Abstract
Type I IFNs are critical players in host innate and adaptive immunity. IFN signaling is tightly controlled to ensure appropriate immune responses as imbalance could result in uncontrolled inflammation or inadequate responses to infection. It is therefore important to understand how type I IFN signaling is regulated. Here we have investigated the mechanism by which suppressor of cytokine signaling 1 (SOCS1) inhibits type I IFN signaling. We have found that SOCS1 inhibits type I IFN signaling not via a direct interaction with the IFN α receptor 1 (IFNAR1) receptor component but through an interaction with the IFNAR1-associated kinase Tyk2. We have characterized the residues/regions involved in the interaction between SOCS1 and Tyk2 and found that SOCS1 associates via its SH2 domain with conserved phosphotyrosines 1054 and 1055 of Tyk2. The kinase inhibitory region of SOCS1 is also essential for its interaction with Tyk2 and inhibition of IFN signaling. We also found that Tyk2 is preferentially Lys-63 polyubiquitinated and that this activation reaction is inhibited by SOCS1. The consequent effect of SOCS1 inhibition of Tyk2 not only results in a reduced IFN response because of inhibition of Tyk2 kinase-mediated STAT signaling but also negatively impacts IFNAR1 surface expression, which is stabilized by Tyk2.
Keywords: Cytokines/Interferon, Innate Immunity, Jak Kinase, Receptor Regulation, Tyrosine Protein Kinase (Tyrosine Kinase), Ubiquitination, SOCS1
Introduction
Interferons are a family of cytokines that elicit multifaceted effects during the host innate and adaptive immune response. The type I IFN family consists of multiple IFNα subtypes, IFNβ and others, that are produced in response to host viral and bacterial infection and act in an endocrine or autocrine fashion to stimulate cell antiviral responses (1). Type I IFNs signal through a common receptor, the IFN α receptor (IFNAR)2 which is composed of two subunits, IFNAR1 and IFNAR2 (2). The primary signaling events activated by the type I IFNs are mediated by the activation of receptor-associated JAK kinases and the recruitment of STATs to the intracellular domains of the receptor (reviewed in Ref. 3). The kinases Tyk2 and JAK1 are associated with the intracellular domains of IFNAR1 and IFNAR2, respectively (4, 5). The JAK kinases consist of a C-terminal kinase domain, an adjacent pseudo-kinase domain, and N-terminal JH3–7 regions. The kinase domain of Tyk2 contains three conserved and functionally important residues: Tyr-1054 and Tyr-1055, which must be phosphorylated for activity, and a critical Lys-930, which is important for kinase activity (6). The JH3–7 region of Tyk2 allows association of this kinase with IFNAR1 (7). Upon IFN binding, Tyk2 and JAK1 are trans-phosphorylated (on conserved tyrosines within their kinase domain) and activated, resulting in tyrosine phosphorylation of the receptor subunits (8–10). Receptor phosphotyrosines act as docking sites for STATs, which assemble, detach from the receptor, and translocate to the nucleus as the transcription factor complex ISGF3 (composed of STAT1, STAT2, and IRF9) as well as other STAT homo- and heterodimers. Activated STAT transcriptional complexes regulate a complex network of genes and signaling pathways to exhibit cell-specific antiviral, antiproliferative, antitumor, and immunomodulatory activities (reviewed in Ref. 11).
Regulation of type I IFN signaling is critical in maintaining homeostasis and for the generation of an appropriate immune response to viral and bacterial infections. SOCS1 is a potent negative regulator of both type I and type II IFN signaling (12, 13). SOCS1−/− mice are hyperresponsive to type I IFN and have a significantly greater ability than wild-type mice to resolve viral infection independently of IFNγ (13). SOCS1−/− mice also have a severe inflammatory phenotype because of deregulated type I and II IFN signaling. The SOCS1 protein consists of a central SH2 domain, a C-terminal SOCS box, and an N-terminal region that contains the kinase inhibitory region (KIR). The SOCS box is involved in targeted degradation, whereas the SH2 and KIR domain are involved in protein binding and inhibition of kinase activity, respectively (14, 15). SOCS1 expression is induced by the type I IFNs and then acts in a negative feedback loop to inhibit type I IFN signaling. Crosses of SOCS1−/− mice to IFNAR1−/− mice rescue the inflammatory phenotype, whereas crosses to IFNAR2−/− mice do not suggest that SOCS1 inhibits type I IFN signaling exclusively via IFNAR1 (13). Indeed, coimmunoprecipitation studies in our laboratory demonstrate that SOCS1 is associated with IFNAR1 (13).
In other cytokine systems, SOCS proteins have been shown to inhibit signaling either by a direct interaction with intracellular receptors via their phosphotyrosines or indirectly via an interaction with associated JAK kinases. For example, SOCS3 binds directly to phosphotyrosine 757 on gp130 to inhibit signaling via this receptor (16). In vivo studies have demonstrated that this interaction is critical to maintaining the appropriate balance of STAT signaling that otherwise lead to spontaneous diseases that include gastritis and tumors, inflammatory disease of the lungs and hemopoietic abnormalities (17). Furthermore, SOCS1 has been demonstrated to inhibit IFNγ signaling via a direct interaction with phosphotyrosine 441 on IFNGR1 (18). Evidence of indirect inhibition via interaction with JAK kinases has also been shown in vitro where the SH2 domain of SOCS1 binds to a phosphotyrosine residue within the Jak2 kinase domain, whereas modeling studies indicated that the KIR stabilizes this association through an interaction with the JAK catalytic pocket (19–21). SOCS1 has also previously been shown to bind and inhibit phosphorylated Tyk2 through an interaction with its SH2 and KIR domains (14). Because the SOCS proteins, including SOCS1, have the capacity to bind either to a receptor phosphotyrosine or to a JAK family member, we have undertaken to investigate the mechanism whereby SOCS1 regulates type I IFN signaling.
Our results demonstrate that rather than a direct interaction with IFNAR1, SOCS1 inhibits IFNAR signaling through an interaction with Tyk2 via its SH2 and KIR domains. Furthermore, we have discovered a novel role of SOCS1 in the inhibition of an activating Lys-63 polyubiquitination of Tyk2.
EXPERIMENTAL PROCEDURES
Cell Lines and Cell Culture
IFNAR1−/− murine embryonic fibroblasts (MEFs) and HEK293T cells were cultured in DMEM (Invitrogen) supplemented with 10% FCS, 1% L-glutamine, and 1% penicillin/streptomycin and incubated at 37 °C in 5% CO2. Mice used include SOCS1−/−IFNγ−/− and SOCS1+/+IFNγ−/− on a C57BL/6 background as well as wild-type C57BL/6 mice and were housed in specific pathogen free conditions at Monash Medical Centre Animal Facility. Whole thymus from SOCS1−/−IFNγ−/−, SOCS1+/+IFNγ−/−, and wild-type mice were harvested and filtered through 70-μm nylon cell strainers in 1% FCS DMEM to create a single cell suspension. Red blood cells were lysed in Tris-buffered NH4Cl solution for 5 min at 37 °C. Cells were washed twice, counted (Sysmex cell counter), plated at 1 × 107 cells/ml in 1% FCS DMEM, and allowed to rest for 2 h prior to stimulations.
Constructs and Cloning
pEF-BOS-SOCS1-FLAG constructs are as described in (22). pEF-BOS-muIFNAR1 and mutant IFNAR1 constructs are as described in (23). hu-Tyk2-HIS and hu-Tyk2KD-GST (encoding the JH1 domain of Tyk2) were provided by Dr. Isabelle Lucet (Monash University Department of Molecular Biology and Biochemistry). Plasmids pEF-ubiquitin, pEF-Ub-K48-HA and pEF-Ub-K63-HA were a kind gift from Neal Silverman (University of Massachusetts). pEF-Ub-K63-HA contains all lysines mutated to arginine except Lys-63 and thus forms only Lys-63 ubiquitin linkages. pEF-Ub-K48-HA contains all lysines mutated to arginine except Lys-48 and thus forms only Lys-48 ubiquitin linkages. Tyk2-HIS and Tyk2KD-GST were cloned into pEF-BOS. The Tyk2 Y1054F/Y1055F mutations were achieved by site-directed mutagenesis (QuikChange, Stratagene). Mutations were verified by DNA sequencing.
Luciferase Assays
A total 0.5 μg of DNA consisting of IFNAR1 or IFNAR1 mutants (0.3 ng), SOCS1 or SOCS1 mutants (1 ng), interferon stimulated response element (ISRE) luciferase reporter (30 ng), thymidine kinase (TK)-Renilla reporter (100 ng) and pEF-BOS (up to 0.5 μg) were transfected into IFNAR1−/− MEFs with FuGENE6 transfection reagent (Roche) according to the manufacturer's instructions. Transfected cells were stimulated with 50 IU/ml mu-IFNα1 (24) (referred to as IFNα in text) for 7 h. Following stimulation, cells were lysed in reporter lysis buffer (Promega). Luciferase activity was determined using a Promega luciferase assay according to the manufacturer's instructions and TK-Renilla activity using Stop & Glo (Promega). Luminescence of luciferase and TK-Renilla was determined using a FLUOstar Optima microplate reader (BMG Technologies).
Coimmunoprecipitation and Immunoblotting
A total 2.5 μg of DNA consisting of various combinations of IFNAR1 or IFNAR1 mutants (1 μg), SOCS1-FLAG or SOCS1 mutants (500 ng), Tyk2-HIS (2 μg), Tyk2KD-GST or Tyk2KD mutants (500 ng), ubiquitin-HA or ubiquitin mutants (200 ng), and pEF-BOS (up to 2.5 μg) were transfected into HEK293T cells with FuGENE6 transfection reagent according to the manufacturer's instructions. For assays involving ubiquitin, cells were stimulated with MG132 for 4 h prior to harvest. Transfected HEK293T cells were harvested in lysis buffer (50 mm TRIS-HCL (pH 7.4), 1% Nonidet P-40, 150 mm NaCl, 1 mm Na3VO4, 10 mm NaF, 1 mm PMSF, and 1 protease inhibitor tablet per 50 ml of buffer) and precleared with protein G-Sepharose beads (GE Healthcare). Immunoprecipitation was performed with either monoclonal IFNAR1 antibody (Abcam) (with protein-g-Sepharose beads), glutathione beads (GE-Healthcare) (for Tyk2KD-GST) or Nickel affinity Sepharose beads (GE Healthcare) (for Tyk2-HIS). Following immunoprecipitation, Sepharose beads were washed three times in lysis buffer and boiled with 5× β-mercaptoethanol (β-ME) sample buffer to elute the bound protein. Protein lysates were analyzed by SDS-PAGE analyses and Western blotting. Blotting was performed with appropriate antibodies for the detection of IFNAR1 (anti-IFNAR1 (25), rabbit anti-mouse IgG-HRP (Dako)), SOCS1-FLAG (anti-FLAG-HRP (Sigma)), Tyk2KD-GST (anti-GST (Cell Signaling Technology, Inc.), rabbit anti-goat IgG-HRP (Dako)), Tyk2-HIS (anti-HIS (Cell Signaling Technology, Inc.), rabbit anti-mouse IgG-HRP), Tyk2 (anti-Tyk2 (Cell Signaling Technology, Inc.), goat anti-rabbit IgG-HRP (Dako)) or ubiquitin (anti-HA (Rockland), goat anti-rabbit IgG-HRP).
Endogenous Tyk2 Expression
Thymocytes were stimulated with 10,000 IU of IFNα for 0, 30, and 60 min. Cells were washed and lysed in 50 mm Tris (pH 7.4), 1% Nonidet P-40, 10% glycerol, 1 mm EDTA, 1 mm Na3VO4, 1 mm NaF, 1 mm DTT, 10 mm β-glycerophosphate, 1 mm PMSF, and 1× protease inhibitor tablet (Roche) per 50 ml of buffer. Lysates were incubated for 2 h at 4 °C, 5× β-ME sample buffer was added, and the lysates were boiled. Protein lysates were analyzed by SDS-PAGE analyses and Western blotting. Blotting was performed with appropriate antibodies for the detection of Tyk2 (anti-Tyk2, goat anti-rabbit IgG-HRP) or β-tubulin (anti-β-tubulin (Abcam), rabbit anti-mouse IgG-HRP).
Flow Cytometry
Flow cytometry was used to measure and compare the surface levels of IFNAR1 on thymocytes from wild-type, SOCS1+/+IFNγ−/−, and SOCS1−/−IFNγ−/− mice. Where specified, cycloheximide (Sigma Aldrich) (to a final concentration of 20 μg/ml) was added 15 min prior to the addition of mIFNα (to a final concentration of 1,000 IU/ml). One and 2 h after the addition of mIFNα, cells were washed and resuspended in Fc blocking antibody (eBiosciences, diluted to 1:200 in PBS with 2% FCS (wash buffer)) and incubated on ice for 20 min. Cells were then washed twice and resuspended in 100 μl of the primary antibody (either anti-IFNAR1 (25) or isotype control antibody, both diluted to 10 μg/ml in wash buffer, or wash buffer alone for the negative controls) and incubated on ice for 20 min. Cells were then washed twice and resuspended in 100 μl of Alexa Fluour 488 (BD Biosciences, diluted 1:200 in wash buffer) and incubated on ice for 20 min. Following an additional two washes, cells were resuspended in 400 μl of wash buffer containing 1 μg/ml propidium iodide. Fluorescent staining on the cells was analyzed on a BD FACS CANTO II (BD Biosciences).
RESULTS
The SOCS1 SH2 Domain and N-terminal Region Are Critical for Inhibition of Type I IFN Signaling
To determine the critical regions of SOCS1 involved in the interaction with IFNAR1, we generated a series of SOCS1 mutants and tested their effect on IFN signaling using an ISRE luciferase assay (Fig. 1). IFNAR1−/− MEFs were transiently cotransfected with an ISRE luciferase reporter, a TK-Renilla reporter, IFNAR1, and either FLAG-tagged SOCS1 or SOCS1 mutants. We compared the ability of SOCS1 and the SOCS1 mutant proteins to inhibit IFNα-induced luciferase activity. A mutation in a critical region of SOCS1 would abolish its ability to inhibit interferon signaling and thus luciferase activity. In the absence of SOCS1, IFNα-induced luciferase activity was robustly and reproducibly induced approximately 4-fold in this system, and the induced level is normalized to 100%. The addition of SOCS1 significantly reduced luciferase activity to approximately uninduced levels (around 30%). Deletion of the SOCS box plus SH2 domain (S1Δ82–212) was inactive, indicating the importance of these two domains for activity. Furthermore, deletion of the SOCS box alone (S1Δ170–212) retained activity, indicating the importance of the SH2 domain. This was reinforced by the lack of activity of the S1R105K mutant, which contains a mutation within the critical residue of the SOCS1 SH2 domain. The remaining constructs (S1Δ1–76, S1F59A, and S1D64R) demonstrate that deletion or mutation of critical residues in the KIR domain are also important for SOCS activity. Given the importance of the SH2 domain and its characteristic of binding phosphotyrosine residues in the target proteins, we next sought to determine the interacting partner of SOCS1 in the type I IFN signaling complex.
FIGURE 1.

Effect of SOCS1 variants on type I IFN signaling. The histogram shows the relative expression of an ISRE-luciferase reporter in IFNAR1−/− MEFs transiently cotransfected with mu-IFNAR1 and either mu-SOCS1, SOCS1Δ82–212, SOCS1R105K, SOCS1Δ170–212, SOCS1Δ1–76, SOCS1F59A, or SOCS1D64R (represented diagrammatically in the lower panel). C represents the carboxy terminal, and N represents the amino terminal. Cells were stimulated with 50 IU/ml mu-IFNα for 7 h prior to luciferase readings. Luciferase activity is normalized against a TK-Renilla reporter to control for transfection efficiency and then expressed as a percentage of activity in the absence of SOCS1 (-S1), which is denoted as 100%. Addition of SOCS1 resulted in almost complete inhibition of IFN activity to control levels. Data are expressed as mean ± S.E. of three experiments. ***, p < 0.001.
SOCS1 Inhibits Signaling via the Tyk2 Interacting Domain of IFNAR1
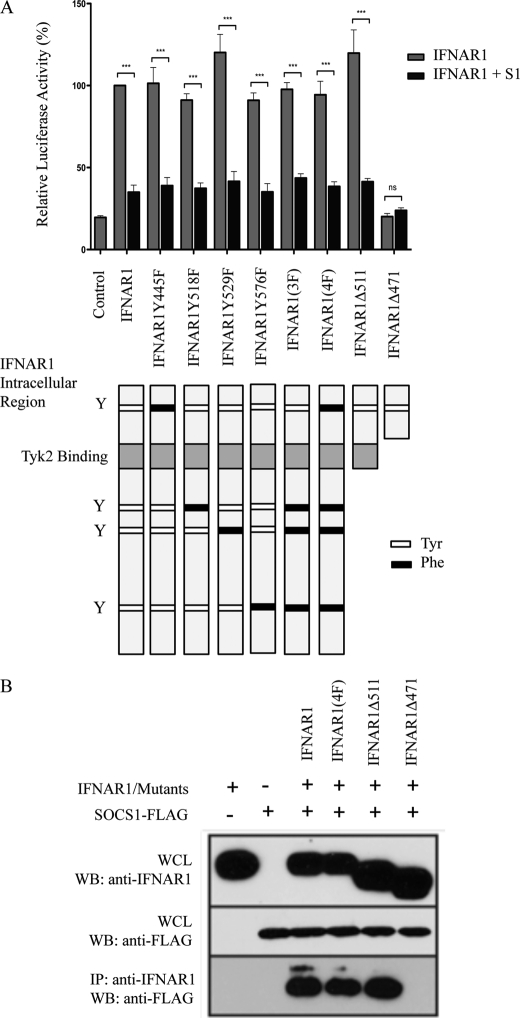
Previous genetic studies involving IFNAR1−/−SOCS1−/− and IFNAR2−/−SOCS1−/− mice indicated that SOCS1 specifically regulates the type I IFN receptor via an interaction with IFNAR1 and not IFNAR2 (13). Because SH2 domains are known to associate with phosphotyrosine residues and because our results above showed that the SH2 domain is critical for SOCS1-mediated inhibition of type I IFN signaling, we investigated whether SOCS1 inhibits activity by binding to a phosphotyrosine within the intracellular domain of IFNAR1, as shown for the type II IFN receptor, IFNGR1 (18). This hypothesis was tested by a luciferase reporter assay (Fig. 2A) and coimmunoprecipitation studies (B). A series of constructs containing single and multiple tyrosine-to-phenylalanine mutations in residues in the intracellular domain of IFNAR1 were utilized to first test the ability of SOCS1 to inhibit signaling in an ISRE luciferase reporter assay (Fig. 2A). IFNAR1−/− MEFs were transiently cotransfected with an ISRE luciferase reporter, a TK-Renilla reporter, IFNAR1, or IFNAR1 tyrosine mutants (as specified) with and without SOCS1-FLAG. The luciferase activity induced by wild-type IFNAR1 in the absence of SOCS1 (again approximately 4-fold in these experiments) is normalized to 100%. All IFNAR1 tyrosine mutant proteins were able to induce luciferase activity to similar levels as wild-type IFNAR1 (in the absence of SOCS1), indicating that the tyrosine residues within the intracellular region of IFNAR1 are not essential for activation of ISRE signaling in this system. Also unexpectedly, in the presence of SOCS1, the luciferase activity induced by IFN stimulation of all IFNAR1 tyrosine mutant proteins was comparable with the inhibition seen with wild-type IFNAR1, including the 4F mutant whereby all four intracellular tyrosine residues of IFNAR1 are mutated to phenylalanine (approximately 30–40%). These results demonstrate that SOCS1 does not interact directly with IFNAR1 phosphotyrosine residues in its intracellular domain but rather uses an alternative mechanism for interaction. Thus, we next generated truncated forms of IFNAR1, terminating at amino acid 471 or 511, that flank the Tyk2 binding site of IFNAR1. The truncation mutant, IFNAR1Δ471, was unable to induce ISRE activity in response to IFN stimulation, consistent with the critical nature of Tyk2 for this activity. Thus, inhibition by SOCS1 could not be determined. The truncation mutant IFNAR1Δ511 was still able to signal at levels comparable with wild-type IFNAR1, with signaling still able to be inhibited by the addition of SOCS1. This suggests that SOCS1 inhibits signaling by binding to a region of IFNAR1 proximal to this truncation site.
FIGURE 2.
Mapping IFNAR1 domains critical for interaction with SOCS1. A, the histogram shows relative expression of an ISRE-luciferase reporter in IFNAR1−/− MEFs transiently cotransfected with either mu-IFNAR1, IFNAR1Y445F, IFNAR1Y518F, IFNAR1Y529F, IFNAR1Y576F, IFNAR1Y518F/Y529F/Y576F(3F), IFNAR1Y445F/Y518F/Y529F/Y576F(4F) IFNAR1Δ511, or IFNAR1Δ471 (represented diagrammatically in the lower panel) with or without mu-SOCS1. Y denotes tyrosine residues. Cells were stimulated with 50 IU/ml mu-IFNα for 7 h prior to luciferase readings. Luciferase activity is normalized against a TK-Renilla reporter to control for transfection efficiency and is expressed relative to wild-type IFNAR1 in the absence of SOCS1, which is denoted as 100%. Data are represented as mean ± S.E. of three experiments. ***, p < 0.001. B, immunoprecipitation (IP) Western blot analyses (WB) of IFNAR1 mutants and SOCS1. IFNAR1, IFNAR1(4F), IFNAR1Δ 511, or IFNAR1Δ471 were cotransfected with SOCS1-FLAG into HEK293T cells. Following expression, immunoprecipitation of IFNAR1 or the IFNAR1 mutants was performed using anti-IFNAR1 antibody. Immunoprecipitates and whole cell lysates (WCL) were separated by SDS-PAGE and immunoblotted with anti-FLAG to detect SOCS1-FLAG. Blots are representative of triplicate experiments.
The putative association of IFNAR1 with SOCS1 was further tested by coimmunoprecipitation studies to confirm the data above (Fig. 2B). We found that SOCS1-FLAG was coimmunoprecipitated with wild-type IFNAR1, IFNAR1(4F), and IFNAR1Δ511 but not IFNAR1Δ471. As SOCS1 was coimmunoprecipitated with IFNAR1(4F), it confirms the above conclusion that SOCS1 does not bind tyrosine residues within the intracellular domain of IFNAR1. Because SOCS1 coimmunoprecipitated with IFNAR1Δ511 but not IFNAR1Δ471, our results suggest that SOCS1 interacts with IFNAR1 either directly or indirectly within this region, likely via Tyk2.
SOCS1 Binds Conserved Tyrosines of Tyk2 and Regulates Tyk2 Expression
To demonstrate that SOCS1 interacted with Tyk2 and to characterize this interaction, we performed coimmunoprecipitation experiments with SOCS1-FLAG and either Tyk2KD-GST or Tyk2KD(2F)-GST in which the tyrosines at residues 1054 and 1055 were mutated to phenylalanines. The latter were included because the SOCS1 SH2 domain dependence of the IFN signal suppression would imply SOCS1-bound phosphotyrosines on Tyk2. Importantly, SOCS1-FLAG is indeed coimmunoprecipitated with Tyk2KD-GST. By contrast, Tyk2KD(2F)-GST interaction is almost completely inhibited (Fig. 3A). These results are consistent with these tyrosine residues being important for SOCS1 docking onto Tyk2. Because our results above demonstrated the importance of the KIR domain in inhibition of signaling, we investigated SOCS1 KIR mutants (SOCS1F59A and SOCS1D64R) for their binding to Tyk2 in a coimmunoprecipitation experiment. Where wild-type SOCS1 bound to Tyk2KD, these mutants failed to interact with wild-type Tyk2KD or Tyk2KD(2F), suggesting that the KIR of SOCS1 is essential for Tyk2 binding and thus inhibition of signaling.
FIGURE 3.
Interaction of SOCS1 with Tyk2 and its degradation. Western blot analyses (WB) and immunoprecipitation (IP) Western blot analyses of Tyk2 interacting with SOCS1. A, Tyk2KD or Tyk2KD(2F) were cotransfected with SOCS1-FLAG, SOCS1F59A-FLAG, or SOCS1D64R-FLAG into HEK293T cells. Following expression, immunoprecipitation of Tyk2 constructs were performed using glutathione-Sepharose beads. Immunoprecipitates and whole cell lysates (WCL) were separated by SDS-PAGE and immunoblotted with indicated antibodies. B, Tyk2-HIS was expressed ± SOCS1-FLAG in HEK293T cells. Cells lysates were separated by SDS-PAGE and probed with the indicated antibodies. C, thymocytes from SOCS1+/+IFNγ−/− and SOCS1−/−IFNγ−/− mice were stimulated with IFNα for 0, 30, or 60 min. Cell lysates were separated by SDS-PAGE and probed with the indicated antibodies. Blots are representative of triplicate experiments. The histogram represents densitometric quantification of the Western blot analyses. Data are represented as mean ± S.E. of three independent experiments.
Because SOCS1 binding can target proteins for degradation and thus impact the levels of these critical signaling molecules, we investigated the effect of SOCS1 on cellular levels of Tyk2. Firstly, Tyk2-HIS was transfected into HEK293T cells in the presence and absence of SOCS1-FLAG. In the presence of SOCS1, Tyk2 expression was reduced (Fig. 3B). Next we analyzed the effect of SOCS1 on the endogenous levels of Tyk2. Analysis of thymocytes from SOCS1−/−IFNγ−/− and SOCS1+/+IFNγ−/− mice demonstrated that SOCS1 modified the Tyk2 expression levels (Fig. 3C). In the SOCS1+/+ thymocytes, Tyk2 levels were significantly reduced by 60 min after stimulation with IFNα. In contrast, in the SOCS1−/− thymocytes, not only was the (SOCS1-mediated) reduction in Tyk2 levels lost, but Tyk2 levels increased with IFN stimulation. Together with our overexpression data it is evident SOCS1 has an important regulatory role in type I IFN signaling through its interaction with Tyk2 and the reduction of its levels in the cell.
SOCS1 Inhibits Lys-63 Polyubiquitination of Tyk2
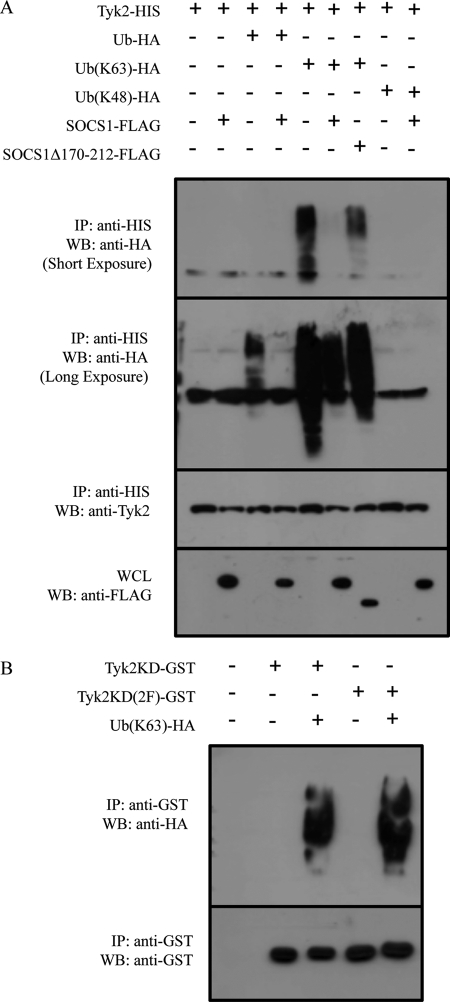
One method of SOCS1-induced degradation of binding partners is via the SOCS box recruitment of E3 ubiquitin ligases (26, 27). Thus, we investigated the ability of Tyk2 to be ubiquitinated in the presence and absence of SOCS1. Firstly, Tyk2-HIS was overexpressed with or without ubiquitin-HA, ubiquitin-(K63)-HA, or ubiquitin-(K48)-HA and/or SOCS1-FLAG or SOCS1Δ170–212-FLAG (Fig. 4A, first through fourth lanes). Following immunoprecipitation of Tyk2 and detection of total ubiquitination using ubiquitin-HA, we found that Tyk2 is indeed spontaneously ubiquitinated in this system and that coexpression with SOCS1 surprisingly inhibited this ubiquitination. We then investigated the type of ubiquitination using mutant ubiquitin constructs, which form Lys-63- or Lys-48-linked ubiquitin chains. These different ubiquitin linkages result in different conformations and activation of alternate pathways (28). Lys-48 linkages are primarily used for targeting the protein for degradation via the proteasome. In contrast, Lys-63 linkages have been implicated in numerous other functions, including activation of NF-κB-inducing kinase (29). As shown in Fig. 4A (fifth through ninth lanes), our data demonstrates that Lys-48-linked polyubiquitination of Tyk2 was not detected, whereas Lys-63-linked polyubiquitination was observed. In addition, the use of ubiquitin-(Lys-63)-HA resulted in increased ubiquitination of Tyk2 compared with wild-type ubiquitin-HA and may be explained either by the greater specificity of this reagent or by the mutation of lysine residues involved in negative regulation. Importantly, SOCS1 coexpression suppressed Lys-63-linked ubiquitination of Tyk2. The expression of SOCS1Δ170–212 (lacking the SOCS box) also inhibited Lys-63-linked polyubiquitination of Tyk2, although to a lesser extent.
FIGURE 4.
The effect of SOCS1 on Tyk2 ubiquitination. Western blots analyses (WB) and immunoprecipitation (IP) Western blot analyses of Tyk2 ubiquitination. A, Tyk2-HIS was expressed in HEK293T cells with ubiquitin-HA, ubiquitin(K63)-HA, or ubiquitin(K48)-HA ± SOCS1-FLAG or SOCS1Δ170–212 in the presence of MG132. Cell lysates were immunoprecipitated with nickel affinity beads. Immunoprecipitates and whole cell lysates (WCL) were separated by SDS-PAGE and probed with the indicated antibodies. The second panel from the top shows a shorter exposure than the top panel. Tyk2 ubiquitination is shown in the top panel, third lane, and Lys-63 ubiquitination in the top panel and the second panel from the top, fifth lane. Inhibition by SOCS1 constructs is evident in the fourth lane (top panel) and the sixth and seventh lanes (top panel or second panel from the top). B, Tyk2KD-GST or Tyk2KD(2F)-GST were expressed in HEK293T cells ± ubiquitin-HA. Cell lysates were immunoprecipitated with glutathione beads. Immunoprecipitates were separated by SDS-PAGE and probed with the indicated antibodies. Blots are representative of triplicate experiments.
We next investigated the impact of whether the phosphotyrosine residues of Tyk2 that were involved in binding SOCS1 were involved in ubiquitination. Our results show that the Tyk2 kinase domain was polyubiquitinated (Fig. 4B). Tyk2KD(2F) was also Lys-63 polyubiquitinated. As Tyk2 requires phosphorylation of these mutated tyrosine residues for its function our results suggest that Lys-63 ubiquitination of the Tyk2 kinase domain is not dependent on Tyk2 phosphorylation and activation.
SOCS1 Expression Reduces IFNAR1 Surface Levels with IFNα Stimulation
Tyk2 has been reported to be involved in stabilization of IFNAR1 at the plasma membrane (30). Because SOCS1 binds Tyk2 and modulates both its degradation and its activating ubiquitination, it is possible that the interaction of SOCS1 with IFNAR1 via its associated Tyk2 modifies cell surface expression levels. Flow cytometry was used to measure the surface expression of IFNAR1 in primary thymocytes from SOCS1−/−IFNγ−/−, SOCS1+/+IFNγ−/−, and wild-type mice (Fig. 5). After IFN stimulation, IFNAR1 surface levels are reduced in all three genotypes. However, in SOCS1−/−IFNγ−/− thymocytes, IFNAR1 surface expression was reduced to a lesser extent than that in thymocytes from SOCS1+/+IFNγ−/− and wild-type mice one and two h after IFN stimulation (Fig. 5A). Thus, SOCS1 either accelerated the internalization or degradation of the receptor or increased recycling/resynthesis. Cycloheximide (CHX) was used, where shown, to block de novo protein synthesis in thymocytes from all three genotypes, thereby allowing us to measure the real rate of internalization in the absence of de novo protein synthesis (Fig. 5B). In the presence of cycloheximide there was again significantly more IFNAR1 present on the surface of SOCS1−/− cells compared with control cells at the 1-h time point. Our results therefore demonstrate that the surface levels of IFNAR1 during a response are modulated by SOCS1, likely through its interaction with Tyk2.
FIGURE 5.
The effect of SOCS1 on surface levels of IFNAR1. The graphs show IFNAR1 surface levels on thymocytes from SOCS1+/+IFNγ−/−, SOCS1−/−IFNγ−/−, and wild-type mice stimulated with IFNα for 0, 1, or 2 h. Cells were probed with anti-IFNAR1 antibody and analyzed by flow cytometry to detect IFNAR1 surface levels in the presence and absence of cycloheximide (CHX). Data are represented as mean ± S.E. of four experiments. ***, p < 0.001; **, p < 0.005.
DISCUSSION
SOCS1 regulation of type I IFN signaling is critical in maintaining an appropriate immune response and to survival. The data presented here complement our previous in vivo data, which demonstrate that SOCS1 regulates signaling through the IFNAR1 component of the receptor. We now add mechanistic data showing how this occurs. Indeed, SOCS1 regulation of type I IFN signaling is mediated via an interaction with the IFNAR1-associated kinase Tyk2 and not by direct interaction with receptor tyrosine kinases. This distinguishes the mechanism of SOCS1 regulation of type I IFN signaling from how it modulates type II IFNγ signaling. This study suggests that in the type I IFN system none of the intracellular tyrosine residues within IFNAR1 are important for the negative regulation of type I IFN signaling via SOCS1. Indeed, they are not required for driving ISRE (STAT1/STAT2/IRF9)-dependent signaling nor for STAT3 signaling as shown recently and thus not for gamma-interferon activated sequence (GAS) element activation (23, 31). It would be interesting to investigate the impact of these residues in the receptors and of SOCS1 on other type I IFN signaling pathways. Consequently, these results demonstrate that for the type I IFN signaling complex the interaction of SOCS1 is mediated indirectly via an adaptor molecule. Within the type I IFN system, SOCS1 requires its KIR and SH2 domains for its inhibitory function, indicating a requirement for SOCS1 interaction with the kinase and phosphotyrosine residues. Our coimmunoprecipitation studies demonstrated that the Tyk2 binding region of IFNAR1 was required for the interaction with SOCS1. Previous studies have found a direct interaction between Tyk2 and SOCS1 and a requirement of the Tyk2 SH2 domain and SOCS1 KIR domain (14), but these studies were not performed in the context of the type I IFN receptor signaling complex as described herein. Here we show that SOCS1 binds conserved tyrosine residues within the Tyk2 catalytic region (Tyr-1054 and Tyr-1055), which are phosphorylated in response to IFNα and are required for Tyk2 activation (6). Mutation of these residues from tyrosine to phenylalanine resulted in almost complete inhibition of the interaction between Tyk2 and SOCS1. Our results therefore suggest that the SOCS1 interaction with Tyk2 is limited to the activated, phosphorylated kinase behaving in a negative feedback manner. In addition to a requirement of the SH2 domain, we also found that the KIR domain of SOCS1 is important for binding to Tyk2 and for its inhibitory action of IFN signaling. Modeling studies have proposed that the KIR domain of SOCS1 mimics the activation loop of Jak2 and obstructs its catalytic groove (19). This region is highly conserved across JAK family members, and thus it is probable that the KIR domain of SOCS1 can also interact with and inhibit Tyk2 catalytic function.
The SOCS box has been implicated previously in the recruitment of E3 ubiquitin ligases and the consequent ubiquitination and destruction of target proteins (26, 27). We therefore aimed to investigate the effect of SOCS1 on Tyk2 ubiquitination and, quite interestingly, found that SOCS1 expression inhibited rather than facilitated the ubiquitination of Tyk2. Analysis of the type of ubiquitination of Tyk2 found that it was Lys-63- linked and not Lys-48-linked ubiquitination. In other signaling systems, Lys-63-linked ubiquitination is associated with activation of target proteins. For example, Lys-63 ubiquitination of the NF-κB-inducing kinase by zinc finger protein 91 contributes to stabilization and tyrosine phosphorylation of the kinase (29). Thus, SOCS1 may exert its negative effects on Tyk2 in part via inhibiting an activating or stabilizing Lys-63 ubiquitination. A mutant SOCS1 protein lacking the SOCS box was also able to inhibit the Lys-63 ubiquitination of Tyk2 and, thus, the SOCS box does not appear to be essential for this function. The mechanism of SOCS1 inhibition of ubiquitination may be via blocking the ubiquitination site of Tyk2, which we found was located within the Tyk2 kinase domain, the same region of Tyk2 to which SOCS1 binds. As SOCS1 coexpression with Tyk2 resulted in reduced Tyk2 levels, it is probable that inhibition of this ubiquitination by SOCS1 destabilizes Tyk2. As SOCS1 did not enhance ubiquitination, as might have been expected, the SOCS1 SOCS box appears to not have a critical role in the regulation of Tyk2. Our luciferase assay supports this conclusion because SOCS1 lacking the SOCS box still enabled significant inhibition of type I IFN signaling. The affinity of the SOCS1 SOCS box for E3 ubiquitin ligases is significantly weaker than that of the other SOCS family members (15). Deletion of the SOCS1 SOCS box in mice, however, resulted in reduced function of SOCS1 in response to IFNγ (32). This may be attributed to the SOCS box stabilizing SOCS1 through recruitment of other proteins. In our overexpression assays we saw slight, although not statistically significant, differences between wild-type SOCS1 and the SOCS box mutant. Perhaps in vivo a more significant role of the SOCS box in type I IFN signaling would be seen.
The Lys-63 linked ubiquitination of Tyk2 is also a novel finding and warrants further investigation. A candidate residue for ubiquitination is lysine 930, located within the Tyk2 kinase domain. A mutation of this residue destabilizes Tyk2 and reduces kinase activity (6).
In addition to facilitating IFN signal transduction by activation of STAT interactions with the receptor, Tyk2 is required for the stabilization of IFNAR1 at the plasma membrane. We postulated that the SOCS1 interaction with Tyk2 may therefore result in the destabilization of Tyk2 and the dissociation of Tyk2 from IFNAR1, leading to reduced surface levels of the receptor. In human cells lacking Tyk2, IFNAR1 is only weakly expressed at the cell surface and is instead found localized in perinuclear endosomal compartments, constituting early and recycling endosomes (30). An internalization motif of human IFNAR1, located within the Tyk2 binding site, is polyubiquitinated and targeted for degradation (33). Increased expression of Tyk2 results in increased localization of IFNAR1 to the cell surface and reduced degradation of IFNAR1 due in part to reduced endocytosis (30). In our overexpression experiments we found that SOCS1 reduced Tyk2 expression and that in primary thymocytes the absence of SOCS1 resulted in increased endogenous Tyk2 expression 1 h after IFN stimulation. Analysis of IFNAR1 internalization in thymocytes demonstrated a role for SOCS1 in controlling IFNAR1 surface expression following IFNα stimulation. Although not significantly different basally, IFNAR1 internalization/recycling is reduced in cells lacking SOCS1 following 1-h IFNα stimulation and correlates with increased endogenous expression of Tyk2. SOCS1 therefore has a role in effecting the surface expression of IFNAR1 during an interferon response. SOCS1 association of Tyk2 following its phosphorylation and activation may result in destabilization of Tyk2 and exposure of the IFNAR1 internalization motif with subsequent IFNAR1 internalization and reduced IFN signaling.
Together these studies demonstrate the mechanism of SOCS1 inhibition of type I IFN signaling. An understanding of this mechanism may lead to novel approaches to manipulate the type I IFN response and thus contribute to better therapeutic treatments of IFN-mediated diseases or enhancement of the beneficial therapeutic effects of IFN.
This work was supported by funding from the National Health and Medical Research Council and the Victorian Government Infrastructure Support Program.
- IFNAR
- IFN α receptor
- SOCS
- suppressor of cytokine signaling
- MEF
- murine embryonic fibroblast
- ISRE
- interferon stimulated response element
- TK
- thymidine kinase
- β-ME
- β-mercaptoethanol
- GAS
- gamma-interferon activated sequence.
REFERENCES
- 1. Samuel C. E. (2001) Clin. Microbiol. Rev. 14, 778–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Weerd N. A., Samarajiwa S. A., Hertzog P. J. (2007) J. Biol. Chem. 282, 20053–20057 [DOI] [PubMed] [Google Scholar]
- 3. Platanias L. C., Fish E. N. (1999) Exp. Hematol. 27, 1583–1592 [DOI] [PubMed] [Google Scholar]
- 4. Colamonici O. R., Uyttendaele H., Domanski P., Yan H., Krolewski J. J. (1994) J. Biol. Chem. 269, 3518–3522 [PubMed] [Google Scholar]
- 5. Domanski P., Fish E., Nadeau O. W., Witte M., Platanias L. C., Yan H., Krolewski J., Pitha P., Colamonici O. R. (1997) J. Biol. Chem. 272, 26388–26393 [DOI] [PubMed] [Google Scholar]
- 6. Gauzzi M. C., Velazquez L., McKendry R., Mogensen K. E., Fellous M., Pellegrini S. (1996) J. Biol. Chem. 271, 20494–20500 [DOI] [PubMed] [Google Scholar]
- 7. Gauzzi M. C., Barbieri G., Richter M. F., Uzé G., Ling L., Fellous M., Pellegrini S. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 11839–11844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barbieri G., Velazquez L., Scrobogna M., Fellous M., Pellegrini S. (1994) Eur. J. Biochem. 223, 427–435 [DOI] [PubMed] [Google Scholar]
- 9. Müller M., Briscoe J., Laxton C., Guschin D., Ziemiecki A., Silvennoinen O., Harpur A. G., Barbieri G., Witthuhn B. A., Schindler C. (1993) Nature 366, 129–135 [DOI] [PubMed] [Google Scholar]
- 10. Constantinescu S. N., Croze E., Wang C., Murti A., Basu L., Mullersman J. E., Pfeffer L. M. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 9602–9606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schindler C., Levy D. E., Decker T. (2007) J. Biol. Chem. 282, 20059–20063 [DOI] [PubMed] [Google Scholar]
- 12. Alexander W. S., Starr R., Fenner J. E., Scott C. L., Handman E., Sprigg N. S., Corbin J. E., Cornish A. L., Darwiche R., Owczarek C. M., Kay T. W., Nicola N. A., Hertzog P. J., Metcalf D., Hilton D. J. (1999) Cell 98, 597–608 [DOI] [PubMed] [Google Scholar]
- 13. Fenner J. E., Starr R., Cornish A. L., Zhang J. G., Metcalf D., Schreiber R. D., Sheehan K., Hilton D. J., Alexander W. S., Hertzog P. J. (2006) Nat. Immunol. 7, 33–39 [DOI] [PubMed] [Google Scholar]
- 14. Narazaki M., Fujimoto M., Matsumoto T., Morita Y., Saito H., Kajita T., Yoshizaki K., Naka T., Kishimoto T. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13130–13134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Babon J. J., Sabo J. K., Zhang J. G., Nicola N. A., Norton R. S. (2009) J. Mol. Biol. 387, 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nicholson S. E., De Souza D., Fabri L. J., Corbin J., Willson T. A., Zhang J. G., Silva A., Asimakis M., Farley A., Nash A. D., Metcalf D., Hilton D. J., Nicola N. A., Baca M. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 6493–6498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jenkins B. J., Grail D., Nheu T., Najdovska M., Wang B., Waring P., Inglese M., McLoughlin R. M., Jones S. A., Topley N., Baumann H., Judd L. M., Giraud A. S., Boussioutas A., Zhu H. J., Ernst M. (2005) Nat. Med. 11, 845–852 [DOI] [PubMed] [Google Scholar]
- 18. Qing Y., Costa-Pereira A. P., Watling D., Stark G. R. (2005) J. Biol. Chem. 280, 1849–1853 [DOI] [PubMed] [Google Scholar]
- 19. Giordanetto F., Kroemer R. T. (2003) Protein Eng. 16, 115–124 [DOI] [PubMed] [Google Scholar]
- 20. Endo T. A., Masuhara M., Yokouchi M., Suzuki R., Sakamoto H., Mitsui K., Matsumoto A., Tanimura S., Ohtsubo M., Misawa H., Miyazaki T., Leonor N., Taniguchi T., Fujita T., Kanakura Y., Komiya S., Yoshimura A. (1997) Nature 387, 921–924 [DOI] [PubMed] [Google Scholar]
- 21. Yasukawa H., Misawa H., Sakamoto H., Masuhara M., Sasaki A., Wakioka T., Ohtsuka S., Imaizumi T., Matsuda T., Ihle J. N., Yoshimura A. (1999) EMBO J. 18, 1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicholson S. E., Willson T. A., Farley A., Starr R., Zhang J. G., Baca M., Alexander W. S., Metcalf D., Hilton D. J., Nicola N. A. (1999) EMBO J. 18, 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao W., Lee C., Piganis R., Plumlee C., de Weerd N., Hertzog P. J., Schindler C. (2008) J. Immunol. 180, 5483–5489 [DOI] [PubMed] [Google Scholar]
- 24. Trajanovska S., Owczarek C. M., Stanton P. G., Hertzog P. J. (2003) J. Interferon Cytokine Res. 23, 351–358 [DOI] [PubMed] [Google Scholar]
- 25. Sheehan K. C., Lai K. S., Dunn G. P., Bruce A. T., Diamond M. S., Heutel J. D., Dungo-Arthur C., Carrero J. A., White J. M., Hertzog P. J., Schreiber R. D. (2006) J. Interferon Cytokine Res. 26, 804–819 [DOI] [PubMed] [Google Scholar]
- 26. Kamura T., Sato S., Haque D., Liu L., Kaelin W. G., Jr., Conaway R. C., Conaway J. W. (1998) Genes Dev. 12, 3872–3881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang J. G., Farley A., Nicholson S. E., Willson T. A., Zugaro L. M., Simpson R. J., Moritz R. L., Cary D., Richardson R., Hausmann G., Kile B. J., Kent S. B., Alexander W. S., Metcalf D., Hilton D. J., Nicola N. A., Baca M. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 2071–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tenno T., Fujiwara K., Tochio H., Iwai K., Morita E. H., Hayashi H., Murata S., Hiroaki H., Sato M., Tanaka K., Shirakawa M. (2004) Genes Cells 9, 865–875 [DOI] [PubMed] [Google Scholar]
- 29. Jin X., Jin H. R., Jung H. S., Lee S. J., Lee J. H., Lee J. J. (2010) J. Biol. Chem. 285, 30539–30547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ragimbeau J., Dondi E., Alcover A., Eid P., Uzé G., Pellegrini S. (2003) EMBO J. 22, 537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russell-Harde D., Wagner T. C., Rani M. R., Vogel D., Colamonici O., Ransohoff R. M., Majchrzak B., Fish E., Perez H. D., Croze E. (2000) J. Biol. Chem. 275, 23981–23985 [DOI] [PubMed] [Google Scholar]
- 32. Zhang J. G., Metcalf D., Rakar S., Asimakis M., Greenhalgh C. J., Willson T. A., Starr R., Nicholson S. E., Carter W., Alexander W. S., Hilton D. J., Nicola N. A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 13261–13265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar K. G., Barriere H., Carbone C. J., Liu J., Swaminathan G., Xu P., Li Y., Baker D. P., Peng J., Lukacs G. L., Fuchs S. Y. (2007) J. Cell Biol. 179, 935–950 [DOI] [PMC free article] [PubMed] [Google Scholar]