Background: β-Amyloid activates presynaptic nicotinic receptors, regulating nerve terminal Ca2+.
Results: Mutating tyrosine 188 but not tyrosine 195 in the ligand-binding domain of α7-nicotinic receptors eliminates activation by β-amyloid.
Conclusion: Tyrosine 188 in the ligand-binding domain of α7-nicotinic receptors plays a key role in β-amyloid-induced activation of the receptors.
Significance: Direct activation of α7-nicotinic receptors may represent a neuromodulatory function of β-amyloid.
Keywords: Alzheimer's Disease, Amyloid, Calcium Imaging, Neuroblastoma, Nicotinic Acetylcholine Receptors, Presynaptic
Abstract
Soluble β-amyloid (Aβ) resides in certain regions of the brain at or near picomolar concentration, rising in level during the prodromic stage of Alzheimer disease. Recently, we identified the homomeric α7 nicotinic acetylcholine receptor (α7-nAChR) as one possible functional target for picomolar Aβ. This study was aimed at addressing which residues in α7-nAChRs potentially interact with Aβ to regulate the presynaptic function of this receptor. Site-directed mutagenesis was carried out to study the key aromatic residues in the mouse α7-nAChR agonist-binding pocket. Mutations of tyrosine188 resulted in a decrease in activation of presynaptic α7-nAChRs by ACh and Aβ but with no change in response to nicotine, indicating the critical role of Tyr-188 in presynaptic regulation by Aβ. Coimmunoprecipitation additionally revealed direct binding of Aβ to α7-nAChRs and to the Tyr-188 mutant receptor. In contrast, mutations of Tyr-195 in α7-nAChR led to decreased activation by nicotine without apparent effects on ACh- or Aβ-induced responses. Agonist-induced responses of Tyr-93 mutant α7-nAChRs indicated possible interactions of nicotine and Aβ with its hydroxyl group, but there was no change in presynaptic responses after mutation of Trp-149. All of the mutants were shown to be expressed on the plasma membrane using cell surface labeling. Together, these results directly demonstrate an essential role for the aromatic residue Tyr-188 as a key component in the agonist binding domain for the activation of α7-nAChRs by Aβ.
Introduction
Alzheimer disease (AD)2 is a neurodegenerative disease characterized by distinct pathologies. Over the course of the disease, there is loss of synapses and neuronal cells in select regions of the brain, particularly those critical for memory and cognitive processes. Histologically, AD pathology is evident in these regions as neuritic plaques, neurofibrillary tangles, and local inflammation. Neuritic plaques are primarily composed of fibrillar β-amyloid (Aβ), but the noted loss of synapses is best correlated with the level of soluble Aβ early in AD (1, 2), followed by the accumulation of neurofibrillary tangles. In the absence of disease, the production of Aβ from synapses (3–5) and the high rate of turnover of soluble Aβ (6) raise the possibility that the peptide is biologically active, perhaps as a neuromodulator (7, 8). Later, as Aβ accumulates, its action may then become disruptive, leading to compromised synaptic signaling (9), possibly via an alteration in the phosphorylation and function of the microtubule-associated protein tau (10, 11), the primary component of neurofibrillary tangles.
To date, the exact cellular targets in brain for Aβ are unknown. One possibility is the nicotinic acetylcholine receptor (nAChR), and Aβ regulation of nAChRs has been reported in numerous studies, most notably for the α7 subtype, but other subtypes have also been implicated (7, 12–15). Homomeric nicotinic α7 nAChRs appeared to display high-affinity binding to Aβ (16, 17), although this remains controversial (18). α7-nAChRs are highly expressed on the presynaptic inputs in the hippocampus and cortex, which are critical regions for synaptic plasticity and memory formation. Specifically, α7-nAChRs located on hippocampal glutamatergic terminals regulate presynaptic glutamate release (19), and α7-nAChRs may facilitate long-term potentiation (20). When applied to rodent hippocampal nerve terminals, pm to nm concentrations of Aβ induced increases in presynaptic calcium, which were largely dependent upon the presence of presynaptic nAChRs, as demonstrated in pharmacological studies and studies using receptor null mutants (21, 22). Picomolar Aβ was also shown to positively modulate synaptic plasticity in the hippocampus via presynaptic α7-nAChRs (7), as measured by an increase in long-term potentiation. In contrast, Aβ blocked acetylcholine (ACh)-induced nicotinic postsynaptic currents when tested on α7-nAChRs in primary cultures of hippocampal neurons (13) and hippocampal slices (14), suggesting an inhibitory effect. Therefore, Aβ may play distinct regulatory roles depending on the location of the nAChRs. In particular, nAChRs at presynaptic sites possibly mediate an agonist-like effect of pm-nm Aβ.
To demonstrate that the agonist-like action of Aβ involved direct activation of nAChRs, α7-nAChRs were expressed in the axonal varicosities of NG108-15 cells, which normally do not express functional nAChRs (23). Aβ applied to NG108-15 varicosities expressing α7-nAChRs induced pronounced and sustained increases in Ca2+, reconstituting the agonist-like effect of Aβ in presynaptic-like structures, and the response could be fully blocked by the selective α7-nAChR antagonist α-bungarotoxin (23). To define the basis for Aβ activation of α7-nAChRs, this study examined candidate residues in the ligand binding domain of α7-nAChRs. Key aromatic residues for ligand binding, part of the so-called “aromatic cluster” in loops A, B, and C (24, 25), were investigated to assess whether they contribute to interactions with Aβ. The structural description of the mouse α7-nAChR ligand binding site was derived from that of the Torpedo nAChR (a muscle-type nAChR) and the ACh binding protein (24).
EXPERIMENTAL PROCEDURES
Cell Cultures and Transfection
NG108-15 cells were cultured in DMEM containing 15% FBS and differentiated in 1% FBS-containing DMEM on exposure to dibutyryl cyclic AMP (1 mm, Sigma Aldrich) for 48 h until the presence of neurites and varicosities were observed (typically 2–3 days) as described (23). Under these conditions, the neurites have a predominantly axonal character, and the varicosities display all of the necessary features of a presynaptic element (26–31). α7-nAChR was expressed in the NG108-15 cells by transfecting the mouse α7 cDNA (α7 cDNA or site-directed mutant α7 cDNA incorporated in pcDNA3.1zeo) with the FuGENE 6 transfection reagent (Roche). Mock cells were treated with FuGENE 6 alone. Successful expression of α7-nAChRs required another 48 h, and functional responses of α7-nAChRs in varicosities were monitored by confocal imaging (23). Moderate expression levels were typically obtained, allowing for detection of negative or positive changes following receptor mutation.
Confocal Imaging
Changes in Ca2+ level in individual varicosities of differentiated NG108-15 cells were monitored by the specific Ca2+ fluorescent dye Fluo-4 as described previously (23). In brief, Fluo-4 was loaded into the cells cultured on Cell-Tak-coated coverslips as the acetoxymethylester derivative (Invitrogen). The cells were then perfused with oxygenated HEPES-buffered saline (HBS) (142 mm NaCl, 2.4 mm KCl, 1.2 mm K2PO4, 1 mm MgCl2, 1 mm CaCl2, 5 mm D-glucose, 10 mm HEPES (pH 7.4), 100 nm tetrodotoxin; 1 μm atropine was included for ACh treatment), and were mounted into a rapid-exchange Warner perfusion chamber. Changes in fluorescence were visualized by a Zeiss LSM 5 Pascal confocal imaging system (excitation, 488 nm; emission, 515–565 nm band-pass; ×40/1.3 epifluorescence, oil-immersion Plan-Neofluar objective). Responses were recorded as a time course of 300 s, comprising 30 frames of confocal images with 10-s intervals. Aβ1–42, ACh, or nicotine was applied at the fifth frame by switching between HBS and HBS containing agonist via a six-channel perfusion valve control system (VC-66CS, Warner Instruments) with DN series constant flow. Four to ten varicosities were identified in captured images, and the fluorescent intensity associated with each varicosity was determined across all frames using ImageJ software. Fluorescence was converted to signal intensity and normalized to baseline (F/F0). Peak responses were F/F0 collected during 120–300 s after activation. For comparison to WT, increased peak (plateau) responses (F/F0-1) of mutant receptors were normalized to those of WT α7-nAChRs.
Site-directed Mutagenesis
The mouse α7-nAChR cDNA sequence was subcloned into the pcDNA3.1zeo plasmid vector (courtesy of Dr. Jerry Stitzel, University of Colorado). Tyrosines and tryptophans of interest were located, and mutations were made by changing original codons to desired codons using the QuikChange II XL site-directed mutagenesis kit (Stratagene) by PCR using the following primers: Y188F, 5′-cctggcaaaaggaatgagaagttctttgaatgctgcaaag-3′; Y188S, 5′-ggcaaaaggaatgagaagttcagtgaatgctgcaaagagccata-3′; Y195F, 5′-aatgctgcaaagagccattcccagatgtcaccta-3′; Y195S, 5′-gttctatgaatgctgcaaagagccaagcccagatgtcacc-3′; Y93F, 5′-aaccagacattctcctctttaacagtgcagatgaacg-3′; Y93S, 5′-gaaaccagacattctcctcagtaacagtgcagatgaacgc-3′; W149F, 5′-caaactgaagtttgggtccttctcctatggagggtgg-3′; and W149A, 5′-actgaagtttgggtccgcgtcctatggagggtgg-3′.
The resultant mutant sequences were confirmed by DNA sequencing (GENEWIZ, Inc., New Brunswick, NJ; Genomics Core Facility at John A. Burns School of Medicine, University of Hawaii; Advanced Studies in Genomics, Proteomics, and Bioinformatics, University of Hawai'i at Manoa), comparing against the mouse α7 subunit nucleotide sequence (NCBI CCDS data base for Mus musculus Chrna7). Numbering of the mouse α7-nAChR amino acid sequence follows that of the processed, mature receptor (minus the 22-amino acid signal sequence).
Construction of the α7 nAChR/5-HT3A Receptor Chimera
A chimeric receptor, α7V202-5HT3A, composed of the extracellular domain of the α7-nAChR subunit and the transmembrane and intracellular domains of the 5HT3A subunit, was constructed to distinguish the role of α7-nAChR extracellular domain. The procedure for construction of the plasmid containing the sequence for the chimeric receptor was adapted from a previously published protocol (32). The N-terminal 224 amino acids of the mouse α7-nAChR (amino acids 1–224 of the proform, the last residue being Val-202 in the mature form) and the C-terminal 242 amino acids of the mouse 5HT3A receptor were amplified by PCR with overlapping ends. Two sets of primer pairs (forward and reverse) were used to amplify the extracellular domain sequence of α7-nAChR from its plasmid and the transmembrane and intracellular domain sequence of the 5HT3A subunit from its respective plasmid. A restriction site, XhoI, was built into the forward primer for the α7 ECD sequence (5′-taggctagcctcgagccggcggacggcgggaca-3′). The reverse primer for the α7 ECD sequence was composed of both the end of extracellular domain sequence of α7 nAChR and the beginning of first transmembrane domain sequence of the 5HT3A receptor (5′-taaaggcctccggcggatgatcactgtgtaggtgacatctgggt-3′). The forward primer for the 5HT3 sequence was simply the reverse complement of the reverse primer (5′-acccagatgtcacctacacagtgatcatccgccggaggccttta-3′). The final primer matched the end of the 5HT3A receptor sequence and had a XmaI restriction site built in (5′-aagcggccgcccgggtcaagaataatgccaaatgga-3′). After the first round of PCR, two pieces with overlapping ends were produced. Further amplification of these two overlapping fragments generated the open reading frame of α7V202-5HT3A, with two restriction sites in both ends, facilitating cloning of the α7V202-5HT3A sequence into a pCI mammalian expression vector. The resultant full chimeric receptor sequence was confirmed by DNA sequencing (Advanced Studies in Genomics, Proteomics, and Bioinformatics, University of Hawai'i at Manoa). Following transfection of differentiated NG108-15 cells, the expression level of the chimeric receptor was checked by both immunostaining and immunoblotting of biotinylated cell surface proteins.
Immunostaining and Immunoblotting following Cell Surface Biotinylation
Immunostaining was used to visualize and compare the expression of wild-type and mutant α7-nAChRs in the varicosities of the differentiated NG108-15 cells following transfection of appropriate plasmids, as described previously (23). Mock-treated cells were used as a background control. Forty-eight hours after transfection, cells were fixed with 4% paraformaldehyde in HBS, washed extensively with TBS, and then incubated with an anti-α7-nAChR antibody (1:200, Millipore). (The specificity of the anti-α7-nAChR antibody was confirmed by comparing immunoprecipitates from a wild-type and an α7-nAChR null mutant (knockout) mouse hippocampus via immunoblot analysis.) FITC-conjugated goat anti-rabbit IgG (1:500, Jackson ImmunoResearch Laboratories, Inc.) was used as the secondary antibody. Stained cells were visualized by the Zeiss LSM 5 Pascal confocal imaging system.
Labeling and isolation of surface proteins on the NG108-15 cells were performed following biotinylation using the Pierce Cell Surface Protein Isolation kit. The presence of surface wild-type and mutant α7-nAChRs in transfected cells, as compared with mock-treated cells, was assessed via immunoblot analysis using an anti-α7-nAChR antibody (1:2000; Millipore) as primary antibody and peroxidase-conjugated goat anti-rabbit IgG as the secondary antibody (1:2500; Jackson ImmunoResearch Laboratories, Inc.). To control for protein loading on the blots, immunolabeling of the transferrin receptor, a ubiquitous cell surface protein, was performed using an anti-transferrin receptor antibody (Abcam, Inc.).
Coimmunoprecipitation
Coimmunoprecipitation of α7-nAChRs with Aβ followed a previously published protocol (15) with the following modifications. NG108-15 cells at 80% confluence in 25 cm2 culture flasks were differentiated as described previously. Plasmids containing cDNA sequences for wild-type or Y188S α7-nAChRs were transfected into the differentiated cells. After 48 h, cells were incubated for 30 min with HBS (control) or 100 nm Aβ42 in HBS at 4 °C with constant shaking. All of the remaining steps were also performed at 4 °C. Cells were recovered from the flasks and pelleted by centrifugation at 1500 × g. Pellets were lysed in 250 μl of cell lysis buffer (25 mm HEPES, 200 mm NaCl, 1 mm EDTA, 0.5% digitonin, 0.2% sodium cholate, 0.5% Nonidet P-40, HaltTM protease inhibitor mixture, 0.02% 2-mercaptoethanol) for 15 min on ice in a bath sonicator, followed by an additional 1-h incubation with continuous shaking on ice. The supernatant was collected following centrifugation of the insoluble fraction at 10,000 × g. The lysates were then incubated with protein G Dynabeads bound with anti-Aβ1–42 monoclonal antibodies, 6E10 (Covance) and BAM10 (Sigma), for 16 h. (Inclusion of both monoclonal antibodies was found to be essential.) The Dynabead immune complexes were isolated via a magnet (DynaMagTM-2), and the remaining lysate was kept for determination of the relative amount of protein by immunoblotting for GAPDH using an anti-GAPDH antibody (Abcam, Inc.). The Dynabead immune complexes were then washed three times with 200 μl of PBS and dissolved in 90 μl of SDS-PAGE sample buffer containing 5% 2-mercaptoethanol. After boiling for 5 min, samples were resolved on 4–20% Tris-HCl SDS gels and then transferred to nitrocellulose. The immunoblot analysis for wild-type and Y188S α7-nAChRs was performed as described in the previous section. The α7-nAChR was detected using a rabbit polyclonal anti-α7-nAChR antibody (1:2000, Millipore), and the 5-HT3A receptor was detected using rabbit polyclonal antibody Ab120 (1:2000, Ref. 33), followed by peroxidase-conjugated goat anti-rabbit IgG (1:2500, Jackson ImmunoResearch Laboratories, Inc.) or IRDye® 680 goat anti-rabbit (1:5000, LI-COR Biosciences) as secondary antibody. Immunoreactive bands were detected by ECLtm detection (GE Healthcare) or Odyssey® Infrared Imaging (LI-COR Biosciences).
Chemicals
Acetylcholine chloride, nicotine tartrate citrate, atropine chloride, digitonin, sodium cholate, and Nonidet P-40 were purchased from Sigma Aldrich. ACh was prepared as a stock solution at 10 mm, stored at −20°C, and diluted to 500 nm with oxygenated HBS before each experiment. 500 nm nicotine was freshly prepared by dissolving in oxygenated HBS before each experiment. Tetrodotoxin was from Calbiochem. The anti-α7-nAChR-specific antibody was purchased from Millipore, and the FITC- and peroxidase-conjugated goat anti-rabbit and anti-mouse antibodies were from Jackson ImmunoResearch Laboratories, Inc. The 6E10 anti-Aβ1–42 monoclonal antibody was from Covance, and the BAM-10 anti-Aβ1–42 monoclonal antibody was from Sigma. Anti-GAPDH and anti-transferrin receptor antibodies were from Abcam, Inc. IRDye® 680 goat anti-rabbit and IRDye® 800 goat anti-mouse antibodies were from LI-COR Biosciences. Fluo-4/AM, protein G Dynabeads, and DynaMagTM-2 were purchased from Invitrogen. The Pierce cell surface protein isolation kit and HaltTM protease inhibitor mixture were from Thermo Scientific.
Aβ
Aβ1–42 was purchased from BACHEM or American Peptide. A stock solution of Aβ1–42 was prepared at 0.1 mm by dissolving solid Aβ peptide in double deionized water as described previously (23). Aβ1–42 for each experiment was diluted (from 1 pm to 100 nm) from the 0.1 mm stock into oxygenated HBS and vortexed to assure full suspension. Under these conditions, the Aβ is largely oligomeric, as assessed by SDS-PAGE and native gel analysis (23). Although monomeric Aβ is also present (e.g. Refs. 34, 35) and may or may not be active, it appears to be a minor component when assessed under native conditions using size exclusion chromatography (36).
Data Analysis and Statistics
The digitized images from confocal time series recordings were analyzed by Image J software. The signal intensity associated with an individual varicosity was expressed as F/F0 for each time point, where F0 is the signal intensity at time zero. Time series were all corrected for photobleaching (typically < 3%) and expressed as mean ± S.E. n represents the number of varicosities examined. Each experiment was replicated at least three times. Multiple groups were compared by one-way analysis of variance with Bonferroni multiple comparison post hoc test. Two-tailed Student's t tests were used for comparison of two groups. p < 0.05 was used as the minimal threshold for significance.
RESULTS
Aβ Activates α7-nAChRs via the Extracellular Domain of the Receptor
The rodent hybrid neuroblastoma cell line NG108-15 was used as a model neuronal system for expression of nAChRs, as they are normally devoid of functional nAChRs. We recently demonstrated the functional expression of nAChRs in differentiated NG108-15 cells following transient transfection of the mouse sequence for α7-nAChR (23). The axonal varicosities of the NG108-15 cells are presynaptic-like structures capable of synapsing on the appropriate postsynaptic target (26–28), and thus expression of nAChRs in the varicosities serves as a reconstituted presynaptic system for examining the agonist-like action of Aβ.
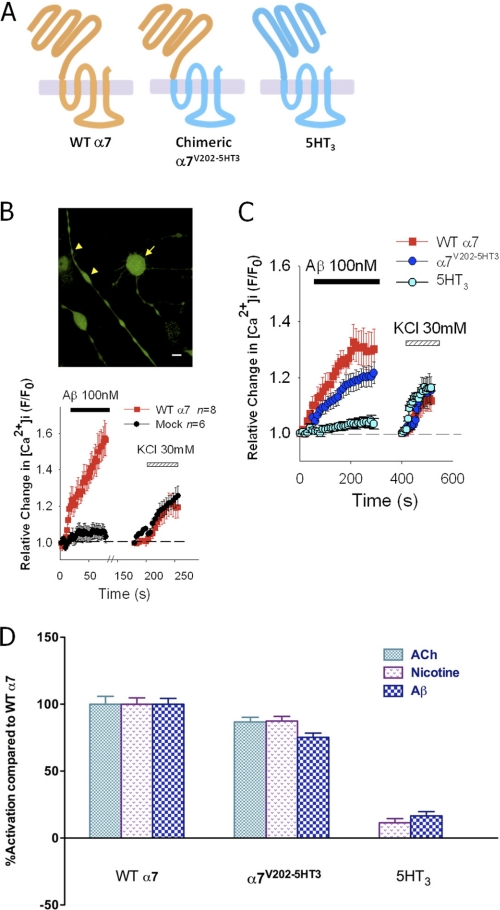
To begin to define the region(s) of the α7-nAChR with which Aβ interacts, we first asked whether the ECD is necessary and sufficient for activation of the receptor by Aβ. To do so, we constructed a chimeric receptor by fusing the nAChR ECD together with the transmembrane and intracellular domains of the 5-HT3 serotonin receptor (13, 37, 38), as the binding site in α7-nAChR for classical nicotinic agonists is located in the ECD, but Aβ may also influence α7-nAChR activity through an additional interaction with the plasma membrane (23), perhaps via lipid-interacting residues in the transmembrane domains (39). Moreover, it was shown previously that the 5-HT3 receptor is unaffected by Aβ (13, 16, 21). The chimeric receptor is denoted by α7V202-5HT3A, as valine (V) at position 202 of mouse α7-nAChR lies at the border between the extracellular and first transmembrane domain of the α7-nAChR (38). This chimera was shown previously to be a fully functional ligand-gated ion channel, activated by nicotinic agonists instead of 5-HT3 agonists (37). Fig. 1A shows a schematic of the chimeric receptor α7V202-5HT3A structure. Consistent with our previous results (21–23), Aβ1–42 or nicotine induced significant increases in [Ca2+]i in the presynaptic-like axonal varicosities expressing wild-type α7-nAChRs but not wild-type 5-HT3 receptors (Fig. 1, C and D). The very small effect of Aβ on varicosities expressing wild-type 5-HT3 receptors was similar to that seen with mock-treated cultures (Fig. 1B) and for the reverse peptide Aβ42–1 (23) and is most likely due to a weak nonspecific peptidergic action, as it is unaffected by the antagonist α-bungarotoxin (23). Nicotine and Aβ were still able to activate the chimeric α7V202-5HT3A receptor (Fig. 1, C and D), demonstrating that Aβ activates presynaptic α7-nAChRs via the ECD of the receptor.
FIGURE 1.
Ca2+ responses evoked by Aβ in the axonal varicosities of NG108-15 cells expressing α7-nAChRs are mediated by the extracellular domain of the receptor. A, schematic of the WT α7-nAChR, the chimeric α7V202-5HT3A receptor, and the 5-HT3A receptor. B, representative micrograph of differentiated NG108-15 cells loaded with Fluo-4 and imaged via confocal microscopy (scale bar = 10 μm). The arrow indicates a typical cell body. The arrowheads indicate two varicosities with different diameters. Representative averaged Ca2+ responses to successive stimulation with 100 nm Aβ1–42 and, after washing with HBS, 30 mm KCl in varicosities expressing α7-nAChRs (WT α7) or varicosities of cultures subjected to mock transfection (Mock) are shown in the graph. As shown previously (23), Aβ-induced Ca2+ responses result from activation of α7-nAChRs inducing Ca2+ influx and concomitant depolarization of voltage-gated calcium channels, followed by the release of Ca2+ from intracellular stores via Ca2+-induced Ca2+ release (27). C, averaged responses to successive stimulation with 100 nm Aβ1–42 and, after washing with HBS, 30 mm KCl in varicosities expressing WT α7-nAChRs, α7V202-5HT3A chimeric receptors or 5-HT3ARs. D, comparison of average peak Ca2+ responses to 500 nm ACh (in the presence of 1 μm atropine), 500 nm nicotine, or 100 nm Aβ1–42 in varicosities expressing WT α7-nAChRs, α7V202-5HT3A chimeric receptors, or 5-HT3A receptors. The weak response to Aβ in varicosities expressing 5-HT3A receptors is comparable with that seen in mock-treated cultures. The signal obtained with nicotine is not significant over the baseline noise. Data are expressed as mean ± S.E., and n represents the total number of varicosities examined across experiments.
Tyrosine at Position 188 Is Critical to Presynaptic Ca2+ Responses Evoked by Aβ and ACh
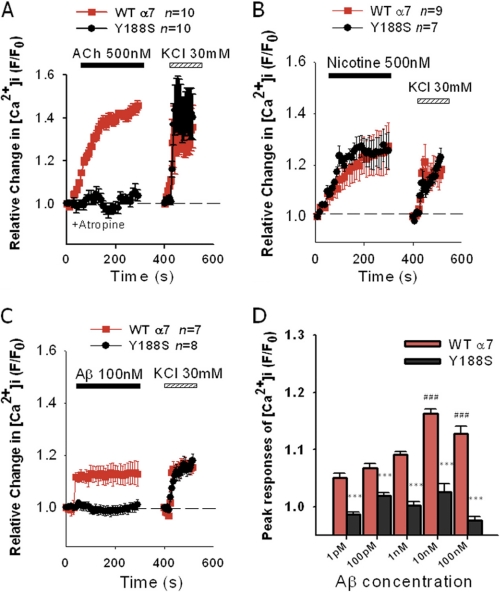
To address whether the activation of α7-nAChRs by Aβ involves a direct interaction with the receptor ligand-binding domain, we first considered tyrosine at position 188 (Tyr-210 in the proform containing the signal sequence). This tyrosine, Tyr-188, resides in loop C in the ligand-binding domain as part of the aromatic cluster (25) and is conserved as residue Tyr-190 in muscle-type nAChRs and Tyr-185 in the ACh binding protein (AChBP). This residue was of particular interest because it was found to interact with the quaternary ammonium of carbamylcholine (as an analog for ACh) but not with nicotine in the resolved crystal structure of the AChBP (40). Moreover, mutation of Tyr-190 in the muscle-type receptor led to a dramatic shift in the EC50 for activation by ACh (41, 42). In our current study, Tyr-188 in α7-nAChR was mutated to serine (S) (Figs. 2 and 3A) and phenylalanine (F) (Fig. 3A). Y188S results in loss of the aromatic group, whereas Y188F results in loss of the hydroxyl group from the tyrosine but retains aromaticity. Ca2+ responses in NG108-15 varicosities expressing Y188S α7-nAChR to Aβ and ACh were almost completely attenuated when compared with responses in varicosities expressing WT α7-nAChRs (Fig. 2A, p < 0.0001; Fig. 2C, p < 0.0001; t tests of averaged peak responses). The attenuation of Aβ-induced responses was evident for pm to nm concentrations of Aβ (Fig. 2D, Bonferroni test, p < 0.001 compared with WT). In contrast, Ca2+ responses in varicosities expressing Y188S evoked by nicotine were identical to those found for WT α7-nAChRs (Fig. 2B). A similar trend was obtained when comparing Ca2+ responses in varicosities expressing Y188F α7-nAChRs to wild-type receptors for these three different agonists (Fig. 3A). However, the attenuation of the ACh- and Aβ-induced Ca2+ responses was partial. K+-depolarization-induced Ca2+ responses elicited following stimulation with the three different agonists were equivalent in varicosities expressing Y188S α7-nAChs as compared with WT α7-nAChRs (Fig. 2), demonstrating the maintained integrity of the preparation. Together, the findings indicate a key role for the aromatic group of Tyr-188 in the activation of the α7-nAChR by Aβ and ACh but not nicotine. As the results for ACh and nicotine are consistent with the prior mutational and structural analyses of the homologous tyrosine in the muscle-type nAChR and the AChBP (40–42), the findings for Aβ might further suggest a potential cation-π interaction between Aβ and Tyr-188 in the α7-nAChR in view of the similar effect of mutation of this tyrosine on receptor activation by Aβ and ACh. Interestingly, α-bungarotoxin, which is a highly selective 8-kDa peptide antagonist of α7-nAChRs, was found to loop a string of amino acid residues (finger II) into the agonist-binding pocket of the α1 subunit, coming into close proximity of Tyr-190, which is equivalent to Tyr-188 in the α7 subunit (43).
FIGURE 2.
Tyrosine at position 188 in α7-nAChRs expressed in the NG108-15 varicosities is essential for Ca2+ responses to Aβ and ACh but not nicotine. Differentiated NG108-15 cells expressing WT α7-nAChRs or Y188S α7-nAChRs were stimulated by 500 nm ACh in the presence of atropine (A), 500 nm nicotine (B), or 100 nm Aβ1–42 (C), each followed by a 100-s wash with HBS, and then stimulated with 30 mm KCl. Averaged data are mean ± S.E., and n represents the total number of varicosities examined. D, peak Ca2+ responses (during 120–300 s after activation) in varicosities expressing WT and Y188S α7-nAChRs to 1 pm (n = 9), 100 pm (n = 9), 1 nm (n = 8), 10 nm (n = 10), and 100 nm (n = 8) Aβ1–42 are compared. ***, p < 0.001 versus WT; ###, p < 0.001versus stimulation of WT by 1 pm Aβ1–42.
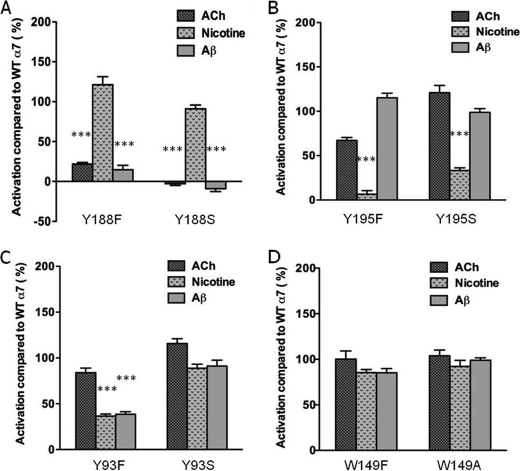
FIGURE 3.
Comparison of Ca2+ responses in varicosities expressing α7-nAChRs with various mutations in the key aromatic residues in the ligand binding domain. Peak Ca2+ responses (plateaus) in varicosities of NG108-15 cells expressing various mutant α7-nAChRs to 500 nm ACh in the presence of atropine, 500 nm nicotine, or 100 nm Aβ1–42 were compared with responses to the same agonists for WT α7-nAChRs. A, Y188F (n = 20); Y188S (n = 20) (B) Y195F (n = 19); Y195S (n = 10) (C) Y93F (n = 12); Y93S (n = 13) (D) W149F (n = 6); W149A (n = 13). Each bar represents averaged responses as a percentage of averaged responses for WT α7-nAChRs for a given agonist, with the standard errors ranging from 1.4% to 10.8%. n represents the total number of varicosities across experiments. ***, p < 0.0001 versus WT.
For comparison, we also mutated the other aromatic residues in the ligand-binding domain, specifically Tyr-195 (loop C), Tyr-93 (loop A), and Trp-149 (loop B). Interestingly, NG108-15 varicosities expressing α7-nAChRs with either Y195F or Y195S displayed robust Ca2+ responses to ACh and Aβ, but the responses to nicotine were strongly attenuated (Fig. 3B, left and right, Bonferroni test, p < 0.0001). These results are essentially the opposite of those found for the Tyr-188 mutants, indicating that Tyr-195 is important for activation by nicotine but not ACh or Aβ. As for Tyr-93, nicotine and Aβ only partially activated presynaptic Y93F α7-nAChRs when compared with the WT receptor (Fig. 3C, Bonferroni test, p < 0.0001), whereas the responses obtained with Y93S α7-nAChRs as compared with WT receptors showed a similar trend but were not significantly different, pointing to a possible interaction of the hydroxyl group in Tyr-93 with Aβ. Finally, we examined the tryptophan at position 149 in loop B of the receptor, another highly conserved residue in the ligand-binding site important for agonist activation (25). W149F and W149A mutants in α7-nAChR did not display changes in presynaptic [Ca2+]i responses to activation by ACh, nicotine, or Aβ that were distinguishable from wild-type receptors (Fig. 3D). K+ depolarization-induced Ca2+ responses observed following agonist stimulation of varicosities expressing mutant α7-nAChRs were similar to those of WT α7-nAChRs (supplemental Fig. S1).
Mutant α7-nAChRs Were Expressed on the Varicosities of the NG108-15 Cells
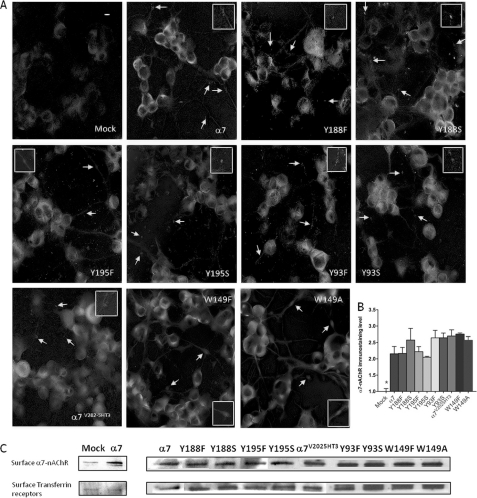
The activation of the various mutant α7-nAChRs by at least one agonist would indicate the functional presence of the receptors. However, to directly assess the expression patterns of the different mutant α7-nAChRs in the NG108-15 cells and to rule out that differences in the agonist-evoked Ca2+ responses might have been due to differing expression levels, we performed immunocytochemistry using a specific anti-mouse α7-nAChR antibody (23). Consistent with results obtained in functional assays, mock-transfected cells displayed weak cytoplasmic immunostaining with no detectable signals on the varicosities (Fig. 4A, Mock), whereas expression of exogenous α7-nAChR resulted in robust immunostaining on the plasma membranes of the cells (evident as sharp fluorescence at the edges of the cells under confocal microscopy) and on the varicosities (arrows and insets, α7). In addition, mutant receptors were detected on the somatic plasma membrane and varicosities to an extent similar to that found for WT α7-nAChRs (Fig. 4, A and B, Bonferroni test, p > 0.05).
FIGURE 4.
Mutant α7-nAChRs were all expressed on the cell surface. A, surface expression levels of α7-nAChR in mock-treated, WT α7-nAChR-transfected, or various mutant α7-nAChR-transfected cultures of differentiated NG108-15 cells were assessed by immunocytochemistry. Positively immunostained varicosities were indicated by arrows and enlarged in the insets. Scale bar = 10 μm. B, the mean signal intensities of four cell bodies from each receptor-expressing culture were normalized to mock-treated cells. *, p < 0.05 Mock versus all receptor-expressing cells and p > 0.05 between receptor-expressing cells. Data are expressed as mean ± S.E. C, surface biotinylated α7-nAChRs extracted from mock-treated, WT α7-nAChR- transfected, or various mutant α7-nAChR-transfected cultures of differentiated NG108-15 cells were detected on immunoblots following isolation via NeutrAvidinTM gel (Pierce). Immunodetection of transferrin receptors was used to control for total protein levels. The surface α7-nAChR detected with mock-treated cultures was similar to that observed for actin, indicating that this signal is due to the very low level background biotinylation of intracellular proteins.
To directly assess cell surface expression, immunoblot analysis of surface biotinylated receptors was also performed. WT and all mutant α7-nAChRs were detected at relatively similar levels when compared with the background from mock-treated cells (Fig. 4C), consistent with the results obtained with immunocytochemistry.
Aβ Coimmunoprecipitates with Wild-type and Mutant α7-nAChRs
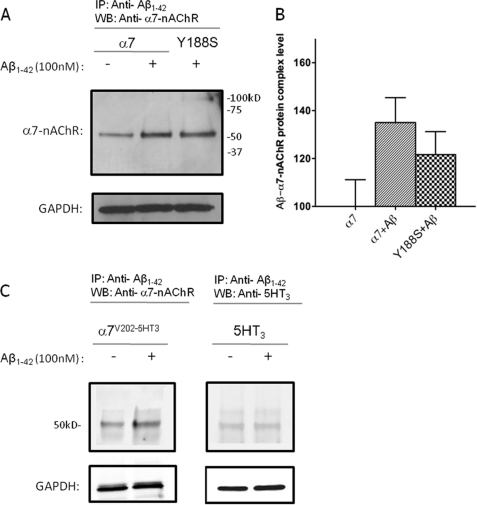
Although mutation of Tyr-188 to serine in the agonist binding site of α7-nAChRs resulted in loss of activation of the receptor by Aβ, it was important to ask whether Aβ still interacted with the mutant receptor as a whole. Interaction of Aβ with α7-nAChRs was assessed by incubation of 100 nm Aβ1–42 with differentiated NG108-15 cells expressing α7-nAChRs, followed by isolation of Aβ·α7·nAChR complexes by coimmunoprecipitation (15). The results show coimmunoprecipitation of α7-nAChRs (Fig. 5A) or α7V202-5HT3A chimeric receptors (Fig. 5C) using an anti-Aβ antibody with extracts from Aβ-treated cells as compared with the control, confirming that Aβ interacts with α7-nAChRs via the ECD. The specificity of this interaction was confirmed by the lack of coimmunoprecipitation of 5-HT3 receptors (Fig. 5C), which are not activated by Aβ over background (Fig. 1, 21). Moreover, the Y188S mutant α7-nAChRs were also coimmunoprecipitated, indicating that the loss of functional response on mutation was likely due to reduced coupling of ligand binding to receptor activation (Fig. 5, A and B).
FIGURE 5.
Coimmunoprecipitation of WT and mutant α7-nAChRs with Aβ. Representative immunoblots for α7-nAChR or Y188S α7-nAChR (A), chimeric α7V202-5HT3A receptor (C, left panel) and 5HT3A receptor following immunoprecipitation (IP) (C, right panel) with anti-Aβ antibodies. Differentiated NG108-15 cells expressing WT α7-nAChRs or Y188S α7-nAChRs, chimeric α7V202-5HT3A receptors, or 5HT3A receptors were incubated for 30 min without (control) or with 100 nm Aβ1–42. Solubilized lysates from the cultures were then subjected to immunoprecipitation using a combination of two anti-Aβ monoclonal antibodies, followed by immunoblotting (WB) for α7-nAChR, chimeric α7V202-5HT3A receptor or 5HT3A receptor, respectively. Immunodetection of GAPDH in the cell lysates was used to control for total protein levels. The level of background signal for α7-nAChR found in the absence of Aβ using this protocol was similar to that found previously (15). To rule out contamination of the α7-nAChR signal with heavy chains from the anti-Aβ antibodies, which would have a very similar Mr (approximately 55 kDa) to that of α7-nAChR, a control was performed without use of the primary anti-α7 antibody and revealed only a very weak background signal. Blots are representative of three experiments. B, quantification of the results shown in A for four separate cultures, normalized to WT.
DISCUSSION
Although previous work indicated that picomolar to nanomolar Aβ directly activates α7-nAChRs at presynaptic sites (22, 23), regulating synaptic plasticity (7), it was also found that this activation was dependent upon the presence of lipid rafts, most likely associated with the nicotinic receptor complex (23). To establish clearly that Aβ interacts directly with presynaptic α7-nAChRs and to define the structural basis for that interaction, we utilized a series of mutant α7-nAChRs, expressed in a neuronal cell line that provides a model presynaptic-like system.
Unlike classical nicotinic agonists, Aβ is a peptide typically 40 or 42 amino acids in length with a substantial hydrophobic domain (last 10–12 residues) and is largely oligomeric at picomolar to nanomolar concentration (9, 23, 34–36). It may thus be possible for Aβ to interact with both the ECD and transmembrane domains of the α7-nAChR. Moreover, lipid interaction with the M4 transmembrane domain of the nAChR regulates its channel kinetics (39), and an interaction between Aβ and the lipid microenvironment around the nAChR may, in turn, regulate the receptor. We therefore first constructed a chimeric receptor containing the ECD from the α7-nAChR and the transmembrane and intracellular domains from the 5-HT3 receptor (13, 37, 38), a ligand-gated ion channel that is very closely related to nicotinic receptors but not activated by Aβ (13, 16, 21). The results using the chimeric receptor (α7V202-5HT3A) showed that the ECD of the α7-nAChR mediates activation of the receptor by Aβ. This is not surprising, as the agonist-binding site is fully contained within the ECD. On the other hand, oligomeric Aβ may still interact with the plasma membrane (18), perhaps specifically with receptor-associated lipid rafts (23).
To demonstrate directly that Aβ activates α7-nAChRs via the agonist-binding domain of the receptor, detailing the specific residues involved, we next constructed mutant receptors carrying substitutions in each of the four key aromatic residues in loops A, B, and C in the ligand-binding domain, as originally identified in muscle-type nAChRs (25). We focused first on Tyr-188 in loop C, as this residue appears to differentially interact with the quaternary amine of ACh as compared with nicotine (40), and, more importantly, its mutation leads to a dramatic reduction in receptor activation by the agonist (41, 42). In line with the latter findings, mutation of Tyr-188 to either phenylalanine or serine led to a near complete loss of responses of the receptor to Aβ and ACh but not to nicotine, demonstrating that this residue is essential for the agonist-like action of Aβ.
Two other key tyrosines were examined: Tyr-195 in loop C and Tyr-93 in loop A. Mutation of Tyr-195 to either phenylalanine or serine had no effect on Aβ-evoked responses while greatly attenuating responses to nicotine, whereas a conservative mutation of Tyr-93 to phenylalanine led to a partial attenuation of Aβ-evoked responses. The Y93F mutant receptor also displayed partially attenuated responses to nicotine, indicating a distinct but possibly lesser role in agonist activation, perhaps via its hydroxyl group. On the other hand, on the basis of the structural information for agonist binding to the AChBP, Tyr-93 lies next to Tyr-188 in the binding pocket (40) whereasTyr-195 lies on the opposite side, as does Trp-149, whose mutation also had no effect. Thus, the partial effect of mutation of Tyr-93 and its close proximity to Tyr-188 may indicate that it affects Tyr-188 rather than necessarily interacting with Aβ. However, distinguishing these possibilities would require more direct structural analysis. In any event, these results further support Tyr-188 as a specific, critical residue for the agonist-like action of Aβ on α7-nAChRs.
As mutations of Tyr-188 to phenylalanine and serine had a similar impact on ACh-evoked responses as that found for Aβ-evoked responses but was without effect on nicotine-evoked responses, it may be that both the aromatic ring and hydroxyl group of this tyrosine provide important contacts with Aβ (Fig. 6). Moreover, with the ACh interaction (using the analog carbamylcholine) with the homologous residue in the AChBP (Tyr-185) occurring through the quaternary amine of the choline ester, it may further indicate an interaction of Tyr-188 with a basic amino acid residue and/or aromatic amino acid residue(s) in Aβ. Although this is highly speculative, there are several histidines, two lysines, and an arginine in the hydrophilic portion of Aβ (approximately 1–28). Of note, Arg-36 in the loop of α-bungarotoxin (finger II), found to interact with the agonist-binding pocket of the α1-nAChR, is part of a cation-π interaction with Tyr-190 (equivalent to Tyr-188 in α7-nAChR). It will thus be of particular interest to determine the residues in Aβ important for its agonist-like action on α7-nAChRs. Preliminarily, we have found that the hydrophilic domain of Aβ was sufficient for its agonist-like action.3
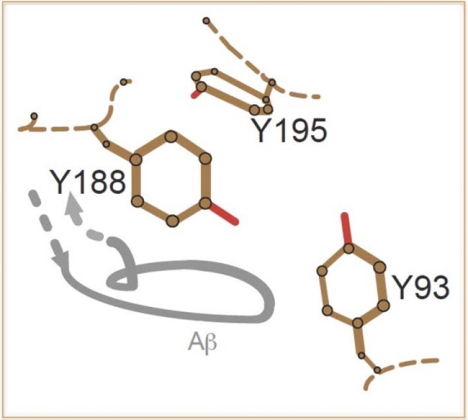
FIGURE 6.
Model of the interaction of a putative loop from Aβ (gray) with the ligand binding domain in the α7-nAChR. The positions of the three key tyrosines from loop C (Tyr-188 and Tyr-195) and loop A (Tyr-93) involved in agonist binding are based on the relative positions of the equivalent residues in the deduced structure of the extracellular domain of the α1-nAChR subunit (43). Trp-149 is not included, as mutations of this residue had no impact on responses evoked by Aβ. The position of a putative loop of Aβ (peptide backbone) relative to these tyrosines is based on the structural interaction of a loop from α-bungarotoxin (finger II) with the equivalent tyrosines in α1-nAChR subunit (43). The model indicates primary juxtaposition and interaction of Aβ with Tyr-188, with potential interaction with Tyr-93 but not Tyr-195. Hydroxyl groups are shown in red. The portions of the connecting peptide backbone in the α1-nAChR are shown as dotted lines.
Curiously, there was no impact of mutation of Trp-149. However, this residue has been found to play a primary role in agonist binding to chicken α7-nAChRs (44). As noted previously, this tryptophan lies on the other side of the agonist binding pocket from Tyr-188, on the basis of the AChBP structure, and thus may not make contact with Aβ. In addition, Tyr-188 has been noted to undergo a large shift on agonist binding, more clearly linked with activation (see 45).
Although direct assays for specific binding of Aβ on nAChRs are problematic (18), it was important to ask whether mutation of Tyr-188 affected the general interaction of Aβ with the receptor. Using coimmunoprecipitation (15), it was found that Aβ still associated with the Tyr-188S mutant of the α7-nAChR. Thus, as would be expected, there are likely multiple points of contact between Aβ and the receptor, with Tyr-188 possibly playing a primary role in receptor activation rather than Aβ binding affinity (41, 42). However, coimmunoprecipitation requires substantial stability of the immune complexes, and hence does not accurately reflect binding to the agonist-binding domain. This point may be more clearly addressed by examining the impact of various fragments of Aβ while assessing their interaction with the receptor in conventional binding assays.
Acknowledgments
We thank Dr. Jerry Stitzel of the University of Colorado for providing the mouse α7 sequence in the pcDNA3.1 expression vector and Dr. Cedomir Todorovic for critical reading of the manuscript.
This work was supported, in whole or in part, by grants from the American Health Assistance Foundation, The Hawaii Community Foundation, and the National Institute on Aging Grant AG21586. Support for the confocal microscopy core at the University of Hawaii was provided by the National Center for Research Resources of the National Institutes of Health via Research Centers in Minority Institutions Grant G12 RR003061 and Centers of Biomedical Research Excellence Grant P20 RR016453.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
M. Tong, J. L. M. Lawrence, T. Sherrin, M. Robles, M. Contarino, M. M. White, R. Bellinger, C. Todorovic, and R. A. Nichols, in preparation.
- AD
- Alzheimer disease
- Aβ
- β amyloid
- nAChR
- nicotinic acetylcholine receptor
- ACh
- acetylcholine
- HBS
- HEPES-buffered saline
- ECD
- extracellular domain
- AchBP
- acetylcholine binding protein.
REFERENCES
- 1. McLean C. A., Cherny R. A., Fraser F. W., Fuller S. J., Smith M. J., Beyreuther K., Bush A. I., Masters C. L. (1999) Ann. Neurol. 46, 860–866 [DOI] [PubMed] [Google Scholar]
- 2. Lue L. F., Kuo Y. M., Roher A. E., Brachova L., Shen Y., Sue L., Beach T., Kurth J. H., Rydel R. E., Rogers J. (1999) Am. J. Pathol. 155, 853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lazarov O., Lee M., Peterson D. A., Sisodia S. S. (2002) J. Neurosci. 22, 9785–9793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheng J. G., Price D. L., Koliatsos V. E. (2002) J. Neurosci. 22, 9794–9799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cirrito J. R., Yamada K. A., Finn M. B., Sloviter R. S., Bales K. R., May P. C., Schoepp D. D., Paul S. M., Mennerick S., Holtzman D. M. (2005) Neuron 48, 913–922 [DOI] [PubMed] [Google Scholar]
- 6. Bateman R. J., Munsell L. Y., Morris J. C., Swarm R., Yarasheski K. E., Holtzman D. M. (2006) Nat. Med. 12, 856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puzzo D., Privitera L., Leznik E., Fà M., Staniszewski A., Palmeri A., Arancio O. (2008) J. Neurosci. 28, 14537–14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abramov E., Dolev I., Fogel H., Ciccotosto G. D., Ruff E., Slutsky I. (2009) Nat. Neurosci. 12, 1567–1576 [DOI] [PubMed] [Google Scholar]
- 9. Selkoe D. J. (2008) Behav. Brain Res. 192, 106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberson E. D., Scearce-Levie K., Palop J. J., Yan F., Cheng I. H., Wu T., Gerstein H., Yu G. Q., Mucke L. (2007) Science 316, 750–754 [DOI] [PubMed] [Google Scholar]
- 11. Zempel H., Thies E., Mandelkow E., Mandelkow E. M. (2010) J. Neurosci. 30, 11938–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dineley K. T., Westerman M., Bui D., Bell K., Ashe K. H., Sweatt J. D. (2001) J. Neurosci. 21, 4125–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Q., Kawai H., Berg D. K. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 4734–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pettit D. L., Shao Z., Yakel J. L. (2001) J. Neurosci. 21, RC120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang H. Y., Stucky A., Liu J., Shen C., Trocme-Thibierge C., Morain P. (2009) J. Neurosci. 29, 10961–10973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H. Y., Lee D. H., Davis C. B., Shank R. P. (2000) J. Neurochem. 75, 1155–1161 [DOI] [PubMed] [Google Scholar]
- 17. Wang H. Y., Lee D. H. S., D'Andrea M. R., Peterson P. A., Shank R. P., Reitz A. B. (2000) J. Biol. Chem. 275, 5626–5632 [DOI] [PubMed] [Google Scholar]
- 18. Small D. H., Maksel D., Kerr M. L., Ng J., Hou X., Chu C., Mehrani H., Unabia S., Azari M. F., Loiacono R., Aguilar M. I., Chebib M. (2007) J. Neurochem. 101, 1527–1538 [DOI] [PubMed] [Google Scholar]
- 19. Sharma G., Vijayaraghavan S. (2003) Neuron 38, 929–939 [DOI] [PubMed] [Google Scholar]
- 20. Kenney J. W., Gould T. J. (2008) Mol. Neurobiol. 38, 101–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dougherty J. J., Wu J., Nichols R. A. (2003) J. Neurosci. 23, 6740–6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehta T. K., Dougherty J. J., Wu J., Choi C. H., Khan G. M., Nichols R. A. (2009) J. Neurochem. 109, 1452–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khan G. M., Tong M., Jhun M., Arora K., Nichols R. A. (2010) Eur. J. Neurosci. 31, 788–796 [DOI] [PubMed] [Google Scholar]
- 24. Brejc K., van Dijk W. J., Klaassen R. V., Schuurmans M., van der Oost J., Smit A. B., Sixma T. K. (2001) Nature 411, 269–276 [DOI] [PubMed] [Google Scholar]
- 25. Dougherty D. A., Lester H. A. (2001) Nature 411, 252–253, 255 [DOI] [PubMed] [Google Scholar]
- 26. Nelson P., Christian C., Nirenberg M. (1976) Proc. Natl. Acad. Sci. U.S.A. 73, 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rondé P., Dougherty J. J., Nichols R. A. (2000) J. Physiol. 529, 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rondé P., Nichols R. A. (2001) Neuroscience 102, 979–987 [DOI] [PubMed] [Google Scholar]
- 29. Wu G., Fang Y., Lu Z. H., Ledeen R. W. (1998) J. Neurocytol. 27, 1–14 [DOI] [PubMed] [Google Scholar]
- 30. McGee R., Simpson P., Christian C., Mata M., Nelson P., Nirenberg M. (1978) Proc. Natl. Acad. Sci. U.S.A. 75, 1314–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han H. Q., Nichols R. A., Rubin M. R., Bähler M., Greengard P. (1991) Nature 349, 697–700 [DOI] [PubMed] [Google Scholar]
- 32. Grandori R., Struck K., Giovanielli K., Carey J. (1997) Protein Eng. 10, 1099–1100 [DOI] [PubMed] [Google Scholar]
- 33. Spier A. D., Wotherspoon G., Nayak S. V., Nichols R. A., Priestley J. V., Lummis S. C. R. (1999) Mol. Brain Res. 67, 221–230 [DOI] [PubMed] [Google Scholar]
- 34. Bell K. A., O'Riordan K. J., Sweatt J. D., Dineley K. T. (2004) J. Neurochem. 91, 349–361 [DOI] [PubMed] [Google Scholar]
- 35. Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Nature 416, 535–539 [DOI] [PubMed] [Google Scholar]
- 36. Sandberg A., Luheshi L. M., Söllvander S., Pereira de Barros T., Macao B., Knowles T. P., Biverstål H., Lendel C., Ekholm-Petterson F., Dubnovitsky A., Lannfelt L., Dobson C. M., Härd T. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 15595–15600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eiselé J. L., Bertrand S., Galzi J. L., Devillers-Thiéry A., Changeux J. P., Bertrand D. (1993) Nature 366, 479–483 [DOI] [PubMed] [Google Scholar]
- 38. Gee V. J., Kracun S., Cooper S. T., Gibb A. J., Millar N. S. (2007) Br. J. Pharmacol. 152, 501–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barrantes F. J. (2004) Brain Res. Rev. 47, 71–95 [DOI] [PubMed] [Google Scholar]
- 40. Celie P. H., van Rossum-Fikkert S. E., van Dijk W. J., Brejc K., Smit A. B., Sixma T. K. (2004) Neuron 41, 907–914 [DOI] [PubMed] [Google Scholar]
- 41. O'Leary M. E., White M. M. (1992) J. Biol. Chem. 267, 8360–8365 [PubMed] [Google Scholar]
- 42. Aylwin M. L., White M. M. (1994) FEBS Lett. 349, 99–103 [DOI] [PubMed] [Google Scholar]
- 43. Dellisanti C. D., Yao Y., Stroud J. C., Wang Z. Z., Chen L. (2007) Nat. Neurosci. 10, 953–962 [DOI] [PubMed] [Google Scholar]
- 44. Galzi J. L., Bertrand D., Devillers-Thiéry A., Revah F., Bertrand S., Changeux J. P. (1991) FEBS Lett. 294, 198–202 [DOI] [PubMed] [Google Scholar]
- 45. Gay E. A., Yakel J. L. (2007) J. Physiol. 584, 727–733 [DOI] [PMC free article] [PubMed] [Google Scholar]