Gαq primarily through an allosteric mechanism that involves release of PH domain-mediated autoinhibition (25). However, in cells, it is also possible that Gαq recruits p63RhoGEF to specific locales on the PM (26) or repositions the RhoGEF at the membrane so that it can more productively interact with its substrate RhoA.
Herein we demonstrate that p63RhoGEF is anchored to the PM via palmitoylation at a cluster of conserved cysteines at the N terminus of the protein. Disruption of these palmitoylation sites not only redistributes p63RhoGEF to the cytoplasm but also greatly reduces its basal activity in cells. Targeting palmitoylation-defective mutants of p63RhoGEF to the PM by fusion with PLC-δ1 PH domains restores their basal activity. We show that wild-type Gαq, but not its palmitoylation-deficient (palm−) C9S/C10S mutant, can recruit palm− p63RhoGEF to the PM. Palm− Gαq is unable to activate p63RhoGEF in cells, indicating that Gαq must be targeted to the cell membrane to promote full activation of p63RhoGEF. Because wild-type Gαq can achieve levels of activation comparable with deletion of the p63RhoGEF PH domain, even in the case of palm− p63RhoGEF, the chief mechanism regulating p63RhoGEF activity seems to be allosteric and probably does not involve localization to specific locales on the membrane surface or reorientation of p63RhoGEF for more optimal interactions with RhoA.
EXPERIMENTAL PROCEDURES
Plasmids and Mutagenesis
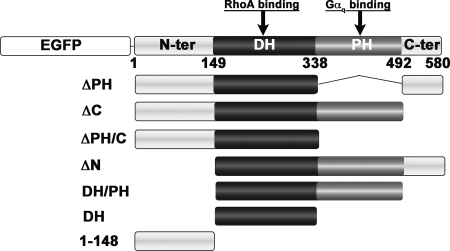
The cloning of p63RhoGEF into pEGFP-C1 (Clontech) was described previously (25). Vectors encoding all p63RhoGEF truncations were constructed by cloning the corresponding PCR fragment into pEGFP-C1 using the XhoI and HindIII restriction sites. The truncations correspond to residues 149–580 (ΔN), 1–492 (ΔC), 1–338/492–580 (ΔPH), 1–338 (ΔPH/C), 149–492 (DH/PH), 149–338 (DH), and N-terminal fragments corresponding to residues 1–148, 1–114, and 1–75. Cys to Ser mutations were introduced using QuikChange mutagenesis (Stratagene) and EGFP-p63RhoGEF as the template. For constructs of p63RhoGEF C-terminally fused to EGFP, we cloned PCR fragments into the pEGFP-N3 plasmid (Clontech) using the XhoI and HindIII restriction sites. The construction of EGFP-p63RhoGEF-2xPLCδ1PH was described previously (25), and this plasmid was used as template to create Cys to Ser mutations. For detection of palmitoylation, we used N-terminal c-Myc-tagged p63RhoGEF cloned into the pCMV-Tag3B plasmid (13) and created Cys to Ser mutants in this construct. Wild-type pCMV-Gαq, a gift from Dr. T. Kozasa (University of Illinois), was used as template to create the Gαq-C9S/C10S double mutant, and these two constructs were used as templates to generate wild-type and C9S/C10S Gαq fragments, respectively, for cloning into mRFP-C1 (Clontech). SRE.L firefly luciferase and pRL-thymidine kinase Renilla luciferase reporter plasmids were described previously (27). All plasmids were verified by DNA sequencing.
Cell Culture and Transfection
Human embryonic kidney 293 (HEK293) cells were grown to 60–80% confluence in high glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) in the presence of 100 units/ml penicillin and 100 μg/ml streptomycin and were incubated at 37 °C in a humidified atmosphere with 5% CO2. Cells were then switched to antibiotic-free medium before transfection using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
SRE.L Luciferase Reporter Assay
The reporter assay was conducted using the Dual-Luciferase reporter assay System (Promega) according to the manufacturer's protocol. In this assay, HEK293 cells were seeded into 96-well plates 24 h before transfection. In antibiotic-free medium, cells were co-transfected with the indicated plasmids (EGFP fusion constructs), pSRE.L, pRL-TK, and EGFP (to adjust total DNA amount to 240 ng/well). One day after transfection, cells were starved in DMEM containing 0.5% FBS and incubated for an additional 24 h. Cells were then washed with phosphate-buffered saline (PBS) and lysed with freshly diluted 1× Lysis Buffer (Promega). Lysed cells were transferred to a white polystyrene 96-well reading plate (Corning Costar) and assayed for luciferase activity. Luminescence was read on a Victor plate reader with dual injectors (PerkinElmer). Firefly activities were measured in triplicate for each transfection mix and then normalized to Renilla activities, and the results are reported as -fold activation over the basal level, corresponding to the activity of the empty EGFP vector.
Confocal Microscopy
HEK293 cells were plated on poly-d-lysine (Sigma)-precoated coverslips in 12-well plates. After attachment, cells were transfected with 250 ng/well of the indicated plasmids. 24 h after transfection, cells were washed with PBS and fixed with 4% paraformaldehyde. For localization studies in HEK293T, coverslips were directly mounted on glass slides using Vectashield Mounting Medium (Vector Laboratories). Images were captured using an Olympus FluoView 500 laser-scanning confocal microscope (Olympus America, Inc.) with a ×60 oil immersion objective (Nikon, Japan) and processed with Adobe Photoshop.
2-Bromopalmitate (2BP) Treatment
After transfection of HEK293 cells on coverslips, the medium was switched to fresh medium supplemented with 100 μm 2BP (Sigma) and incubated for at least 12 h. Treated HEK293 cells were then washed with PBS and fixed with 4% paraformaldehyde before analysis by confocal microscopy.
Detection of Palmitoylation Using Cu(I)-catalyzed Azide-Alkyne Cycloaddition Reaction (Click Chemistry)
HEK293 cells were transfected with Myc-tagged wild-type or C23S/C25S/C26S p63RhoGEF using Effectene (Qiagen) according to the manufacturer's instructions and were cultured in DMEM with 10% FBS for 42 h. Cells were then incubated in fresh medium supplemented with 100 μm 17-octadecynoic acid (17-ODYA) or palmitic acid (PA) for 6 h. Cells were washed with PBS and lysed in 20 mm Hepes-NaOH, pH 7.4, 100 mm NaCl, 3 mm MgCl2, and 1% Nonidet P-40 buffer supplemented with protease inhibitors (3 μg/ml leupeptin and 1 mm phenylmethylsulfonyl fluoride). Cleared lysates were immunoprecipitated with an anti-Myc monoclonal antibody (ABM) and Protein G-Sepharose (GE Healthcare) for 4 h. The resins were washed three times with lysis buffer and suspended in 94 μl of PBS and 6 μl of freshly premixed click chemistry reagent (final 10 μm Alexa Fluor® 488-azide (Invitrogen), 1 mm tris(2-carboxyethyl)phosphine, 100 μm tris-(benzyltriazolylmethyl)amine, and 1 mm CuSO4) (28, 29). After a 1-h incubation at room temperature, the resins were washed three times with PBS containing 1% Nonidet P-40 and treated with sample buffer for SDS-PAGE. Alexa488-labeled proteins were detected by in-gel fluorescence.
Data Analysis
SRE.L luciferase activities are representative of at least three independent experiments, each performed in triplicate, and are presented as mean ± S.D. Data are compared by two-way analysis of variance with Bonferroni post-tests, using GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA).
RESULTS
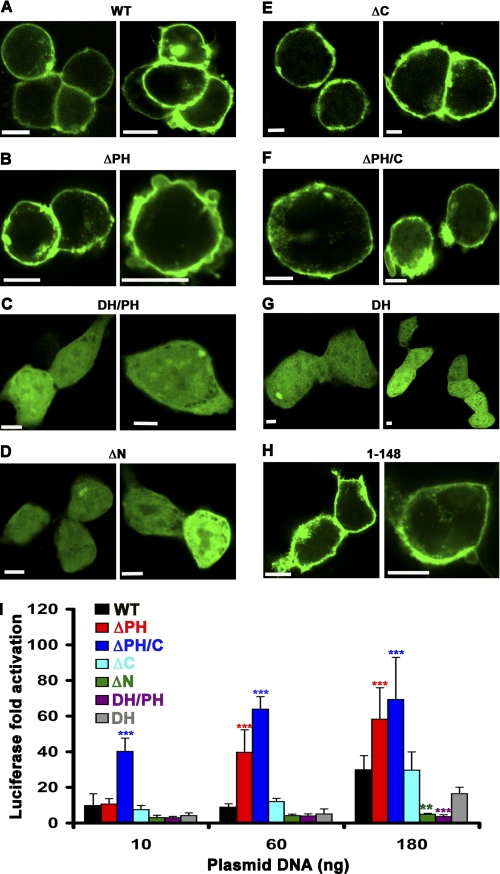
To identify the region responsible for targeting p63RhoGEF to the PM (Fig. 2A), we designed a series of truncation and deletion mutants with an N-terminal EGFP fusion (Fig. 1) and examined their subcellular localization by confocal microscopy. Although PH domains are widely recognized for their ability to interact with phospholipid headgroups (30, 31), deletion of the PH domain did not disrupt PM localization of p63RhoGEF (Fig. 2B). Because the EGFP-p63RhoGEF DH/PH truncation (residues 149–492) was cytoplasmic (Fig. 2C), we individually deleted the N- and C-terminal extensions of the DH/PH tandem domains. Deletion of the N-terminal region (residues 1–148) clearly redistributed p63RhoGEF from the PM to the cytoplasm (Fig. 2D), whereas deletion of the C-terminal region (residues 493–580) had no effect (Fig. 2E). The N-terminal region (residues 1–148) was able to localize the DH domain (residues 149–338) to the PM (Fig. 2F), which on its own was cytoplasmic (Fig. 2G) and also targeted EGFP to the PM (Fig. 2H). Together these data show that the N-terminal region is necessary and sufficient for PM targeting of p63RhoGEF. Next, we determined if there is a correlation between the subcellular localization of p63RhoGEF and its activity in cells (Fig. 2I). Both ΔN and DH/PH, which localized to the cytoplasm, lost most of their ability to activate RhoA in cells. Likewise, the basal activity of the cytoplasmic DH domain was less than that of the full-length enzyme and either ΔPH or ΔPH/C, despite the loss of the autoinhibitory PH domain. In contrast, PM-localized ΔC retained wild-type basal activity, and PM-localized ΔPH and ΔPH/C had the highest activity of all, consistent with loss of the PH domain. Thus, there are motifs within the N-terminal region that are responsible for PM localization and enhance basal activity in cells.
FIGURE 2.
The N-terminal extension of p63RhoGEF is necessary and sufficient for PM association and for high basal activity in cells. A–H, confocal microscopy images of HEK293 cells transfected with 250 ng of DNA encoding the indicated EGFP-p63RhoGEF variant. A, wild-type p63RhoGEF (WT); B, ΔPH; C, DH/PH; D, ΔN; E, ΔC; F, ΔPH/C; G, DH; H, 1–148. Only variants that contain the N-terminal 148 amino acids of p63RhoGEF are localized to the PM. Data shown are representative of 3–5 independent experiments. Scale bar, 10 μm. I, membrane localization correlates with high basal SRE activity in HEK293 cells. EGFP-p63RhoGEF-induced SRE activity is reported as the -fold increase of normalized luciferase over that of EGFP alone as a function of the increasing amount of transfecting EGFP-p63RhoGEF plasmid. ΔN and DH/PH, which are not PM-associated, have negligible activity. The DH domain, which is localized to the cytoplasm, shows increased activity compared with DH/PH due to the absence of autoinhibition imposed by the PH domain but still exhibits lower activity than wild-type p63RhoGEF. The highest activities are obtained for ΔPH and ΔPH/C, presumably due to loss of PH domain-mediated inhibition combined with retention of membrane localization. The data shown are representative of four independent experiments with each data point measured in triplicate and reported as the mean ± S.D. (error bars). Asterisks indicate a significant difference from wild type (**, p < 0.01; ***, p < 0.001).
FIGURE 1.
Schematic representation of EGFP-p63RhoGEF variants used to test subcellular localization of p63RhoGEF and its ability to activate RhoA in HEK293 cells. p63RhoGEF is composed of central tandem DH and PH domains, bracketed by N-terminal (N-ter) and C-terminal (C-ter) extensions with relatively low sequence complexity. The DH domain is the catalytic center of the enzyme and represents the primary RhoA binding site, whereas the PH domain is the primary binding site of Gαq. For visualization with confocal microscopy, EGFP was fused to the N terminus of each p63RhoGEF variant (shown only for wild-type p63RhoGEF).
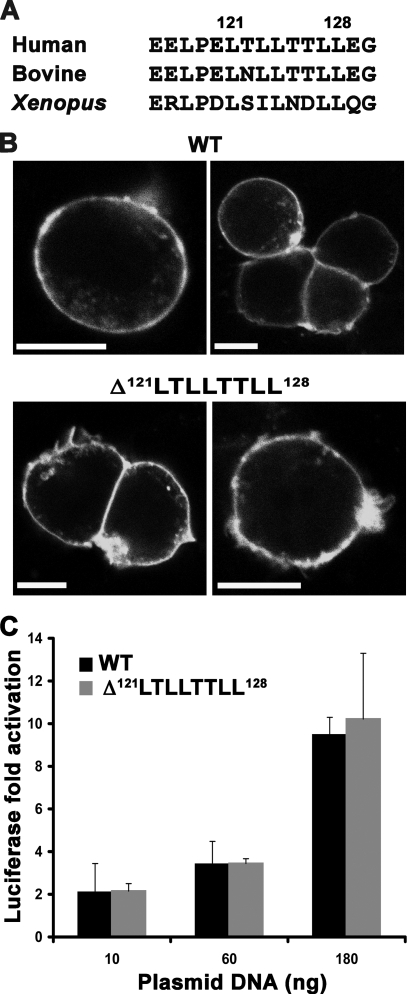
The most obvious conserved feature in the N-terminal region is an unusual hydrophobic 121LTLLTTLL128 cluster (Fig. 3A). For many proteins, di-Leu-based motifs are known to play a role in trafficking and PM targeting (32, 33). However, deletion of this motif in p63RhoGEF did not perturb PM localization (Fig. 3B) or RhoGEF activity in cells (Fig. 3C).
FIGURE 3.
A conserved hydrophobic di-Leu-based motif in the N terminus of p63RhoGEF is not required for PM association or for SRE activity in cells. A, amino acid sequence alignment showing conservation of the di-Leu-based hydrophobic motif in human (Q86VW2), bovine (XP_002687606.1), and Xenopus (NP_001123746.1) orthologs. B, deletion of the 121LTLLTTLL128 motif does not disrupt PM localization of EGFP-p63RhoGEF. Data shown are representative of four experiments. Scale bar, 10 μm. C, the Δ121LTLLTTLL128 variant also retains wild-type basal activity in cells. The data shown are representative of five independent experiments performed in triplicate. Error bars, S.D.
Because there were no other regions of strong sequence conservation among p63RhoGEF family members, we made additional N-terminal truncations of p63RhoGEF to further delimit the region(s) responsible for membrane targeting. Deletion of either the first 114 or the first 75 amino acids also resulted in loss of both PM localization and basal GEF activity (data not shown). These results indicate that the determinants for PM targeting reside within the first 75 amino acids of p63RhoGEF.
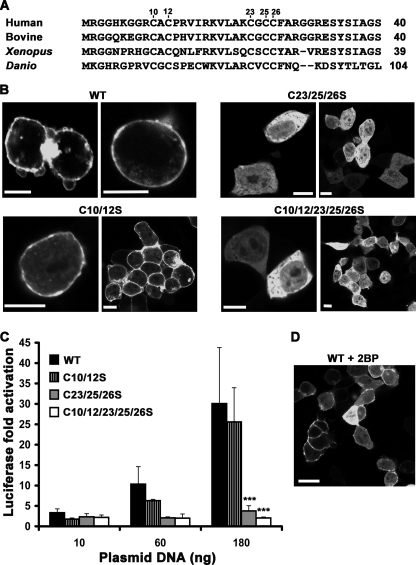
At the N terminus of p63RhoGEF, there are two Cys strings (Cys-10/12 and Cys-23/25/26) that are highly conserved in p63RhoGEF orthologs from most higher eukaryotes but not in Trio or Duet (Fig. 4A). Because these residues are candidates for palmitoylation, we mutated either Cys string to serine residues. The C10S/C12S double mutant had no detectable effect on PM localization as measured by confocal microscopy. However, the C23S/C25S/C26S triple mutant was cytoplasmic (Fig. 4B), indicating that the second Cys string is the primary determinant of PM targeting. Elimination of both Cys strings (C10S/C12S/C23S/C25S/C26S) also relocated EGFP-p63RhoGEF to the cytoplasm (Fig. 4B). Accordingly, the basal activities of C23S/C25S/C26S and C10S/C12S/C23S/C25S/C26S were significantly decreased (***, p < 0.001), whereas the C10S/C12S mutant had only slightly reduced activity (Fig. 4C). Consistent with the data obtained for the N-terminal EGFP fusions, C-terminal EGFP fusions of wild-type and C10S/C12S retained their PM association and basal activity, whereas the C23S/C25S/C26S mutant relocated to the cytoplasm and showed abolished basal activity (data not shown). Therefore, placement of the EGFP fusion does not affect PM localization or activity. To identify which individual Cys residues were most important for membrane targeting, we examined the localization of the C23S, C23S/C25S, C25S, and C26S mutants of EGFP-p63RhoGEF with confocal microscopy. The C23S, C25S/C26S, and C26S mutations disrupted PM association of EGFP-p63RhoGEF, whereas C25S disrupted PM localization of ∼40% of transfected cells (data not shown). These results suggest that all of the cysteine residues in the second Cys string contribute to PM localization of EGFP-p63RhoGEF but that Cys-23 and Cys-26 are the most dominant.
FIGURE 4.
A conserved N-terminal Cys string is required for PM association and optimal basal activity. A, alignment of the N-terminal region of p63RhoGEF orthologs reveals two conserved Cys strings. Accession numbers are Q86VW2 (human), XP_002687606.1 (bovine), NP_001123746.1 (Xenopus), and XP_698049.4 (Danio). B, subcellular distribution of EGFP-p63RhoGEF Cys string mutants. The C23S/C25S/C26S mutant (C23/25/26S) is found in the cytoplasm, whereas the C10S/C12S mutant (C10/12S) is retained at the PM. Mutation of both Cys strings to Ser also confers cytoplasmic localization. Data shown are representative of four experiments. Scale bar, 10 μm. C, SRE activities are abolished for the C23S/C25S/C26S and C10S/C12S/C23S/C25S/C26S mutants and reduced for the C10S/C12S mutant of p63RhoGEF compared with wild type. The data shown are representative of 3–5 independent experiments performed in triplicate. ***, significant difference at the p < 0.001 level compared with wild type. D, 2BP partially inhibits membrane targeting of p63RhoGEF. Confocal microscopy shows that about 50% of 2BP-treated cells exhibit cytoplasmic distribution of wild-type EGFP-p63RhoGEF. Data shown are representative of two experiments. Scale bar, 20 μm. Error bars, S.D.
To test if palmitoylation contributes to PM targeting of p63RhoGEF, we treated HEK293 cells overexpressing wild-type p63RhoGEF with 2BP, a commonly used inhibitor of palmitoylation (36). Consistent with the role of palmitoylation in promoting recruitment to the PM, p63RhoGEF partially redistributed to the cytoplasm in 2BP-treated cells (Fig. 4D). We therefore hypothesized that palmitoylation of the Cys-23/25/26 string is responsible for PM localization and that this localization is important for p63RhoGEF basal activity.
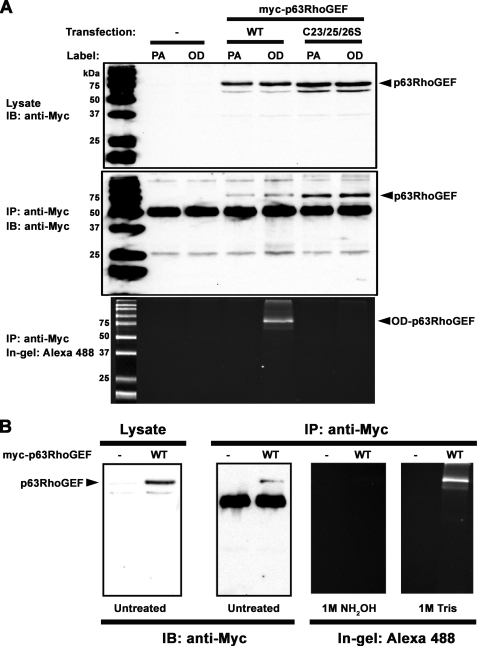
To unambiguously confirm that p63RhoGEF is palmitoylated, we performed a cell-based palmitoylation assay by testing the ability of p63RhoGEF to incorporate the PA analog 17-ODYA, which can be detected using “click chemistry” on immunoprecipitated protein (28). Click chemistry is an efficient and selective Cu2+-catalyzed cycloaddition reaction between an alkyne group, in this case in 17-ODYA, and an azide, in this case in Alexa Fluor® 488-azide (28, 29). As opposed to its analog 17-ODYA, PA cannot be modified and is therefore not detectable via in-gel fluorescence of Alexa Fluor® 488. Similar to their equivalent EGFP fusions (Fig. 4C), wild-type Myc-p63RhoGEF, but not the C23S/C25S/C26S mutant, was able to activate RhoA in cells (data not shown). Both wild-type and C23S/C25S/C26S Myc-p63RhoGEF were similarly expressed (Fig. 5A, top), and both could be immunoprecipitated by anti-c-Myc antibody (Fig. 5A, middle). Wild-type but not C23S/C25S/C26S Myc-p63RhoGEF could be modified with 17-ODYA, as visualized by in-gel fluorescence of samples resolved on an SDS denaturing gel (Fig. 5A, bottom). Labeling with Alexa Fluor® 488 was dependent on the addition of 17-ODYA but not of PA, which cannot be modified via click chemistry. Consistent with thioester linkage of 17-ODYA to Cys residues, pretreatment of the gel with 1 m hydroxylamine resulted in loss of fluorescence from wild-type Myc-p63RhoGEF. In contrast, pretreatment of the gel with 1 m Tris did not prevent detection of 17-ODYA incorporated into wild-type Myc-p63RhoGEF (Fig. 5B). The fact that 17-ODYA incorporation was completely eliminated in the C23S/C25S/C26S mutant (Fig. 5A, bottom) also indicates that the Cys-10/12 string does not represent a significant palmitoylation site, consistent with PM localization of p63RhoGEF-C10S/C12S (Fig. 4B). Thus, we conclude that p63RhoGEF is palmitoylated in cells at Cys-23, Cys-25, and/or Cys-26, and that modification with palmitate is responsible for its PM association.
FIGURE 5.
p63RhoGEF is palmitoylated at Cys-23/25/26 in cells. A, HEK293 cells transfected with either wild-type (WT), C23S/C25S/C26S (C23/25/26S), or no (control) Myc-p63RhoGEF were incubated in medium containing 100 μm PA or 100 μm 17-ODYA (OD) for 6 h. Shown are immunoblots (IB) of lysates (top) and immunoprecipitates (IP) (middle) using an anti-Myc antibody showing expression and pull-down of WT and C23S/C25S/C26S, respectively. In-gel fluorescence detection of Alexa-488 in immunoprecipitated samples (bottom) shows a fluorescent band only for wild-type p63RhoGEF when labeled with OD (OD-p63RhoGEF). The data shown are representative of three independent experiments. B, confirmation of thioester linkage at Cys-23/25/26. HEK293 cells expressing Myc-p63RhoGEF were incubated in medium containing 100 μm 17-ODYA for 6 h. Lysates were immunoprecipitated with anti-Myc antibody, and 17-ODYA-labeled p63RhoGEF was detected with Alexa488-azide using click chemistry. Samples were resolved by SDS-PAGE on triplicate gels. One gel was soaked in 1 m Tris (pH 7.5), and another was soaked in 1 m NH2OH (pH 7.5) for 16 h before detection of in-gel fluorescence. The third gel was transferred to a nitrocellulose membrane for immunoblotting. The fluorescent band corresponding to 17-ODYA-labeled p63RhoGEF is eliminated upon treatment with 1 m NH2OH, pH 7.5, but unaffected by treatment with 1 m Tris, pH 7.5. The data shown are representative of two experiments.
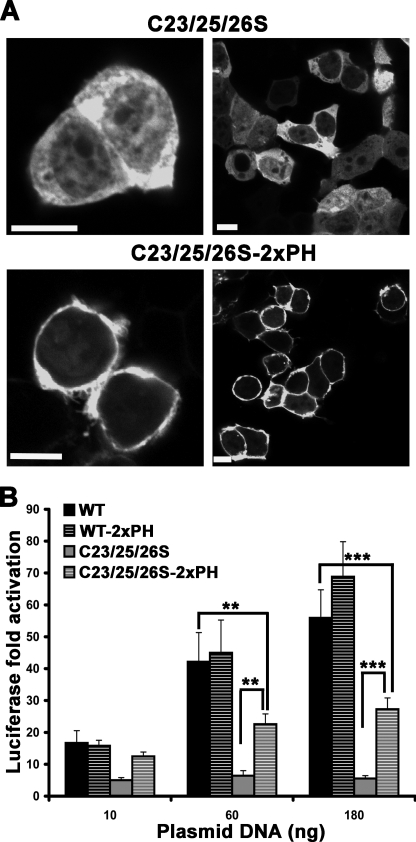
To determine whether loss of the ability to activate RhoA by C23S/C25S/C26S (palm−) p63RhoGEF is due to disruption of PM association or to loss of palmitoylation, we targeted palm− p63RhoGEF to the PM by fusion with two tandem PH domains of PLCδ1 and analyzed its subcellular localization and GEF activity. Our data show that this fusion was recruited to the PM (Fig. 6A) and that this recruitment partially rescued the activity of palm− p63RhoGEF (Fig. 6B). As expected, wild-type p63RhoGEF, which was already localized to the PM, did not show significant differences in activity upon fusion with the two tandem PH domains of PLCδ1 (2xPH) (Fig. 6B). Thus, PM targeting is sufficient for the observed basal activity of exogenously expressed p63RhoGEF in cells, and the loss of activity in palm− p63RhoGEF is due to the loss of PM association and not explicitly to the loss of palmitoylation.
FIGURE 6.
Targeting palm− p63RhoGEF to the PM rescues its activity. A, confocal images of HEK293 cells showing PM translocation of palm− EGFP-p63RhoGEF (C23/25/26S) when fused to two tandem PH domains from PLCδ1 (C23/25/26S-2xPH). Data shown are representative of three experiments. Scale bar, 10 μm. B, SRE activities of wild-type and palm− EGFP-p63RhoGEF in the absence (WT and C23/25/26S, respectively) or presence (WT-2xPH and C23/25/26S-2xPH, respectively) of the two tandem PH domains from PLCδ1. The data shown are representative of four independent experiments, each performed in triplicate. Significance between the indicated columns is indicated by asterisks (**, p < 0.01; ***, p < 0.001). Error bars, S.D.
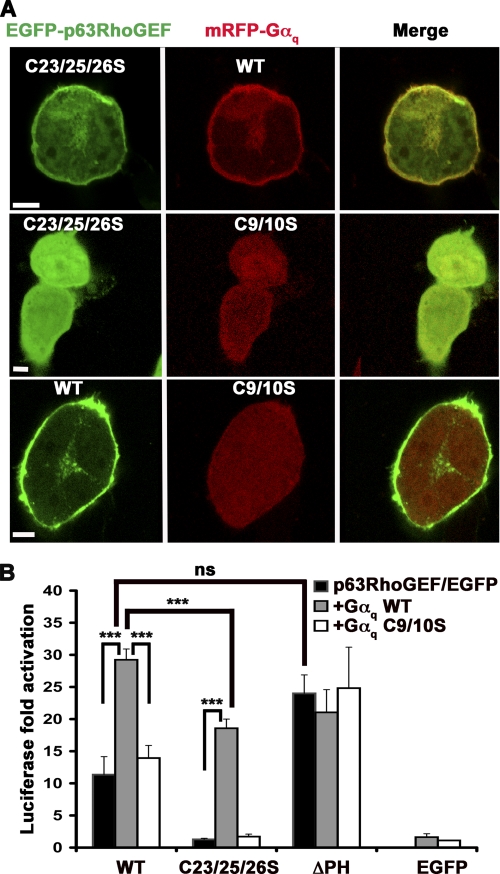
We next tested if the ability of p63RhoGEF to be activated by Gαq is affected by palmitoylation. Gαq binds to the PH domain of p63RhoGEF and relieves its autoinhibition in vitro (13, 25). Gαq itself is targeted to the PM via palmitoylation at Cys-9 and Cys-10, and mutation of these residues to Ser relocates Gαq-GFP to the cytoplasm (37, 38). Thus, exogenous expression of Gαq should be able to recruit the palm− mutant of p63RhoGEF to the PM and potentially rescue activity. Indeed, palm− EGFP-p63RhoGEF was partially recruited to the PM when co-expressed with wild-type mRFP-Gαq in HEK293 cells (Fig. 7A, top panels) but retained its cytoplasmic distribution when co-expressed with palm− mRFP-Gαq (Fig. 7A, middle panels), confirming that palmitoylation of Gαq can drive membrane targeting of p63RhoGEF. However, wild-type p63RhoGEF and palm− Gαq did not seem to co-localize (Fig. 7A, bottom panels). The SRE response of wild-type p63RhoGEF was activated ∼3-fold in a synergistic fashion by co-expression with wild-type Gαq. However, co-expression with palm− Gαq resulted in no increase over wild-type p63RhoGEF basal activity (Fig. 7B), consistent with their non-overlapping distribution in cells (Fig. 7A, bottom panels). Co-expression of wild-type Gαq with palm− p63RhoGEF resulted in a ∼20-fold increase in SRE activity but to lower levels than co-expression with wild-type p63RhoGEF (Fig. 7B). As expected, palm− Gαq was unable to activate palm− p63RhoGEF (Fig. 7B) because both were localized to the cytoplasm (Fig. 7A, middle panels). EGFP-p63RhoGEF-ΔPH, which was PM-localized (Fig. 2B) and unable to bind Gαq, exhibited elevated activity compared with wild type due to the loss of autoinhibition mediated by the PH domain and was unaffected by the co-expression of any Gαq variant (Fig. 7B). These results demonstrate that palmitoylated and membrane-localized Gαq is required for maximal stimulation of p63RhoGEF activity and that Gαq-mediated activation of p63RhoGEF exhibits higher fold activation when p63RhoGEF is cytoplasmic in its basal state, presumably because membrane targeting contributes to activation in addition to the known allosteric activation by Gαq. Our data also suggest that Gαq activates wild-type p63RhoGEF primarily via an allosteric mechanism, because the ΔPH construct exhibits comparable GEF activity under the same conditions in which we observe maximal Gαq stimulation. Based on these data, Gαq does not seem to recruit exogenously expressed p63RhoGEF to specific membrane locales or help reorient the enzyme at the membrane surface. Activation by Gαq is only manifested when Gαq is itself membrane-targeted, perhaps because membrane targeting is required for efficient activation of Gαq by Gq-coupled receptors (39).
FIGURE 7.
Gαq rescues the membrane localization and activity of palm− p63RhoGEF in a palmitoylation-dependent manner. A, confocal images of HEK293 cells co-transfected with EGFP-p63RhoGEF and mRFP-Gαq. Wild-type (WT) Gαq, but not Gαq-C9S/C10S (C9/10S), recruits C23S/C25S/C26S p63RhoGEF (C23/25/26S) to the PM, whereas wild-type p63RhoGEF does not co-localize with Gαq-C9/10S. Scale bar, 20 μm. B, SRE activities of either EGFP-p63RhoGEF variants or EGFP alone when co-expressed in the presence or absence of pCMV-Gαq. Gαq-WT significantly activates both the WT and C23S/C25S/C26S variants of p63RhoGEF. In contrast, Gαq-C9S/C10S is unable to efficiently activate any of the p63RhoGEF variants. Co-expressed Gαq variants have no effect on the ΔPH variant, which is unable to bind Gαq. The data shown are representative of four experiments performed in triplicate. ***, significant difference between the indicated columns (p < 0.001). Error bars, S.D. ns, not significant.
DISCUSSION
Heterotrimeric G-protein-regulated RhoGEFs represent one link between activated GPCRs and monomeric small GTPases. Tight regulation of these RhoGEFs is required to control the activation/deactivation balance of their signaling pathways. Proper localization of heterotrimeric G-protein-regulated RhoGEFs with respect to their substrate Rho GTPases is one potential layer of regulation. RH-RhoGEFs show significant basal activity and perhaps as a consequence are sequestered away from the PM in their basal state. Thus, a large component of their activation by Gα12/13 seems to consist of facilitating their membrane recruitment. This would be consistent with the fact that direct activation has been difficult to demonstrate in vitro for the RH-RhoGEF family members with the exception of p115RhoGEF (22). Accordingly, little (40) to no (27) synergy in the SRE response has been reported for co-expression of Gα12/13 and RH-RhoGEFs (19, 27). This is presumably because Gα12/13 expression alone generates a robust SRE response from endogenous RH-RhoGEF proteins found in cultured cells. On the other hand, autoinhibition of p63RhoGEF by its PH domain keeps it in a relatively quiescent state, and thus, from a regulatory standpoint, it matters less whether it is constitutively co-localized with its substrate at the PM. Activation by Gαq, which is itself localized at the PM, could consist of multiple events, including binding to and relieving autoinhibition imposed by the PH domain, recruitment to specific membrane locales, reorientation of the RhoGEF, or a combination. The homologous C-terminal RhoGEF domains of Trio and Duet are also activated by Gαq, presumably through a similar mechanism (13, 14, 41). However, because these proteins lack an obvious equivalent to the Cys string found in the N terminus of p63RhoGEF, they must have alternative mechanisms for membrane localization (42, 43).
Our data show that p63RhoGEF is tethered to the PM via palmitoylation of the Cys-23/25/26 string and that disruption of palmitoylation not only abolishes p63RhoGEF association with the PM but also markedly alters its ability to activate RhoA when exogenously expressed in cells. Palmitoylation of two or three of these cysteines appears to be important for efficient localization to the PM. Multiple palmitoylation at clusters of cysteine residues has also been observed in other signaling proteins (17, 34, 35). Although the activity of the DH domain is higher than that of DH/PH due to release of autoinhibition, it still has less SRE activity than full-length p63RhoGEF. However, when the DH domain retains an intact N-terminal region, as in the ΔPH/C variant, it exhibits the highest rates of basal activity we observe in our assays (Fig. 2I). This proves that targeting p63RhoGEF to the PM greatly enhances GEF activity. Targeting palm− p63RhoGEF to the PM by fusion with the two tandem PH domains of PLCδ1 (2xPH) rescues its activity (Fig. 6B), and Gαq co-expression with palm− p63RhoGEF results in higher fold activation than with wild-type p63RhoGEF (Fig. 7B), indicating that 1) the inability of palm− p63RhoGEF to activate RhoA was due to defects in PM localization and not to the absence of palmitoylation, 2) activation by Gαq does not require palmitoylation of p63RhoGEF, and 3) Gαq-mediated PM recruitment and activation of p63RhoGEF strictly require palmitoylation of Gαq. This is reminiscent of the regulation of p115-RhoGEF, which requires palmitoylated Gα13 for activation (44). However, for p63RhoGEF, there is an additional layer of regulation in that membrane-localized p63RhoGEF still exhibits relatively low levels of RhoA activity due to autoinhibition by its PH domain. Because deletion of the PH domain (e.g. in the ΔPH protein) both eliminates Gαq binding and leads to maximal activity (Fig. 7B), Gαq does not seem to function by relocating p63RhoGEF to specific locales on the membrane or by reorienting the catalytic core. However, because both p63RhoGEF and Gαq are palmitoylated, it is possible that they are already found in the same lipid microdomains (26, 45, 46), and we also cannot rule out the possibility that locale-specific recruitment may be important for endogenous p63RhoGEF.
To the best of our knowledge, p63RhoGEF is the only member of the Dbl family RhoGEFs that has been shown to be constitutively associated with the PM by palmitoylation. Its low basal activity allows the enzyme to remain responsive to extracellular signals even when it is co-localized with its substrate, RhoA. It is possible that the membrane localization of p63RhoGEF serves other purposes, such as allowing for more rapid activation of RhoA upon stimulation of Gq-coupled receptors because a membrane translocation step is not required. It may also simply reflect the fact that p63RhoGEF has a relatively simple regulatory mechanism compared with other GPCR-regulated RhoGEFs, including its homologs Trio and Duet, which are more complex proteins and can potentially be recruited to the PM via multiple mechanisms. For example, PDZ-RhoGEF and LARG have N-terminal PDZ domains that are recruited to plexin receptors (47–49), and the PH domains of RH-RhoGEFs bind to activated RhoA in a feed-forward mechanism of activation (21, 50). It will be interesting to identify the palmitoyltransferase(s) responsible for carrying out posttranslational modification of p63RhoGEF and determine whether this occurs in a signal- or locale-dependent manner. In this regard, a future direction would be to investigate whether the palmitoylation of p63RhoGEF is modulated by Gq-coupled receptors. It would also be interesting to determine whether p63RhoGEF palmitoylation is dynamic and results in cycling of the protein between internal membranes and the PM as has been observed for other peripheral membrane proteins modified with palmitate (51).
Acknowledgments
We thank Dr. Richard Neubig for use of the Victor plate reader, the Center for Chemical Genomics at the Life Sciences Institute for use of the PHERAstar plate reader, and the laboratory of Dr. Alan Saltiel for use of the Olympus confocal microscope.
This work was supported, in whole or in part, by National Institutes of Health Grants HL071818 and HL086865 (to J. J. G. T.) and GM051466 (to M. E. L.). This research used the DNA Sequencing Core of the Michigan Diabetes Research and Training Center supported by National Institutes of Health Grant DK20572.
- RhoGEF
- Rho guanine nucleotide exchange factor
- 2BP
- 2-bromopalmitate
- DH
- Dbl homology
- EGFP
- enhanced green fluorescent protein
- GPCR
- G protein-coupled receptor
- HEK293
- human embryonic kidney 293
- LARG
- leukemia-associated RhoGEF
- mRFP
- monomeric red fluorescent protein
- 17-ODYA
- 17-octadecynoic acid
- PA
- palmitic acid
- palm−
- palmitoylation-deficient
- PH
- pleckstrin homology
- PLCδ1
- phospholipase C-δ1
- PM
- plasma membrane
- RGS
- regulator of G protein signaling
- RH
- RGS homology
- RH-RhoGEF
- RH domain containing RhoGEF
- SRE
- serum response element.
REFERENCES
- 1. Burridge K., Wennerberg K. (2004) Cell 116, 167–179 [DOI] [PubMed] [Google Scholar]
- 2. Heasman S. J., Ridley A. J. (2008) Nat. Rev. Mol. Cell Biol. 9, 690–701 [DOI] [PubMed] [Google Scholar]
- 3. Jaffe A. B., Hall A. (2005) Annu. Rev. Cell Dev. Biol. 21, 247–269 [DOI] [PubMed] [Google Scholar]
- 4. Rossman K. L., Der C. J., Sondek J. (2005) Nat. Rev. Mol. Cell Biol. 6, 167–180 [DOI] [PubMed] [Google Scholar]
- 5. Bos J. L., Rehmann H., Wittinghofer A. (2007) Cell 129, 865–877 [DOI] [PubMed] [Google Scholar]
- 6. Aittaleb M., Boguth C. A., Tesmer J. J. (2010) Mol. Pharmacol. 77, 111–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sternweis P. C., Carter A. M., Chen Z., Danesh S. M., Hsiung Y. F., Singer W. D. (2007) Adv. Protein Chem. 74, 189–228 [DOI] [PubMed] [Google Scholar]
- 8. Suzuki N., Hajicek N., Kozasa T. (2009) Neuro-Signals 17, 55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki N., Tsumoto K., Hajicek N., Daigo K., Tokita R., Minami S., Kodama T., Hamakubo T., Kozasa T. (2009) J. Biol. Chem. 284, 5000–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Z., Singer W. D., Danesh S. M., Sternweis P. C., Sprang S. R. (2008) Structure 16, 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hart M. J., Jiang X., Kozasa T., Roscoe W., Singer W. D., Gilman A. G., Sternweis P. C., Bollag G. (1998) Science 280, 2112–2114 [DOI] [PubMed] [Google Scholar]
- 12. Hajicek N., Kukimoto-Niino M., Mishima-Tsumagari C., Chow C. R., Shirouzu M., Terada T., Patel M., Yokoyama S., Kozasa T. (2011) J. Biol. Chem. 286, 20625–20636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lutz S., Shankaranarayanan A., Coco C., Ridilla M., Nance M. R., Vettel C., Baltus D., Evelyn C. R., Neubig R. R., Wieland T., Tesmer J. J. (2007) Science 318, 1923–1927 [DOI] [PubMed] [Google Scholar]
- 14. Rojas R. J., Yohe M. E., Gershburg S., Kawano T., Kozasa T., Sondek J. (2007) J. Biol. Chem. 282, 29201–29210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberts P. J., Mitin N., Keller P. J., Chenette E. J., Madigan J. P., Currin R. O., Cox A. D., Wilson O., Kirschmeier P., Der C. J. (2008) J. Biol. Chem. 283, 25150–25163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chisari M., Saini D. K., Kalyanaraman V., Gautam N. (2007) J. Biol. Chem. 282, 24092–24098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pedone K. H., Hepler J. R. (2007) J. Biol. Chem. 282, 25199–25212 [DOI] [PubMed] [Google Scholar]
- 18. Marrari Y., Crouthamel M., Irannejad R., Wedegaertner P. B. (2007) Biochemistry 46, 7665–7677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer B. H., Freuler F., Guerini D., Siehler S. (2008) J. Cell. Biochem. 104, 1660–1670 [DOI] [PubMed] [Google Scholar]
- 20. Siehler S. (2009) Br. J. Pharmacol. 158, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhattacharyya R., Banerjee J., Khalili K., Wedegaertner P. B. (2009) Cell. Signal. 21, 996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kozasa T., Jiang X., Hart M. J., Sternweis P. M., Singer W. D., Gilman A. G., Bollag G., Sternweis P. C. (1998) Science 280, 2109–2111 [DOI] [PubMed] [Google Scholar]
- 23. Jaiswal M., Gremer L., Dvorsky R., Haeusler L. C., Cirstea I. C., Uhlenbrock K., Ahmadian M. R. (2011) J. Biol. Chem. 286, 18202–18212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sprang S. R., Chen Z., Du X. (2007) Adv. Protein Chem. 74, 1–65 [DOI] [PubMed] [Google Scholar]
- 25. Shankaranarayanan A., Boguth C. A., Lutz S., Vettel C., Uhlemann F., Aittaleb M., Wieland T., Tesmer J. J. (2010) Cell. Signal. 22, 1114–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crouthamel M., Abankwa D., Zhang L., DiLizio C., Manning D. R., Hancock J. F., Wedegaertner P. B. (2010) Mol. Pharmacol. 78, 767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aittaleb M., Gao G., Evelyn C. R., Neubig R. R., Tesmer J. J. (2009) Cell. Signal. 21, 1569–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin B. R., Cravatt B. F. (2009) Nat. Methods 6, 135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Charron G., Zhang M. M., Yount J. S., Wilson J., Raghavan A. S., Shamir E., Hang H. C. (2009) J. Am. Chem. Soc. 131, 4967–4975 [DOI] [PubMed] [Google Scholar]
- 30. Lemmon M. A. (2008) Nat. Rev. Mol. Cell Biol. 9, 99–111 [DOI] [PubMed] [Google Scholar]
- 31. Lemmon M. A., Ferguson K. M. (2000) Biochem. J. 350, 1–18 [PMC free article] [PubMed] [Google Scholar]
- 32. Carrel D., Hamon M., Darmon M. (2006) J. Cell Sci. 119, 4276–4284 [DOI] [PubMed] [Google Scholar]
- 33. Steenhuis P., Herder S., Gelis S., Braulke T., Storch S. (2010) Traffic 11, 987–1000 [DOI] [PubMed] [Google Scholar]
- 34. Smotrys J. E., Linder M. E. (2004) Annu. Rev. Biochem. 73, 559–587 [DOI] [PubMed] [Google Scholar]
- 35. Tsutsumi R., Fukata Y., Noritake J., Iwanaga T., Perez F., Fukata M. (2009) Mol. Cell. Biol. 29, 435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Webb Y., Hermida-Matsumoto L., Resh M. D. (2000) J. Biol. Chem. 275, 261–270 [DOI] [PubMed] [Google Scholar]
- 37. Wedegaertner P. B., Chu D. H., Wilson P. T., Levis M. J., Bourne H. R. (1993) J. Biol. Chem. 265, 25001–25008 [PubMed] [Google Scholar]
- 38. Hughes T. E., Zhang H., Logothetis D. E., Berlot C. H. (2001) J. Biol. Chem. 276, 4227–4235 [DOI] [PubMed] [Google Scholar]
- 39. Hepler J. R., Biddlecome G. H., Kleuss C., Camp L. A., Hofmann S. L., Ross E. M., Gilman A. G. (1996) J. Biol. Chem. 271, 496–504 [DOI] [PubMed] [Google Scholar]
- 40. Evelyn C. R., Wade S. M., Wang Q., Wu M., Iñiguez-Lluhí J. A., Merajver S. D., Neubig R. R. (2007) Mol. Cancer Ther. 6, 2249–2260 [DOI] [PubMed] [Google Scholar]
- 41. Bellanger J. M., Estrach S., Schmidt S., Briançon-Marjollet A., Zugasti O., Fromont S., Debant A. (2003) Biol. Cell 95, 625–634 [DOI] [PubMed] [Google Scholar]
- 42. Sun Y. J., Nishikawa K., Yuda H., Wang Y. L., Osaka H., Fukazawa N., Naito A., Kudo Y., Wada K., Aoki S. (2006) Mol. Cell. Biol. 26, 6923–6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bellanger J. M., Astier C., Sardet C., Ohta Y., Stossel T. P., Debant A. (2000) Nat. Cell Biol. 2, 888–892 [DOI] [PubMed] [Google Scholar]
- 44. Bhattacharyya R., Wedegaertner P. B. (2000) J. Biol. Chem. 275, 14992–14999 [DOI] [PubMed] [Google Scholar]
- 45. Levental I., Grzybek M., Simons K. (2010) Biochemistry 49, 6305–6316 [DOI] [PubMed] [Google Scholar]
- 46. Levental I., Lingwood D., Grzybek M., Coskun U., Simons K. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 22050–22054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aurandt J., Vikis H. G., Gutkind J. S., Ahn N., Guan K. L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 12085–12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perrot V., Vazquez-Prado J., Gutkind J. S. (2002) J. Biol. Chem. 277, 43115–43120 [DOI] [PubMed] [Google Scholar]
- 49. Swiercz J. M., Kuner R., Behrens J., Offermanns S. (2002) Neuron 35, 51–63 [DOI] [PubMed] [Google Scholar]
- 50. Chen Z., Medina F., Liu M. Y., Thomas C., Sprang S. R., Sternweis P. C. (2010) J. Biol. Chem. 285, 21070–21081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rocks O., Gerauer M., Vartak N., Koch S., Huang Z. P., Pechlivanis M., Kuhlmann J., Brunsveld L., Chandra A., Ellinger B., Waldmann H., Bastiaens P. I. (2010) Cell 141, 458–471 [DOI] [PubMed] [Google Scholar]