Background: It is important to understand whether proteasomal dysfunction and endoplasmic reticulum (ER) stress can influence prion propagation.
Results: Both events lead to an increase of PrP aggregates in the secretory pathway and increased pathologic prion protein in infected cells.
Conclusion: Our data suggest a novel pathway that contributes to prion propagation.
Significance: These findings might be of relevance for the pathogenesis of sporadic prion diseases.
Keywords: ER Quality Control, ER Stress, Neurodegenerative Diseases, Prions, Proteasome, Prion Protein, Quality Control
Abstract
A conformational change of the cellular prion protein (PrPc) underlies formation of PrPSc, which is closely associated with pathogenesis and transmission of prion diseases. The precise conformational prerequisites and the cellular environment necessary for this post-translational process remain to be completely elucidated. At steady state, glycosylated PrPc is found primarily at the cell surface, whereas a minor fraction of the population is disposed of by the ER-associated degradation-proteasome pathway. However, chronic ER stress conditions and proteasomal dysfunctions lead to accumulation of aggregation-prone PrP molecules in the cytosol and to neurodegeneration. In this study, we challenged different cell lines by inducing ER stress or inhibiting proteasomal activity and analyzed the subsequent repercussion on PrP metabolism, focusing on PrP in the secretory pathway. Both events led to enhanced detection of PrP aggregates and a significant increase of PrPSc in persistently prion-infected cells, which could be reversed by overexpression of proteins of the cellular quality control. Remarkably, upon proteasomal impairment, an increased fraction of misfolded, fully glycosylated PrP molecules traveled through the secretory pathway and reached the plasma membrane. These findings suggest a novel pathway that possibly provides additional substrate and template necessary for prion formation when protein clearance by the proteasome is impaired.
Introduction
Transmissible spongiform encephalopathies or prion diseases are fatal neurodegenerative disorders in humans and animals. The common hallmark and major component of the infectious agent in the pathogenesis of these diseases is the β-sheet rich and partially protease-resistant protein denoted PrPSc, derived from post-translational conversion of the α-helical, protease-sensitive cellular prion protein (PrPc)3 (1–6). Upon processing and glycosylation, PrPc localizes mainly in cholesterol and sphingolipid-rich domains at the outer leaflet of the plasma membrane (7–9). Direct contact of the two isoforms is a prerequisite for the conformational change of host PrPc into PrPSc (1, 10–12). Although it is assumed that plasma membrane localization of PrPc is mandatory for its conversion into PrPSc (9, 13, 14), much less is known in this respect about events occurring in the endoplasmic reticulum (ER) and in early secretory compartments. Increasing evidence suggests that events taking place in these compartments play a modulating role in the pathogenesis of several neurodegenerative disorders (reviewed in Refs. 15 and 16). Besides processing, folding, and post-translational modification of nascent proteins, ER exerts a stringent quality control that ensures that only those molecules that undergo correct maturation reach their target cellular compartments. Incorrectly assembled proteins are normally retained in the ER and subjected to the ER-associated degradation (ERAD) pathway. This includes retrograde translocation through the ER membrane into the cytosol, deglycosylation, and covalent binding to ubiquitin by its lysine residues. Proteins are thereby marked for degradation by the 26 S proteasome complex (17).
Accumulation of misfolded proteins and alteration of ER homeostasis lead to perturbation of ER functions and ER stress, defined as an imbalance between the cellular demand for ER function and ER capacity to cope with excessive protein load. To reduce this excessive burden, cells trigger the unfolded protein response, which signals attenuation of protein translation, up-regulation of molecular chaperones and folding enzymes, and degradation of malfolded proteins by ERAD (18, 19). ER stress and ERAD are therefore tightly linked. Impairment of the ubiquitin-proteasomal system (UPS) can contribute to ER stress, whereas the UPS itself has been shown to be compromised during acute ER stress (20). An important subject of study for the elucidation of prion pathogenesis and for an effective therapeutic strategy may therefore be found in the early steps of PrPc processing and in the conformational requisites of the PrPc population preferred as a substrate for ongoing prion conversion or even template in de novo prion formation. Alterations in UPS and ER stress have been reported to participate in the pathogenesis of neurodegenerative diseases (21–24). The UPS seems to dispose of misfolded PrP aggregates occurring naturally (25, 26) and of some PrP mutants associated with hereditary forms of transmissible spongiform encephalopathies (27–29). Proteasomal dysfunction in cells overexpressing PrPc leads to accumulation of cytosolic PrP species with aberrant biochemical properties and to neurotoxicity in vivo (30). These molecules might represent ERAD substrates retro-translocated from the ER or arise during acute ER dysfunctions, when a pre-emptive quality control prevents translocation of PrP molecules into the ER and promotes their degradation by the proteasome as a defense mechanism against protein overflow (31). In prion-infected cells, proteasomal impairment caused formation of cytosolic PrPSc aggresomes that triggered apoptosis (32, 33), whereas purified PrPSc preparations impaired proteasomal function (34). Upon ER stress, misfolded PrP molecules were described to reach the plasma membrane and increase the rate of PrPSc replication when used as a substrate for protein misfolding cyclic amplification (35).
In the present study we investigated the involvement of proteasome and ER homeostasis in PrPc processing in the secretory pathway, and in PrPSc propagation in persistently prion-infected cells. We performed studies in different cell lines with physiological, endogenous expression of PrPc or transfected with PrPc. Inhibition of proteasomal activity as well as induction of ER stress significantly affected the total level of PrPc. This resulted in accumulation of aggregated PrP species, which were extensively transported through the secretory pathway to the cell surface. Under these conditions, we detected a significant increase of PrPSc levels in chronically prion-infected cells. Conversely, overexpression of selected molecules of the cellular quality control decreased the accumulation of both aggregated species and PrPSc. Further, we show that deletion of the N-terminal and central domain of PrPc reduced the capacity of the cellular quality control to interact with PrP. These results evidence a new correlation between failures in cellular quality control and PrPSc propagation indicating that misfolded prion protein, which should be a substrate for ERAD degradation, can be recycled to the secretory pathway and become an additional substrate for PrPSc formation. Overall, these studies add to the understanding of molecular requirements for cellular prion propagation and point to mechanisms that also might play a role in prion de novo generation as relevant in sporadic prion diseases.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
All cell culture media and Trypsin-EDTA were purchased from Invitrogen. Protein A-Sepharose was obtained from GE Healthcare. Peptide N-glycosidase F, Pefabloc proteinase inhibitor, FuGENE, and Lipofectamine 2000 transfection reagents were from Roche Molecular Biochemicals (Mannheim, Germany) and Invitrogen, respectively. Lactacystin, MG132, tunicamycin, and thapsigargin were from Calbiochem Merck Biosciences (Nottingham, UK). Monoclonal mouse anti-PrP antibody 4H11 was generated using a dimeric murine PrP as an immunogen (36). Immunoblotting was done using the enhanced chemiluminescence blotting technique (ECL plus) from GE Healthcare. Rabbit anti-Rab6 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-calnexin (Stressgen, Victoria, Canada), and mouse anti-ubiquitin (Santa Cruz Biotechnology) were used for detection of Golgi compartments, ER, and ubiquitinated proteins, respectively.
Cell Culture and Plasmid Construction for Mammalian Expression of PrP Constructs
The mouse neuroblastoma cell line N2a (ATCC CCL 131) has been described previously (37, 38). N2a-22L cells were persistently infected with the prion strain 22L. HpL3-4 cells, which are mouse hippocampal PrP knock-out cells, were kindly provided by Prof. T. Onodera (39). HpL-22L stably express murine wild-type PrP and are persistently infected with the 22L prion strain. L929 cells were purchased from National Collection of Type Cultures. L929-22L cells were persistently infected with the 22L prion strain (40). Cells were maintained in Dulbecco's modified Eagle′s (DMEM) or Opti-MEM medium containing 10% fetal calf serum (FCS), 1% penicillin/streptomycin, and glutamine in a 5% CO2 atmosphere. Wild-type PrPc (wtPrPc) and PrPΔ121 were cloned into the pcDNA3.1/Zeo expression vector (Invitrogen). Construction of PrPΔ121 was done by using bridge-PCR-based standard techniques with mouse wtPrP as a template. Briefly, PrPΔ121 was obtained by deleting amino acids 23–121 of full-length mouse PrP. Constructs were always confirmed by nucleic acid sequencing. Lipofection of cells with recombinant plasmids was carried out according to the manufacturer's directions. The procedures for the production of expression vector for EDEM-3 (41), ERGIC-53 (42), and the fluorescent proteasome reporter substrate UbG76V-GFP (43) have been described elsewhere.
Preparation of Primary Neurons
Primary neurons from hippocampi and astrocytes of C57B/L6 embryonic mice (gestation day 16) were prepared as described elsewhere (44) with some modifications. Briefly, hippocampi were excised, incubated with trypsin, and rinsed in Hanks' balanced salt solution (Invitrogen) complemented with 10% fetal calf serum. Single cell suspensions were obtained by mechanical dissociation. Approximately 1.5 × 106 viable cells were plated in poly-l-lysine-coated 6-well plates in neurobasal medium supplemented with B-27 and antibiotics (Invitrogen). Amounts (10 μm) of the antimitotics uridine and fluorodeoxyuridine (Sigma) were added to prevent astrocyte proliferation. Each week, one-third of the medium was replaced with fresh medium.
Immunoblot Analysis and Detergent Solubility Assay
1 mm EDTA was applied to cultured cells to detach them from dishes. Cells were counted and subsequently centrifuged for 2 min at 1000 rpm and then lysed in cold lysis buffer (100 mm NaCl, 10 mm Tris-HCl, pH 7.5, 10 mm EDTA, 0.5% Triton X-100, 0.5% deoxycholate). Post-nuclear lysates were supplemented with 0.5 mm Pefabloc protease inhibitor prior to precipitation with methanol. Samples were centrifuged for 30 min at 2.500 × g, and the pellets were re-dissolved in TNE buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EDTA) with the addition of gel loading buffer (7% SDS, 30% glycerin, 20% 2-mercaptoethanol, 0.01% Bromphenol Blue in 90 mm Tris-HCl, pH 6.8). After boiling for 10 min at 95 °C, aliquots from equal cell numbers were analyzed on 12.5% SDS-PAGE gels. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane. Membranes were blocked with nonfat dry milk (5%) in TBST (0.05% Tween 20, 100 mm NaCl, 10 mm Tris-Cl, pH 7.8), incubated overnight with appropriate antibody at 4 °C, as indicated, and stained using an enhanced chemiluminescence blotting kit from GE Healthcare. For solubility assay, post-nuclear cell lysates were supplemented with 0.5 mm Pefabloc protease inhibitor and N-lauryl sarcosine to 1%, and centrifuged for 1 h at 100,000 × g, 4 °C in a Beckman TL-100 centrifuge. Soluble fractions (supernatant) were precipitated with methanol. Insoluble fractions (pellet) were resuspended in TNE and analyzed in immunoblot or radioimmune precipitation assays. When necessary, samples were treated with 0.1 unit/μl peptide N-glycosidase F at 37 °C overnight to remove N-linked oligosaccharides and analyzed by SDS-PAGE. Gels were exposed to an x-ray film (Kodak).
Proteinase K Treatment
Aliquots of post-nuclear lysates were incubated for 30 min at 37 °C with 20 μg/ml proteinase K (PK); the proteolysis was stopped by addition of protease inhibitor Pefabloc. Samples were precipitated with methanol and analyzed in an immunoblot assay.
FACS Analysis
For detection of PrP surface expression in compound-treated cells, 1 mm EDTA was applied to detach cells from dishes. Cells were centrifuged for 2 min at 1000 rpm and 4 °C, and then resuspended in FACS buffer (2.5% FCS, 0.05% sodium azide, in PBS) and incubated on ice for 5 min. Cells were incubated with mAb 4H11 (1:10) in FACS buffer for 45 min on ice and washed three times with FACS buffer. Secondary antibodies (FITC-labeled, Dianova) were incubated for 45 min on ice. After washing with FACS buffer, 7-aminoactinomycin D was added for staining of dead cells. For detection of intracellular PrP, cells were fixed in Roti-Histofix 4% (Roth, Karlsruhe, Germany), and the following steps were performed in saponin- buffer (0.1% in FACS buffer). Flow cytometry was performed in a FACS Canto II cell sorter from BD Biosciences.
Confocal Laser Microscopy
N2a or HpL3-4 cells were plated on poly-l-lysine-coated glass coverslips (Marienfeld, Lauda-Königshofen, Germany) at low density 1–3 days prior to staining. 24 h after seeding, HpL3-4 cells were transiently transfected with wtPrP. 16 h before fixing with 4% paraformaldehyde, DMSO, MG132, or tunicamycin were added to the culture medium. After sequential treatment for 10 min each with 50 mm NH4Cl/20 mm glycine, 0.1% Triton X-100, and 0.2% gelatine, mAb 4H11 was added in gelatine for 30 min at room temperature for detection of PrP molecules, while specific antibodies against molecules of the Golgi compartment and ER (as described above) were applied. Cells were washed with PBS and incubated with Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary antisera obtained from Invitrogen. Nuclei were stained with Hoechst (Germany) before mounting the slides on anti-fading solution (Mowiol/DABCO, Roth) and kept at 4 °C. Confocal laser scanning was carried out using a Fluoview FV10i LSM 510 laser scanning microscope (Olympus, Tokyo, Japan).
Immunoprecipitation
For co-immunoprecipitation experiments, a protocol was adapted from Jin and colleagues (45). Transiently transfected HpL3-4 cells were treated for 16 h with 5 μm MG132 or 2.5 μg/ml tunicamycin, and then washed with PBS and lysed on ice in a buffer containing 1% Triton X-100, 150 mm NaCl, 20 mm Tris, pH 7.4. A mixture of protease inhibitors was added immediately after lysis. Cell debris was cleared by centrifugation at 800 × g, and PrP was precipitated from clarified cell lysates with mAb 4H11 for 3 h. Protein A-Sepharose beads were added to the protein-antibody complexes for 90 min at 4 °C. Complexes bound to protein A-agarose were washed with a buffer containing 150 mm NaCl, 10 mm Tris-HCl, pH 7.8, 0.1% N-lauryl sarcosine, and 0.1% Pefabloc, and bound proteins were eluted by boiling in SDS-sample buffer. Precipitates were analyzed by SDS-PAGE and developed with the indicated antibodies for detection of proteins bound to PrP.
Crude Membrane Fractionation Preparation
Membrane preparation was performed according to a previous study (46). HpL3-4 cells transiently transfected with wtPrP or deletion constructs were rinsed three times in PBS and once in Hepes 10 mm, pH 7.4, and then incubated for 15 min in Hepes supplemented with protease inhibitors, on ice. Cells were scraped off in homogenization buffer (20 mm Hepes, pH 7.4, 250 mm sucrose, 1 mm EDTA, 1 mm dithiothreitol, and protease inhibitors) and homogenized with a 22-gauge needle. Homogenates were centrifuged for 5 min at 4 °C and 3,000 × g, the post-nuclear supernatant were then spun for 15 min at 100,000 × g and 4 °C in a TL 100.2 rotor centrifuge. The supernatant (cytosolic fraction) was removed, and the pellets (membrane fraction) were resuspended in homogenization buffer. Proteins were analyzed with 4H11 by SDS-PAGE and immunoblot.
Surface Biotinylation Assay
Surface localization of PrPc and aggregates was assessed by biotinylation. Upon reaching 70–80% confluence, transiently transfected HpL3-4 cells were rinsed with cold PBS. After 20-min incubation on ice with 250 μg/ml membrane-impermeable Sulfo-biotin-X-NHS (Pierce), the cells were rinsed with cold PBS. Cells were incubated with 20 mm glycine/50 mm NH4Cl 10 min on ice for quenching and rinsed again with cold PBS before harvesting with lysis buffer on ice. Post-nuclear lysates were subjected to solubility assay as described. Insoluble fractions were resuspended in 100 μl of radioimmune precipitation assay buffer (0.5% Triton X-100, 0.5% deoxycholate in PBS) supplemented with 1% SDS before boiling at 95 °C for 10 min then brought to 1000 μl with lysis buffer and supplemented with Pefabloc and N-lauryl sarcosine to 1%. All samples were incubated with mAb 4H11 (1:100) overnight at 4 °C. Protein A-Sepharose beads were added to the protein-antibody complexes for 90 min at 4 °C. Immunoadsorbed proteins were washed in cold radioimmune precipitation assay buffer with 1% SDS at 4 °C and subsequently boiled in gel loading buffer to recover precipitated PrP before analysis by 12.5% SDS-PAGE.
Photodensitometric Analysis and Statistics
Photodensitometric analysis of immunoblots was performed using the ImageQuant TL software (GE Healthcare). Quantitative data are shown as mean ± S.D. For evaluation of statistical significance of the data, we used Prism (GraphPad, San Diego, CA) and applied the paired t test. p values less than 0.05 were considered as significant.
RESULTS
ER Stress and Impairment of Proteasomal Activity Affect Endogenous PrPc Expression and Result in Accumulation of PrP Aggregates
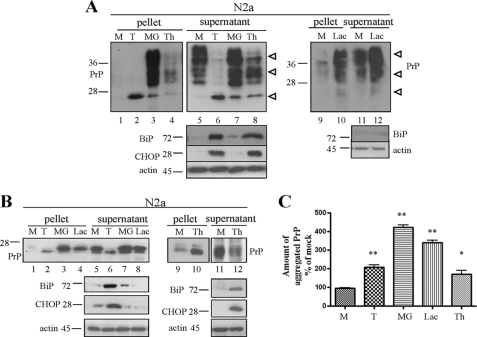
In line with the work done by other groups, we set out to analyze how proteasomal dysfunction and ER stress are involved in the processing and turnover of PrPc in cell culture models, thereby focusing on PrP in the secretory pathway. Perturbation of cellular environment and clearance pathways can lead to accumulation of misfolded, aggregated PrP species. Conventionally, aggregation of PrP molecules is evaluated by their solubility in non-ionic detergent upon high speed centrifugation of cell lysates. For this purpose, mouse neuroblastoma N2a cells expressing endogenous PrP were treated for 16 h with the proteasomal inhibitors MG132 or lactacystin (5 μm) or ER stress was induced with tunicamycin (2,5 μg/ml), an agent that prevents N-glycosylation, or with thapsigargin (1 μm), which depletes ER-Ca2+. Upon lysis, we performed ultracentrifugation in the presence of 1% Sarkosyl to separate aggregated proteins in the pellets from soluble ones in the supernatants. Soluble and insoluble PrP levels in post-nuclear lysates of equal number of cells upon treatment were visualized in immunoblot analysis with anti-PrP mAb 4H11 (Fig. 1A). Induction of ER stress was verified by detection of the specific markers Grp78/BiP and GAD153/CHOP (Fig. 1A, lower panels), whereas analysis of cells transfected with a vector coding for a GFP-tagged reporter for proteasomal activity confirmed proteasomal impairment upon MG132 treatment (data not shown). In mock treated cells, PrP partitioned mainly in the soluble fraction (Fig. 1A, lanes 1 and 5). Conversely, all compounds used increased formation of aggregated PrP molecules (Fig. 1A, lanes 2–4 and 10). As expected, tunicamycin-induced aggregates were unglycosylated PrP (Fig. 1A, lane 2), whereas MG132 and lactacystin led to accumulation of insoluble PrP molecules comprising mainly fully glycosylated isoforms (Fig. 1A, lanes 3 and 10). For better comparison of the signals, proteins were deglycosylated with peptide N-glycosidase F in an additional experiment (Fig. 1, B and C), and comparable results were obtained with L929 fibroblasts (supplemental Fig. S1). Taken together, these studies confirm that induction of acute ER stress as well as inhibition of proteasomal degradation lead to an extensive accumulation of detergent-insoluble PrP aggregates in all cell lines tested here.
FIGURE 1.
ER stress and proteasomal impairment result in accumulation of insoluble PrP aggregates. A, N2a cells were treated for 16 h with tunicamycin (T), MG132 (MG), lactacystin (Lac), or thapsigargin (Th). Post-nuclear lysates from equal number of cells were ultracentrifuged in the presence of 1% Sarkosyl to separate soluble (supernatant) from insoluble (pellet) fractions. Samples were subjected to immunoblotting, and PrP was detected with mAb 4H11. Induction of ER stress was verified with antibodies for Grp78/BiP and GAD153/CHOP. Equal protein loading was verified with actin, shown in the lowest panels. Molecular size markers are depicted on the left. Arrowheads on the right indicate un-, mono-, and diglycosylated PrP isoforms. M, mock; T, tunicamycin; MG, MG132; Th, thapsigargin; and Lac, lactacystin. B, solubility assay with N2a cells, as described above. Proteins in post-nuclear lysates were additionally deglycosylated with peptide N-glycosidase F to facilitate quantification. The doublet bands for deglycosylated PrP most likely originate from incomplete deglycosylation. C, densitometric evaluation of immunoblots from the experiment described above (performed in triplicate). Bars represent amount of insoluble PrP compared with mock treated cells.
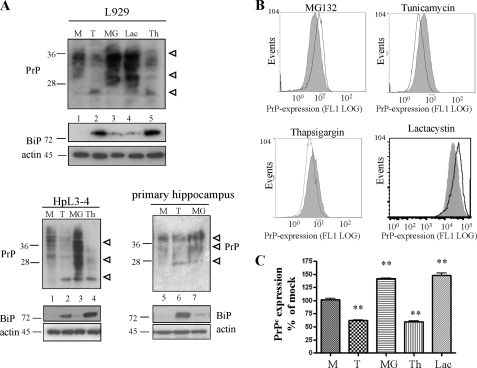
In a subsequent set of experiments we monitored the repercussion of proteasomal impairment and ER stress on the expression levels of total cellular PrP. We therefore treated L929 fibroblasts expressing endogenous PrP for 16 h with MG132, tunicamycin, lactacystin, or thapsigargin and visualized PrP amount in post-nuclear lysates of equal number of cells in immunoblot analysis with mAb 4H11 (Fig. 2A). Up-regulation of Grp78/BiP (Fig. 2A, lower panels) confirmed ER stress induction. In tunicamycin- and thapsigargin-treated cells PrP levels were attenuated compared with mock treated cells (Fig. 2A, lanes 1, 2, and 5). Conversely, treatment with MG132 or lactacystin led to a significant increase of all PrP-glycoforms in the tested cell line (Fig. 2A, lanes 3 and 4). These findings were verified in HpL3-4 mouse hippocampal cells transiently transfected with PrPc and primary hippocampal neurons of C57BL/6 mice in immunoblot (Fig. 2A, lower part) and in FACS analysis in N2a (Fig. 2, B and C), L929, and HpL3-4 (data not shown) cells. We subsequently confirmed that the increase in cellular PrPc was not due to up-regulation of PrP mRNA levels by comparing the relative transcript levels by real-time PCR (supplemental Fig. S1). Taken together, these studies show that ER stress and proteasomal impairment affect cellular PrP levels in different ways.
FIGURE 2.
ER stress reduces and proteasomal inhibition increases expression of total PrPc. A, L929 cells, HpL3-4 (HpL3-4 cells transiently transfected with mouse wtPrP), and primary hippocampus neurons were treated for 16 h with 2,5 μg/ml tunicamycin or 5 μm MG132. Cells were counted before lysis. PrPc amounts in post-nuclear lysates from equal cell numbers were analyzed by immunoblotting using anti-PrP mAb 4H11. Induction of ER stress was measured by probing samples with antibodies for Grp78/BiP. Actin loading controls are shown in the lowest panels. M, mock; T, tunicamycin; MG, MG132; Th, thapsigargin; and Lac, lactacystin. B, total PrPc expression levels were measured by FACS analysis with permeabilized cells in L929 fibroblasts upon treatment with the indicated compounds for 16 h, using mAb 4H11 (open line), and compared with mock treated cells (gray curves). C, evaluation of FACS analysis of two different experiments performed in triplicate confirms significant difference (p ≤ 0.005) in PrPc expression between mock and compound-treated cells. PrP expression in compound-treated cells was expressed as the percentage of expression in mock treated cells.
PrPc Trafficking in the Secretory Pathway Is Affected by Proteasomal Activity and ER Homeostasis
The findings described so far confirm once again the importance of the early secretory pathway for PrP metabolism. Our subsequent experiments concentrated on the cellular localization where PrP aggregates accumulate under these conditions.
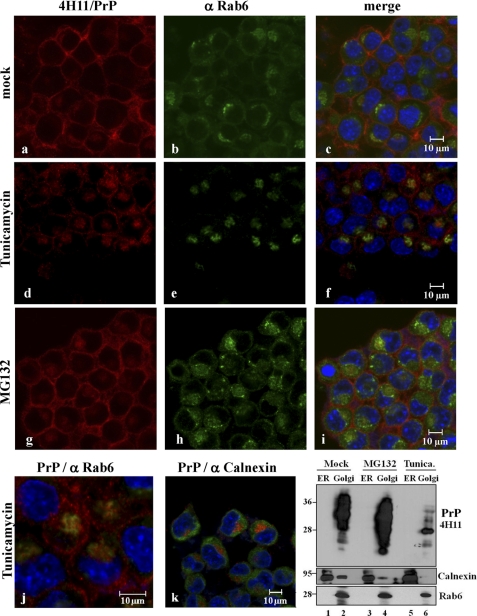
We therefore investigated PrP localization in confocal microscopy studies (Fig. 3). N2a cells were treated with tunicamycin or MG132 for 16 h and stained with 4H11 for detection of PrP, after permeabilization. As expected, in mock treated cells, PrP showed the characteristic surface localization (Fig. 3, a–c). Interestingly, upon induction of ER stress, cell surface localization of PrP decreased and PrP partitioned intracellularly and partially in the Golgi compartments (as visualized by specific staining for the Golgi protein Rab6) (Fig. 3, d–f). Conversely, proteasomal impairment led to an increase of PrP signal throughout the cell. The PrP pattern was particularly prominent at the plasma membrane but also in Golgi compartments (Fig. 3, g–i). Fig. 3j shows partial co-localization of Golgi markers and PrP under tunicamycin treatment at higher magnification, whereas Fig. 3k denotes no detectable co-localization of PrP with ER markers under these conditions. In line with this, biochemical ER-Golgi fractionation showed higher amounts of PrP in MG132-treated cells, with no detectable PrP expression in ER in immunoblot (Fig. 3l). Immunofluorescence results were confirmed in thapsigargin- and tunicamycin-treated N2a cells and in HpL3-4 cells.
FIGURE 3.
Proteasomal activity and ER stress influence PrP transport through the secretory pathway. Confocal microscopy analysis in N2a cells upon treatment with tunicamycin or MG132 is shown. Localization of PrPc was detected with anti-PrP mAb 4H11 (red). Upon tunicamycin treatment PrP surface expression decreased and PrP shows a more pronounced intracellular distribution (d–f). Upon MG132 treatment a bright PrP staining can be seen throughout the cell, including Golgi apparatus, and at the cell surface (g–i). Golgi vesicles were detected with an antibody for Rab6, green (b, e, h, and j); ER was visualized with anti calnexin antibody (green, k). The lower right panel depicts a representative ER-Golgi fractionation experiment for PrP populations under these conditions. Immunoblotting with calnexin and Rab6 was used for characterizing fractions.
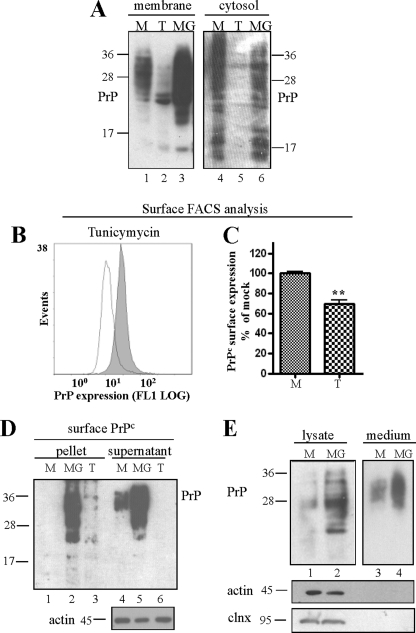
To support and further characterize these findings, we performed the next series of experiments in transfected HpL3-4 to ensure a sufficient detectable amount of PrPc was attained. We separated membrane bound from cytosolic proteins by crude membrane fraction preparation in mock and tunicamycin- and MG132-treated cells and visualized PrPc by immunoblot. The accumulation of PrP molecules detected upon treatment with either drug concerned mainly the membrane-bound fraction (Fig. 4A). This suggests that a significant portion of PrP that accumulated upon inactivation of proteasomal activity comprises luminal species. Plasma membrane expression of PrP upon tunicamycin treatment was subsequently measured in surface FACS analysis in non-permeabilized cells (Fig. 4B). As suggested by our confocal microscopy studies, ER stress induction negatively affected plasma membrane localization of PrP (Fig. 4C). Cell surface expression upon proteasomal impairment was investigated in FACS analysis with lactacystin in HpL3-4 and L929 cells (supplemental Fig. S2) and in a surface biotinylation assay coupled with ultracentrifugation (Fig. 4D). Specificity for cell surface biotinylation was confirmed using the cytosolic protein Grb2 as a control (data not shown). These experiments uniformly revealed that PrP aggregates reach the plasma membrane at a significant extent. In an additional experiment we collected higher amounts of PrPc secreted into the culture medium when cells were treated with MG132, compared with mock treatment (Fig. 4E). To ensure that proteins collected in the medium fraction were not derived from cell contamination, lysates were probed with antibodies for actin and for the ER chaperone calnexin (Fig. 4E, lower panels). In summary, these findings confirm that inhibition of proteasomal degradation positively affects transport of PrPc along the secretory pathway and enhances plasma membrane localization and secretion of fully glycosylated PrPc.
FIGURE 4.
PrP aggregates accumulating upon proteasomal inhibition increasingly localize at the cell surface. A, immunoblot of PrP-transfected HpL3-4 cells upon crude membrane fraction preparation. Tunicamycin (T) and MG132 (MG) treatment did not increase the amount of membrane-bound PrPc (compared with mock treated (M) cells). B and C, tunicamycin treatment (open line) resulted in reduced cell surface localization of PrPc when measured in surface FACS analysis with non-permeabilized HpL3-4 cells. D, transiently PrP-transfected HpL3-4 cells were biotinylated upon treatment with tunicamycin or MG132. Aggregated and insoluble PrP were separated by ultracentrifugation assay and precipitated with mAb 4H11. Surface-expressed, biotinylated PrP was monitored by probing blots with streptavidin. Equal protein loading was confirmed with actin (bottom panel). E, the amount of secreted PrP was measured in culture medium of mock and MG132-treated cells upon immunoblotting. Significantly more PrP was collected in the medium of cells treated with MG132. Cell contamination in the medium was excluded by probing cell lysates and media fraction with antibodies for non-secretory proteins calnexin and actin.
Perturbation of ER Homeostasis and Inhibition of UPS Pathway Result in Increased Accumulation of PrPSc in Prion-infected Cells
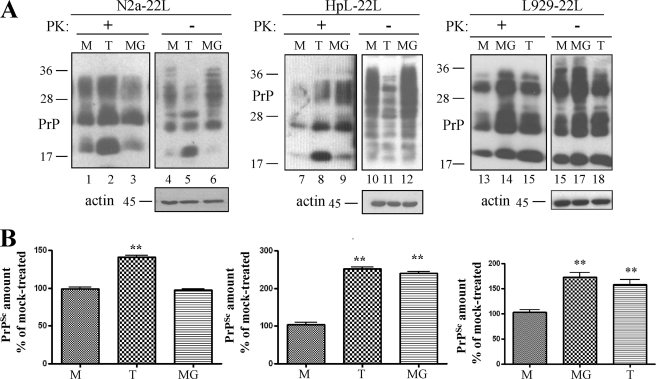
The findings that proteasomal impairment and induction of ER stress affect PrPc trafficking and aggregation state in the secretory pathway led to the assumption that these phenomena might have repercussions on PrPSc replication. This hypothesis was analyzed in N2a cells, in HpL3-4 cells stably transfected with wtPrPc and in L929 cells, all cell lines persistently infected with the 22L prion strain and denoted N2a-22L, HpL-22L, and L929-22L (40), respectively. Upon treatment with tunicamycin or MG132 for 16 h (Fig. 5A), cells were harvested and subjected to PK digestion to visualize PrPSc content. Post-nuclear lysates from equal cell numbers were analyzed by SDS-PAGE followed by immunoblot and probed with mAb 4H11. Loading of equal amounts of total proteins was confirmed with anti-actin antibody, in non-PK-digested lysates. In all tunicamycin-treated cells the amount of PrPSc was significantly higher than in mock treated ones (Fig. 5, A (lanes 2, 8, and 15) and B). Conversely, MG132 induced accumulation of PrPSc in HpL-22L and L929-22L cells (Fig. 5A, lanes 3, 9, and 14). We confirmed these results in a second approach with thapsigargin or lactacystin (supplemental Fig. S3). Taken together, these experiments show that proteasomal activity, possibly in a cell-specific manner, and ER stress are important mediators of PrPSc accumulation in cells.
FIGURE 5.
ER stress and proteasomal dysfunction enhance accumulation of PrPSc in prion-infected cells. A, the amount of PrPSc in N2a-22l, HpL-22L, and L929-22L cells treated with tunicamycin (T) or MG132 (MG) was compared with that in mock treated (M) cells. Immunoblots show total PrP- (−PK) and PrPSc-content (+PK) in post-nuclear lysates probed with mAb 4H11. The lowest panels show actin-loading controls. B, densitometric evaluation of at least two independent experiments performed in triplicate. PrPSc in compound-treated cells was plotted as the percentage of PrPSc measured in mock treated cells (p < 0,005).
Overexpression of Quality Control Molecules Prevents Both PrPc Aggregation and PrPSc Accumulation
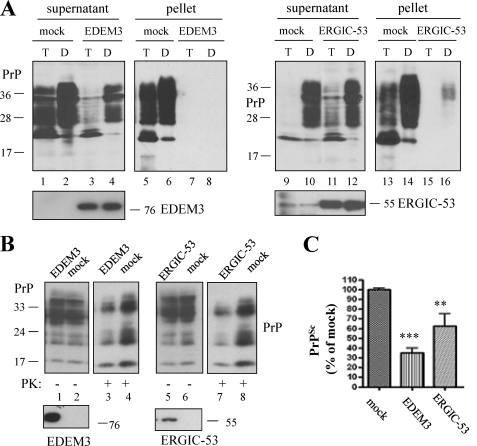
In the above results we have shown that ER stress and consequent accumulation of PrP aggregates result in increased amounts of PrPSc in prion-infected cells. Several studies show that enhancing cellular quality control of PrPc can influence its conversion into PrPSc. Therefore, we investigated whether we can counteract the described effects by overexpressing molecules playing a role in the cellular quality control. Among these, EDEM-3 is a member of the EDEM protein family (ER degradation-enhancing α-mannosidase-like proteins) and releases misfolded glycoproteins from the calnexin/calreticulin cycle, promoting translocation into the cytosol for proteasomal degradation (47). The other molecule investigated was ERGIC-53, a cargo receptor for selected transport of glycoproteins from the ER to secretory compartments (42). Overexpression of this molecule in L929 cells led to a significant reduction of PrP aggregation when ER stress was triggered with tunicamycin or DTT, an ER stress inducer (Fig. 6A, lanes 5–8 and 13–16). In persistently prion-infected L929-22L cells, upon overexpression of either molecule used in this analysis, we detected significantly less PrPSc than in mock transfected control cells (Fig. 6, B (lanes 3, 4, 7, and 8) and C). There was no detectable effect on PrPc (lanes 1 and 2, and 5 and 6). Efficient expression of the constructs was verified using specific antibodies. In summary, these data show that selected molecules of the cellular quality can counteract accumulation of prion protein aggregates and PrPSc in infected cells.
FIGURE 6.
Overexpression of ER quality control molecules reverses formation of PrPc aggregates and PrPSc accumulation. A, N2a cells were transiently transfected with EDEM-3 (lanes 3, 4, 7, and 8) or ERGIC-53 (lanes 11, 12, 15, and 16). 16 h before harvesting, cells were treated with tunicamycin (T) or DTT (D) to induce ER stress and subsequently subjected to solubility assay. Immunoblots show the amount of PrP collected in soluble and insoluble fractions probed with mAb 4H11. Significantly less aggregated PrP was found in cells overexpressing ERGIC-53 or EDEM-3. the lowest panels confirm successful transfection with the indicated constructs. B, prion-infected L929-22L cells transiently overexpressing EDEM-3 or ERGIC-53 were harvested 72 h post transfection and subjected to PK digestion to isolate PrPSc. Upon SDS-PAGE, amounts of PrPSc were analyzed by immunoblot. All cells overexpressing transfected proteins contain less PrPSc than mock transfected ones (lanes 3 and 4, and 7 and 8). Expression of quality control molecules is shown in the lowest panels with specific antibodies. C, evaluation of two independent experiments performed in triplicate confirms significant reduction of PrPSc in prion-infected cells overexpressing EDEM-3 or ERGIC-53.
Cellular Quality Control Only Partially Recognizes N-terminally Deleted PrPc
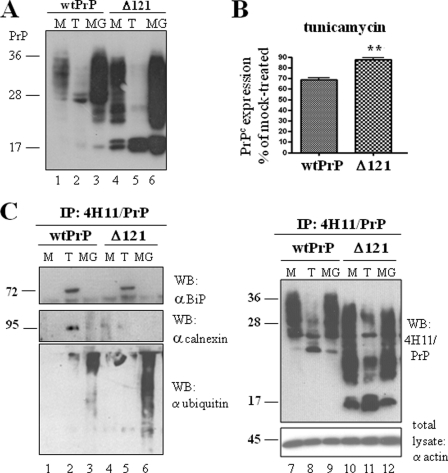
The work shown so far highlights that events involved in the early steps of protein maturation as well as a functioning disposal pathway have important consequences for downstream trafficking and functioning of authentic PrPc in the secretory pathway. To further characterize these processes, we focused on the disposal pathway of a PrP molecule lacking N-terminal and central portions (i.e. amino acids 23–121; termed Δ121) and how this might affect its cellular quality control under stress situations. Expression of wild-type PrPc (wtPrP) and Δ121 in transiently transfected HpL3-4 cells showed that the N-terminally deleted PrP was fully glycosylated (Fig. 7A, lane 4) and reached the cell surface, as revealed by FACS analysis and biotinylation assays (data not shown). Although from this blot it may appear that the expression level of Δ121 is higher than that of wtPrP, follow-up studies showed that Δ121 is not reproducibly elevated when compared with wtPrP. In general, the expression levels of both constructs were similar, and we do not assume that the effects described in the following are due to differences in expression levels. Δ121 expression was also affected by treatment of cells with tunicamycin or MG132 (Fig. 7, lanes 5 and 6). Nevertheless, FACS analysis upon tunicamycin treatment revealed that ER stress had a weaker effect on Δ121 (Fig. 7B).
FIGURE 7.
N-terminally deleted PrP is not affected by cellular quality control. A, HpL3-4 cells transiently transfected with wtPrP or Δ121 were treated with tunicamycin (T) or MG132 (MG) for 16 h (or left untreated, M), and expression of transfected proteins was determined by SDS-PAGE and immunoblot with anti-PrP mAb 4H11. From the shown blot it appears that the expression level of Δ121 is higher than that of wtPrP. Follow-up studies showed that Δ121 is not reproducibly elevated when compared with wtPrP. So, in general the expression levels of both constructs are similar. B, reduction of expression levels of wtPrP and Δ121 upon treatment with tunicamycin in comparison to mock treated cells was measured in transfected, permeabilized HpL3-4 cells with FACS analysis. The bars show the relative amount of PrP expressed as a percentage of expression in mock treated cells (for each construct). Reduction of expression was less prominent for Δ121 than for wtPrP (p ≤ 0,005). C, transfected prion proteins were precipitated with mAb 4H11 under non-denaturing conditions from transfected HpL3-4 cells. Immunoblots of precipitates were subsequently developed with specific antibodies to determine protein complexes. In contrast to wtPrP, Δ121 bound to BiP but not to calnexin upon induction of ER stress (lanes 2 and 5). Conversely, Δ121 was strongly ubiquitinated when proteasomal function was impaired by MG132 treatment (left, bottom panel, lanes 3 and 6). Successful precipitation of prion proteins was confirmed by probing immunoblot with mAb 4H11 (right, upper panel), equal protein loading (from total lysates) with actin (right, bottom panel).
We tried to analyze this phenomenon by co-precipitating wt and deleted PrPs with ER chaperones BiP and calnexin, both playing an important role in cellular ER stress response and quality control (Fig. 7C). wtPrP and Δ121 were immune-precipitated under non-denaturing conditions with mAB 4H11 prior to SDS-PAGE analysis. Immunoblots were then probed with anti-BiP, anti-calnexin antibody for detection of protein complexes or with mAb 4H11 (to confirm isolation of PrP molecules). A 78-kDa band corresponding to BiP co-precipitated with all PrP constructs upon induction of ER stress with tunicamycin. Conversely, calnexin (∼90 kDa) was only recovered in cells expressing wtPrP (Fig. 7C, lane 2). These results suggest that the N-terminal deletion in the PrP sequence prevents certain cellular chaperones from binding to or recognizing PrP. Covalent attachment of polyubiquitin chains to lysine residues of the proteins often precedes proteasomal degradation and results in the formation of a molecular mass ladder. We therefore investigated the ubiquitination state of PrP during ER stress and proteasomal impairment and compared it to Δ121 (Fig. 7C, lower left panel). PrP immunoprecipitates were probed with anti-ubiquitin antibody in immunoblot analysis. Polyubiquitinated PrP species were found in all cells treated with MG132 with a drastic increase in cells expressing Δ121 (Fig. 7C, lanes 3 and 6). In summary, these data demonstrate that deletion of amino acids 23–121 attenuates quality control response to PrP but makes PrP a significant substrate for proteasomal degradation.
DISCUSSION
In infectious forms of prion diseases a direct interaction between the PrPSc inoculum and PrPc seems to underlie this conformational change. The cellular mechanisms underlying sporadic transmissible spongiform encephalopathies remain mostly unknown and are difficult to assess in experimental systems. Several models propose the existence of a PrP isoform more prone to conversion into PrPSc (11, 48, 49). The important role of the ER environment and of the ERAD pathway in metabolism and turnover of wild-type and mutant PrP has been highlighted in the past especially with regard to implications for prion diseases (50–54). Whereas work done by other groups mainly focused on aberrant PrP moieties in the cytosol or in aggresomes and its possible impact in execution of neurodegeneration, the aim of our study was to investigate how perturbations of ER homeostasis and UPS affect PrPc metabolism in the secretory pathway and PrPSc biogenesis. Our results support previous data and propose additional new mechanisms favoring PrPSc formation.
Perturbation of ER Homeostasis and UPS Enhance PrP Aggregates in the Secretory Pathway and PrPSc Formation
We found that induction of ER stress with different compounds and in different cell lines resulted in a general attenuation of PrPc level. This finding has previously been described (31) and is probably due to activation of the cellular unfolded protein response as a protective response to stress. However, we found in addition aggregated PrP species that localized mainly in secretory compartments and at the cell surface. Conversely, inhibition of proteasomal function led to a significant increase of total PrPc level and to accumulation of detergent-soluble and insoluble PrPc isoforms. PrP species detected under these conditions were fully glycosylated (and resisted enzymatic de-glycosylation with endoglycosidase H; data not shown), were processed through the secretory pathway, and localized at the outer leaflet of the plasma membrane. This was the case in cells with endogenous PrP expression, in primary neurons as well as in PrP-transfected cells. It can therefore be concluded that this phenomenon is not due to overexpression of PrPc or to external promoter activity (although particularly pronounced in transfected HpL3-4 cells). The majority of the studies conducted on proteasomal degradation of PrP describe cytosolic accumulation of toxic PrP aggregates upon inhibition of this pathway (25, 30, 26). Nevertheless, trafficking of PrP through the secretory pathway under these conditions has been mentioned in previous studies (26, 33, 35). Although not extensively investigated for PrP metabolism, this phenomenon has been described for other proteins (55), and it was assumed that ER and quality control compartments are connected to the secretory pathway. Events occurring in the early secretory compartments must play a role, because PrP molecules were increased when we coupled MG132 treatment of cells with brefeldin-A, which inhibits trafficking of proteins to the cell surface (data not shown). Alternatively, PrP molecules accumulating in our experiments might result from retro-transport from post-ER compartments. Indeed, as reported for other proteins (56, 57), similar findings have also been suggested for PrPc (26, 58, 59). Of note, in our study proteasomal inhibition did not cause detectable ER stress, as visualized by the fact that we did not see up-regulation of BiP or CHOP in cells treated with MG132 or lactacystin. Conversely, analysis of cells expressing a reporter vector revealed that the concentrations used to induce ER stress did not compromise proteasomal activity (data not shown). Therefore, in the experiments performed here we analyzed two distinct pathways that, although physiologically connected, independently affected PrPc and PrPSc metabolism. Both pathways led to accumulation of insoluble PrP species in the secretory pathway, but the events underlying their formation might be different, as are the effects on PrPc localization and expression.
PrPc Aggregation and PrPSc Accumulation in the Secretory Pathway Can Be Reversed by Activation of Cellular Quality Control
In our experiments, inhibition of proteasomal activity amplified PrPSc levels in persistently prion-infected cells in a cell line-specific manner. To our knowledge the direct correlation between proteasome and PrPSc accumulation in cells represents a new aspect in prion metabolism. Previous studies reported formation of large cytoplasmic PrPSc aggregates, which associated with aggresomes and led to apoptotic death in prion-infected neurons after mild inhibition of the proteasome (32). In addition, purified PrPSc preparations were seen to inhibit the proteolytic activity of the proteasome (34). These data support the view of a cytosolic localization for portions of PrPSc either by retro-translocation or by endolysosomal membrane destabilization. In our study, upon proteasomal inhibition, PrPc and detergent-insoluble aggregates are extensively transported to the cell surface, one of the putative sites for prion formation. These PrP molecules could represent additional substrate binding to existing PrPSc seeds and leading to the extensive formation of PrPSc detected in our analysis. In line with a different model (58), PrPSc might represent a natural substrate for the UPS degradation via retro-transport from the secretory pathway or by destabilization of the endolysosomal system and would thereby accumulate when this pathway is compromised. A different phenomenon is the extensive amount of PrPSc found when we elicited ER stress. Our data in persistently prion-infected cells support work done by other groups that used protein misfolding cyclic amplification to show increased potential for prion conversion under such conditions (35). Other studies performed in the past had suggested a faster kinetic of conversion into PrPSc when cells were treated with tunicamycin, i.e. possibly favoring unglycosylated PrP for prion conversion (60). Because induction of ER stress led to an attenuation of PrPc expression and a reduction of cell surface localization, our results support the hypothesis that PrPc and PrPSc levels in quantity do not always correlate (61). It is therefore worth speculating that this PrP population represents a substrate with a conformation more adequate to bind to and/or being converted into PrPSc.
We further underlined the fundamental role of the early secretory pathway in folding and transporting of PrPc with respect to prion formation by overexpressing molecules known to promote cellular quality control and transport of proteins. This approach has proved successful for other neurodegenerative diseases, because overexpression of Grp78/BiP, calnexin, or Orp150/Grp170 suppressed formation of β-amyloid peptide (Aβ), the major cause of Alzheimer disease (62). GrP78/BiP was also seen to chaperone maturation of PrPc (45). Chemical chaperones known to stabilize proteins in their native conformation reduced the rate and extent of PrPSc formation in prion-infected cells (63). Overexpression of Grp58/Erp57 was seen to protect cells from the toxic activity of prion scrapie material (64). In agreement with these approaches, overexpression of EDEM-3 or ERGIC-53 significantly reduced PrP aggregates and PrPSc in infected cells. EDEM proteins are ER-resident lectins that recognize N-linked glycans on aberrantly folded proteins, accelerate their release from the calnexin/calreticulin cycle, and sort them for ERAD degradation (47, 65). It is therefore plausible that, by enhancing ERAD degradation of PrP aggregates, EDEM-3 subtracts the substrate necessary for prion conversion. A similar explanation for the reduction of PrPSc could apply to ERGIC-53, which selectively transports functionally folded proteins from the ER to ERGIC vesicles (42) and also operates in the quality control of glycoproteins. ERGIC-53 might therefore promote proper folding of PrPc and selectively transport this cargo to the cell surface. This PrPc population would have a more stable conformation and be less efficiently converted into PrPSc. Of note, such conversion favoring or disfavoring cellular conditions might also be of relevance for the pathogenesis of sporadic Creutzfeldt-Jakob disease, where initial conversion might take place without a bona fide PrPSc template.
Cellular Quality Control Fails to Recognize N-terminally Deleted PrP
Aberrant conformation of, or absent and/or masking of, specific protein domains might prevent efficient recognition by the cellular quality control (66). This seems to be the case when we delete the N-terminal and central segment of PrPc. Induction of ER stress and activation of unfolded protein response resulted in a minor reduction of protein expression compared with wtPrP and, despite sustained binding to BiP, we failed to detect a consistent interaction of the mutant protein with calnexin. Interaction of this ER chaperone with wtPrPc is a novel finding. In addition to functioning as a lectin binding to sugar residues of glycoproteins, according to the “dual binding” model and similarly to other chaperones, calnexin can promote folding by binding to hydrophobic segments of non-native glycoproteins (67–69). We can speculate that PrPc lacking its N terminus is only partially recognized by the folding ER machinery and by defense mechanisms of the unfolded protein response. Conversely, Δ121 is a significant substrate for proteasomal degradation, as seen by accumulation of ubiquitinated material upon inhibition of this pathway. The deleted segment comprises elements identified to be of importance for trafficking and metabolism of PrP as also shown previously by us (70). These include the octapeptide repeats, a charged cluster, and a hydrophobic transmembrane core, which can span the lipid bilayer in transmembrane PrP conformers and has been implicated in sorting of PrP (71, 52, 72, 73). Recent work done in vivo showed that deletions within these domains lead to a progressive lethal myelin degeneration and severe neurodegenerative illness (74–76). The results provided by our work suggest that failures in the recognition by cellular quality control mechanisms might also account for the neurotoxic phenotype caused by these and other pathogenic PrP mutants.
Taken together our data once more support the notion that the ER environment as well as cellular quality control mechanisms tightly modulate PrP maturation and PrPSc formation. We show that, possibly in a cell-specific manner, proteasomal degradation and ERAD play a physiological role for endogenous PrPc in the secretory pathway. Impairments in this pathway as well as disturbances in ER homeostasis cause accumulation of PrP aggregates, which are increasingly recycled through the secretory pathway, resulting in enhanced PrPSc replication. This represents a yet unexplored puzzle in prion metabolism, which raises new questions, e.g. in scenarios of sporadic prion diseases, and will require detailed characterization in future studies.
Supplementary Material
Acknowledgments
We are grateful to Prof. Takashi Onodera for providing HpL3-4 cells. We thank Dr. Nobuko Hosokawa (Kyoto University, Japan) for providing the expression vector for EDEM-3 and Prof. Hans-Peter Hauri (University of Basel, Switzerland) for the ERGIC-53 expression vector.
This work was also supported by Deutsche Forschungsgemeinschaft Scha594/7-1, SFB-596 (Project A8), and Alberta Prion Research Institute, Alberta, Canada (Projects 20080238 and 20090122).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- PrPc
- protease-sensitive cellular prion protein
- ER
- endoplasmic reticulum
- ERAD
- ER-associated degradation
- UPS
- ubiquitin-proteasomal system
- wt
- wild type
- PK
- proteinase K.
REFERENCES
- 1. Cohen F. E., Pan K. M., Huang Z., Baldwin M., Fletterick R. J., Prusiner S. B. (1994) Science 264, 530–531 [DOI] [PubMed] [Google Scholar]
- 2. Prusiner S. B., Scott M. R., DeArmond S. J., Cohen F. E. (1998) Cell 93, 337–348 [DOI] [PubMed] [Google Scholar]
- 3. Collinge J. (2001) Annu. Rev. Neurosci. 24, 519–550 [DOI] [PubMed] [Google Scholar]
- 4. Aguzzi A., Polymenidou M. (2004) Cell 116, 313–327 [DOI] [PubMed] [Google Scholar]
- 5. Winklhofer K. F., Tatzelt J., Haass C. (2008) EMBO J. 27, 336–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castilla J., Saá P., Hetz C., Soto C. (2005) Cell 121, 195–206 [DOI] [PubMed] [Google Scholar]
- 7. Borchelt D. R., Scott M., Taraboulos A., Stahl N., Prusiner S. B. (1990) J. Cell Biol. 110, 743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taraboulos A., Scott M., Semenov A., Avrahami D., Laszlo L., Prusiner S. B., Avraham D. (1995) J. Cell Biol. 129, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caughey B., Raymond G. J. (1991) J. Biol. Chem. 266, 18217–18223 [PubMed] [Google Scholar]
- 10. Prusiner S. B., Scott M. R. (1997) Annu. Rev. Genet. 31, 139–175 [DOI] [PubMed] [Google Scholar]
- 11. Prusiner S. B. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horiuchi M., Caughey B. (1999) EMBO J. 18, 3193–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gilch S., Winklhofer K. F., Groschup M. H., Nunziante M., Lucassen R., Spielhaupter C., Muranyi W., Riesner D., Tatzelt J., Schätzl H. M. (2001) EMBO J. 20, 3957–3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taraboulos A., Raeber A. J., Borchelt D. R., Serban D., Prusiner S. B. (1992) Mol. Biol. Cell 3, 851–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindholm D., Wootz H., Korhonen L. (2006) Cell Death. Differ. 13, 385–392 [DOI] [PubMed] [Google Scholar]
- 16. Lehman N. L. (2009) Acta Neuropathol. 118, 329–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ellgaard L., Molinari M., Helenius A. (1999) Science 286, 1882–1888 [DOI] [PubMed] [Google Scholar]
- 18. Shen X., Zhang K., Kaufman R. J. (2004) J. Chem. Neuroanat. 28, 79–92 [DOI] [PubMed] [Google Scholar]
- 19. Schrder M., Kaufman R. J. (2005) Annu. Rev. Biochem. 74, 739–789 [DOI] [PubMed] [Google Scholar]
- 20. Menéndez-Benito V., Verhoef L. G., Masucci M. G., Dantuma N. P. (2005) Hum. Mol. Genet. 14, 2787–2799 [DOI] [PubMed] [Google Scholar]
- 21. Ciechanover A., Brundin P. (2003) Neuron 40, 427–446 [DOI] [PubMed] [Google Scholar]
- 22. Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B. A., Yuan J. (2000) Nature 403, 98–103 [DOI] [PubMed] [Google Scholar]
- 23. Imai Y., Soda M., Inoue H., Hattori N., Mizuno Y., Takahashi R. (2001) Cell 105, 891–902 [DOI] [PubMed] [Google Scholar]
- 24. Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K., Hori S., Kakizuka A., Ichijo H. (2002) Genes Dev. 16, 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma J., Lindquist S. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 14955–14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yedidia Y., Horonchik L., Tzaban S., Yanai A., Taraboulos A. (2001) EMBO J. 20, 5383–5391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh N., Zanusso G., Chen S. G., Fujioka H., Richardson S., Gambetti P., Petersen R. B. (1997) J. Biol. Chem. 272, 28461–28470 [DOI] [PubMed] [Google Scholar]
- 28. Zanusso G., Petersen R. B., Jin T., Jing Y., Kanoush R., Ferrari S., Gambetti P., Singh N. (1999) J. Biol. Chem. 274, 23396–23404 [DOI] [PubMed] [Google Scholar]
- 29. Campana V., Sarnataro D., Fasano C., Casanova P., Paladino S., Zurzolo C. (2006) J. Cell Sci. 119, 433–442 [DOI] [PubMed] [Google Scholar]
- 30. Ma J., Wollmann R., Lindquist S. (2002) Science 298, 1781–1785 [DOI] [PubMed] [Google Scholar]
- 31. Kang S. W., Rane N. S., Kim S. J., Garrison J. L., Taunton J., Hegde R. S. (2006) Cell 127, 999–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kristiansen M., Messenger M. J., Klöhn P. C., Brandner S., Wadsworth J. D., Collinge J., Tabrizi S. J. (2005) J. Biol. Chem. 280, 38851–38861 [DOI] [PubMed] [Google Scholar]
- 33. Dron M., Dandoy-Dron F., Farooq Salamat M. K., Laude H. (2009) J. Gen. Virol. 90, 2050–2060 [DOI] [PubMed] [Google Scholar]
- 34. Kristiansen M., Deriziotis P., Dimcheff D. E., Jackson G. S., Ovaa H., Naumann H., Clarke A. R., van Leeuwen F. W., Menéndez-Benito V., Dantuma N. P., Portis J. L., Collinge J., Tabrizi S. J. (2007) Mol. Cell 26, 175–188 [DOI] [PubMed] [Google Scholar]
- 35. Hetz C., Castilla J., Soto C. (2007) J. Biol. Chem. 282, 12725–12733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ertmer A., Gilch S., Yun S. W., Flechsig E., Klebl B., Stein-Gerlach M., Klein M. A., Schätzl H. M. (2004) J. Biol. Chem. 279, 41918–41927 [DOI] [PubMed] [Google Scholar]
- 37. Butler D. A., Scott M. R., Bockman J. M., Borchelt D. R., Taraboulos A., Hsiao K. K., Kingsbury D. T., Prusiner S. B. (1988) J. Virol. 62, 1558–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schätzl H. M., Laszlo L., Holtzman D. M., Tatzelt J., DeArmond S. J., Weiner R. I., Mobley W. C., Prusiner S. B. (1997) J. Virol. 71, 8821–8831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuwahara C., Takeuchi A. M., Nishimura T., Haraguchi K., Kubosaki A., Matsumoto Y., Saeki K., Matsumoto Y., Yokoyama T., Itohara S., Onodera T. (1999) Nature 400, 225–226 [DOI] [PubMed] [Google Scholar]
- 40. Heiseke A., Aguib Y., Riemer C., Baier M., Schätzl H. M. (2009) J. Neurochem. 109, 25–34 [DOI] [PubMed] [Google Scholar]
- 41. Hirao K., Natsuka Y., Tamura T., Wada I., Morito D., Natsuka S., Romero P., Sleno B., Tremblay L. O., Herscovics A., Nagata K., Hosokawa N. (2006) J. Biol. Chem. 281, 9650–9658 [DOI] [PubMed] [Google Scholar]
- 42. Appenzeller C., Andersson H., Kappeler F., Hauri H. P. (1999) Nat. Cell Biol. 1, 330–334 [DOI] [PubMed] [Google Scholar]
- 43. Dantuma N. P., Lindsten K., Glas R., Jellne M., Masucci M. G. (2000) Nat. Biotechnol. 18, 538–543 [DOI] [PubMed] [Google Scholar]
- 44. Lopes M. H., Hajj G. N., Muras A. G., Mancini G. L., Castro R. M., Ribeiro K. C., Brentani R. R., Linden R., Martins V. R. (2005) J. Neurosci. 25, 11330–11339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jin T., Gu Y., Zanusso G., Sy M., Kumar A., Cohen M., Gambetti P., Singh N. (2000) J. Biol. Chem. 275, 38699–38704 [DOI] [PubMed] [Google Scholar]
- 46. Ganley I. G., Pfeffer S. R. (2006) J. Biol. Chem. 281, 17890–17899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hosokawa N., Wada I., Hasegawa K., Yorihuzi T., Tremblay L. O., Herscovics A., Nagata K. (2001) EMBO Rep. 2, 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saborío G. P., Soto C., Kascsak R. J., Levy E., Kascsak R., Harris D. A., Frangione B. (1999) Biochem. Biophys. Res. Commun. 258, 470–475 [DOI] [PubMed] [Google Scholar]
- 49. Daude N., Lehmann S., Harris D. A. (1997) J. Biol. Chem. 272, 11604–11612 [DOI] [PubMed] [Google Scholar]
- 50. Narwa R., Harris D. A. (1999) Biochemistry 38, 8770–8777 [DOI] [PubMed] [Google Scholar]
- 51. Ivanova L., Barmada S., Kummer T., Harris D. A. (2001) J. Biol. Chem. 276, 42409–42421 [DOI] [PubMed] [Google Scholar]
- 52. Stewart R. S., Drisaldi B., Harris D. A. (2001) Mol. Biol. Cell 12, 881–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tatzelt J., Schätzl H. M. (2007) FEBS J. 274, 606–611 [DOI] [PubMed] [Google Scholar]
- 54. Gu Y., Verghese S., Mishra R. S., Xu X., Shi Y., Singh N. (2003) J. Neurochem. 84, 10–22 [DOI] [PubMed] [Google Scholar]
- 55. Kamhi-Nesher S., Shenkman M., Tolchinsky S., Fromm S. V., Ehrlich R., Lederkremer G. Z. (2001) Mol. Biol. Cell 12, 1711–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nehls S., Snapp E. L., Cole N. B., Zaal K. J., Kenworthy A. K., Roberts T. H., Ellenberg J., Presley J. F., Siggia E., Lippincott-Schwartz J. (2000) Nat. Cell Biol. 2, 288–295 [DOI] [PubMed] [Google Scholar]
- 57. Nichols B. J., Kenworthy A. K., Polishchuk R. S., Lodge R., Roberts T. H., Hirschberg K., Phair R. D., Lippincott-Schwartz J. (2001) J. Cell Biol. 153, 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Béranger F., Mangé A., Goud B., Lehmann S. (2002) J. Biol. Chem. 277, 38972–38977 [DOI] [PubMed] [Google Scholar]
- 59. Hetz C. A., Soto C. (2006) Curr. Mol. Med. 6, 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taraboulos A., Rogers M., Borchelt D. R., McKinley M. P., Scott M., Serban D., Prusiner S. B. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 8262–8266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vorberg I., Raines A., Story B., Priola S. A. (2004) J. Infect. Dis. 189, 431–439 [DOI] [PubMed] [Google Scholar]
- 62. Hoshino T., Nakaya T., Araki W., Suzuki K., Suzuki T., Mizushima T. (2007) Biochem. J. 402, 581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tatzelt J., Prusiner S. B., Welch W. J. (1996) EMBO J. 15, 6363–6373 [PMC free article] [PubMed] [Google Scholar]
- 64. Hetz C., Russelakis-Carneiro M., Wälchli S., Carboni S., Vial-Knecht E., Maundrell K., Castilla J., Soto C. (2005) J. Neurosci. 25, 2793–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Olivari S., Galli C., Alanen H., Ruddock L., Molinari M. (2005) J. Biol. Chem. 280, 2424–2428 [DOI] [PubMed] [Google Scholar]
- 66. Ashok A., Hegde R. S. (2009) PLoS. Pathog. 5, e1000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Williams D. B. (2006) J. Cell Sci. 119, 615–623 [DOI] [PubMed] [Google Scholar]
- 68. Ihara Y., Cohen-Doyle M. F., Saito Y., Williams D. B. (1999) Mol. Cell 4, 331–341 [DOI] [PubMed] [Google Scholar]
- 69. Ware F. E., Vassilakos A., Peterson P. A., Jackson M. R., Lehrman M. A., Williams D. B. (1995) J. Biol. Chem. 270, 4697–4704 [DOI] [PubMed] [Google Scholar]
- 70. Nunziante M., Gilch S., Schätzl H. M. (2003) J. Biol. Chem. 278, 3726–3734 [DOI] [PubMed] [Google Scholar]
- 71. Hegde R. S., Mastrianni J. A., Scott M. R., DeFea K. A., Tremblay P., Torchia M., DeArmond S. J., Prusiner S. B., Lingappa V. R. (1998) Science 279, 827–834 [DOI] [PubMed] [Google Scholar]
- 72. Wüthrich K., Riek R. (2001) Adv. Protein Chem. 57, 55–82 [DOI] [PubMed] [Google Scholar]
- 73. Uelhoff A., Tatzelt J., Aguzzi A., Winklhofer K. F., Haass C. (2005) J. Biol. Chem. 280, 5137–5140 [DOI] [PubMed] [Google Scholar]
- 74. Shmerling D., Hegyi I., Fischer M., Blättler T., Brandner S., Götz J., Rülicke T., Flechsig E., Cozzio A., von Mering C., Hangartner C., Aguzzi A., Weissmann C. (1998) Cell 93, 203–214 [DOI] [PubMed] [Google Scholar]
- 75. Baumann F., Tolnay M., Brabeck C., Pahnke J., Kloz U., Niemann H. H., Heikenwalder M., Rülicke T., Bürkle A., Aguzzi A. (2007) EMBO J. 26, 538–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li A., Christensen H. M., Stewart L. R., Roth K. A., Chiesa R., Harris D. A. (2007) EMBO J. 26, 548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.