Abstract
Study Objectives:
Evaluate the long-term durability of treatment response and safety of a nasal expiratory positive airway pressure (EPAP) device used to treat obstructive sleep apnea (OSA).
Design:
A prospective, multicenter, single-arm, open-label extension to a 3-month EPAP vs sham randomized clinical trial
Setting:
13 sites including both academic and private sleep disorder centers
Patients:
OSA patients in the EPAP arm of the EPAP vs sham randomized study who used the EPAP device ≥ 4 h per night, ≥ 5 nights per week on average during months 1 and 2 of the 3-month trial and had ≥ 50% reduction in AHI or AHI reduction to < 10 documented by polysomnography, comparing the 3-month device-on PSG to the week-one device-off PSG.
Interventions:
Treatment with a nasal EPAP device (N = 41) for 12 months. Polysomnography (PSG) on the patients wearing the device was performed after 12 months of treatment. The month 12 device-on PSG data from the analyzable subject cohort (N = 34) was compared to the week 1 device-off PSG from the EPAP vs sham trial.
Measurements and Results:
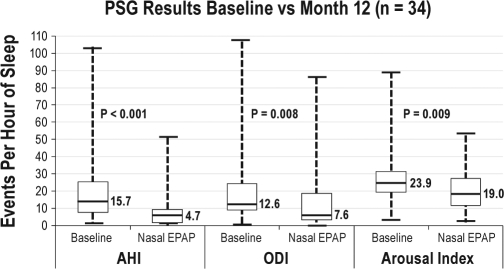
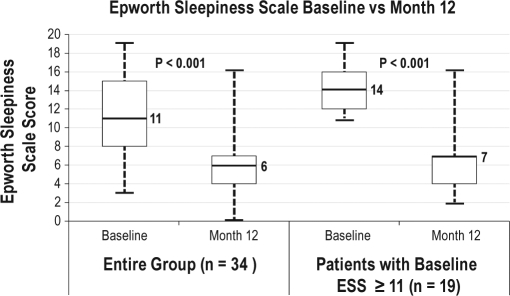
Of the 51 patients eligible, 34 were still using the EPAP device at the end of 12 months. Median AHI was reduced from 15.7 to 4.7 events/h (week 1 device-off versus month 12 device-on). The decrease in the AHI (median) was 71.3% (p < 0.001). The median proportion of sleep time with snoring was reduced by 74.4% (p < 0.001). Over 12 months of EPAP treatment, the Epworth Sleepiness Scale decreased (11.1 ± 4.2 to 6.0 ± 3.2, p < 0.001), and the median percentage of reported nights used (entire night) was 89.3%.
Conclusions:
Nasal EPAP significantly reduced the AHI, improved subjective daytime sleepiness and reduced snoring after 12 months of treatment. Long-term adherence to EPAP was excellent in those who had a positive clinical response at month 3 of the EPAP vs sham study.
Clinical Trial Registration:
ClinicalTrials.gov
Trial name: Extension Study of Original Protocol AERO C009 for Obstructive Sleep Apnea-hypopnea (AERO C009E).
URL: http://clinicaltrials.gov/ct2/show/NCT00849043?term=Ventus+Medical&rank=2.
Registration Number: NCT00849043.
Citation:
Kryger MH; Berry RB; Massie CA. Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment for obstructive sleep apnea (OSA). J Clin Sleep Med 2011;7(5):449-453.
Keywords: Obstructive sleep apnea, expiratory positive airway pressure, CPAP
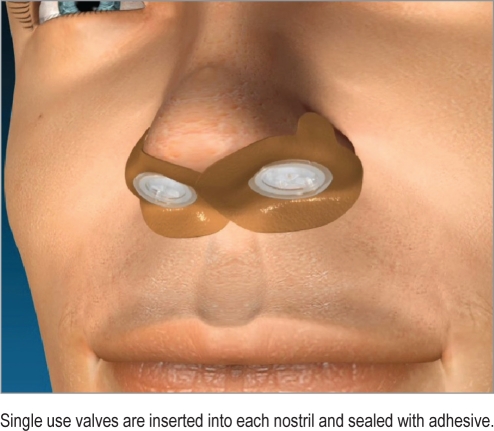
Obstructive sleep apnea (OSA) is a common disorder1 often resulting in adverse cardiovascular consequences, daytime sleepiness, and disturbed nocturnal sleep of the patient and bed partner.2,3 An expiratory positive airway pressure (EPAP) nasal device (Provent Sleep Apnea Therapy, Ventus Medical Inc., Belmont, CA) has been developed to provide a new therapeutic option for OSA patients (Figure 1). Prior studies4–8 have documented safety and efficacy, including a large multicenter, randomized, double-blind, sham-controlled trial with 3-month follow-up (EPAP vs sham study). An open label extension of the 3-month EPAP vs sham study was conducted to evaluate the long-term durability of EPAP treatment response after 12 months of follow-up.
Figure 1.
Nasal EPAP device
Single use valves are inserted into each nostril and sealed with adhesive.
METHODS
Study Design
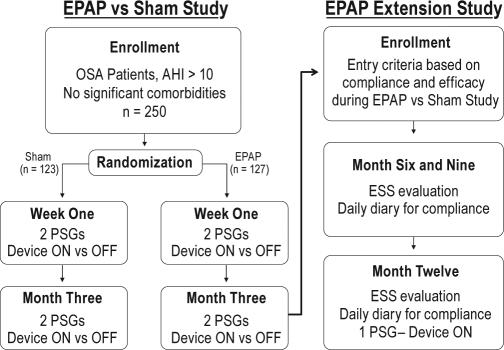
The study was a prospective, 13-site, single-arm, open-label extension clinical trial (study investigators listed in Acknowledgments). The study design illustrating the connection between the EPAP vs sham 3-month study and the EPAP open label extension study with 12-month follow-up is shown in Figure 2. The study was registered on clinicaltrials.gov (NCT00849043). The local institutional review board (or authorized national institutional review board) at each site approved the study.
Figure 2.
EPAP vs sham and EPAP open label extension study design
BRIEF SUMMARY
Current Knowledge/Study Rationale: Because there are few alternatives to using positive airway pressure in OSAS, a non-invasive nasal expiratory positive pressure device was developed; this treatment showed efficacy in some patients at 3 months. This research was done to explore long term efficacy and evaluated patient after one year.of treatment.
Study Impact: Although CPAP treatment remains the treatment of choice in OSAS, the nasal expirartory positive pressure device is an additional treatment option in some patients. Because compliance and efficacy may be maintained after 12 months of use, this treatment may be considered for long term use in selected patients.
Patient Recruitment and Study Initiation
Patients in the EPAP arm of the EPAP vs Sham study8 who met adherence and efficacy criteria were enrolled. The inclusion and exclusion criteria are provided in the Supplement, Table S1. Inclusion criteria included ≥ 50% reduction in AHI or an AHI reduction to < 10 documented by polysomnography, comparing the 3-month device-on PSG to the week 1 device-off PSG. The other key inclusion criteria related to compliance, defined as EPAP device use ≥ 4 h per night, ≥ 5 nights per week on average during months 1 and 2 of the 3-month trial. After signing informed consent, patients were instructed to continue nightly use of the nasal EPAP device.
Nasal EPAP Device
The nasal EPAP device consists of a single-use valve inserted into each nostril and held in place by adhesive. The valve has minimal inspiratory resistance but an expiratory resistance of 80 cm H2O/L/sec at a flow rate of 100 mL/sec. The adhesive substrate, similar to that found in adhesive bandages, was applied to the outer edges of the nares, resulting in a leak-free seal between the valve and the nose.
Treatment Evaluations
During the 4-week period preceding the 6-, 9-, and 12-month office visits, patients were asked to complete a daily diary entry each morning after awakening, documenting if the device was in place in the morning. Each follow-up office visit included a medical evaluation by the study physician as well as completion of the Epworth Sleepiness Scale (ESS),9 a subjective measure of propensity to fall asleep in common situations.
Polysomnography
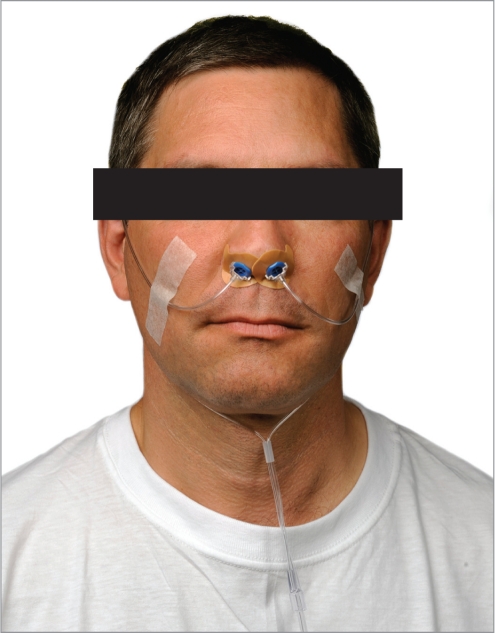
Attended polysomnography (PSG) was performed using standard techniques including monitoring of EEG derivations (frontal, central, and occipital), right and left electrooculographic derivations, a chin electromyographic (EMG) derivation, nasal pressure, an oral thermal sensor, chest and abdominal effort belts, a body position monitor, a left leg EMG derivation, a single ECG channel, and pulse oximetry. A quantitative snoring sound volume meter with a microphone was also used (Q-Snor, Braebon Medical Corporation). A specially designed nasal cannula (Ventus Medical Inc., Belmont, CA) was used to securely attach to the nasal EPAP device to allow for standard measurement of nasal airflow via nasal pressure during PSG (Figure 3).
Figure 3.
Nasal cannula attached to EPAP device
The polysomnographic and snore data were analyzed by a central scoring center. Sleep was manually staged in 30-sec epochs, and arousals and respiratory events were scored using the recommended criteria published in the American Academy of Sleep Medicine Scoring Manual.10 Hypopneas were defined as reductions in airflow ≥ 30% from baseline with a duration > 10 sec associated with a drop in arterial oxygen saturation (SpO2) ≥ 4%. The arterial oxygen desaturation index (ODI) was the number of desaturations ≥ 3% per hour of total sleep time (TST).
The reported snore data was derived by calculating the percentage of TST during which the sound level detected by the Q-Snor device was > 10 dB above the ambient sound level. This approach was employed to reconcile site differences in the Q-Snor calibration of noise minimum values and thus absolute dB values of sound level recorded by the Q-Snor device.
Statistical Analysis
Analysis was performed on the analyzable subject cohort that included all patients in whom data was available. The primary and secondary endpoints were established a priori. The primary end point of the study was the percent change in AHI between the week 1 device-off PSG (from the EPAP vs sham study) to the month 12 device-on PSG. The secondary endpoint was the change in ESS between baseline of the EPAP vs sham study and month 12 of the EPAP open label extension study.
The paired t-test and the sign-rank test were used to test the null hypothesis that the mean within-subject measurement was equal to zero. A p < 0.05 was considered statistically significant. Results are presented as means ± standard deviation or median (25th, 75th percentile).
RESULTS
Patient Flow, Demographics, and Dropouts
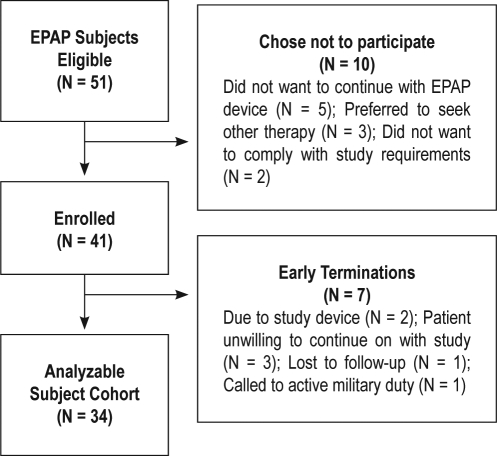
A patient flow diagram is provided in Figure 4. Forty-one (41) patients were enrolled.
Figure 4.
Diagram of patient flow
The patient demographics are shown in Table 1.
Table 1.
Patient demographics
| N = 41 | |
|---|---|
| Age (years) | 50.1 ± 13.6 |
| Gender | 63.4% male |
| BMI (kg/m2) | 32.5 ± 7.5 |
There were 7 patients who terminated early from the study, leaving a cohort of 34 analyzable subjects. The 7 early terminations were due to patient unwillingness to continue on with the study (N = 3), study device (N = 2), lost to follow-up (N = 1), and called to active military duty (N = 1).
AHI, ODI, and Arousal Index
There were statistically significant reductions in the median AHI, ODI, and arousal index when comparing the week 1 device-off to month 12 device-on results. The data are presented in Figure 5.
Figure 5.
Baseline vs month 12 PSG results
Snoring
The median proportion of sleep time spent snoring was reduced by 74.4% (p < 0.001).
Sleep Architecture and Effect of Position and Sleep Stage
There was a statistically significant increase in the amount of stage N2 sleep; however, this was not clinically significant. The amount of stage N1, N3, and REM sleep did not have a statistically significant change. The EPAP device significantly reduced the AHI during both NREM and REM sleep, as well as in the supine and non-supine positions.
The TST and sleep stage durations (% of TST), as well as the effects of body position and sleep stage, are provided in the Supplement, Table S2.
Impact on Subjective Sleepiness
Use of the nasal EPAP device resulted in a significant long-term reduction in subjective sleepiness as measured by the Epworth Sleepiness Scale (ESS). The data are presented in Figure 6.
Figure 6.
ESS – Baseline vs Month 12
Adherence to Therapy
The median percentage (25, 75 percentile) of reported nights the device was worn for the entire night was 89.3% (81.8, 95.2). The median percentage of nights that the diary was completed was 98.8% (97.6, 100.0).
Adverse Events
There were no serious device-related adverse events. Device-related adverse events were reported by 42% (17/41) of patients. The most frequently reported events were difficulty exhaling, nasal discomfort, dry mouth, headache, and insomnia.
DISCUSSION
The major finding of the study was that the patients who were compliant with the EPAP device at month three in the EPAP vs sham study, and had a positive response to it, continued to show benefit at twelve months. Of the 51 patients eligible, 34 were still using the EPAP device at the end of 12 months. AHI was significantly reduced and subjective sleepiness was significantly decreased compared to device-off nights on the week 1 sleep study of the EPAP vs sham study. The unwanted effects of EPAP treatment were mild. Median device use (adherence), as reported by patient diary, was excellent, with the nasal EPAP device worn all night for approximately 89% of nights.
We found that the effectiveness of nasal EPAP is similar or compares favorably to other treatments. Although CPAP is considered the gold standard of treatment, inadequate adherence to CPAP is common.11,12 In a study comparing oral appliances and CPAP, the mean AHI dropped from baseline of 21.3/hour to 4.8/hour with CPAP and to 14.0/hour with an oral appliance.12
Our study has a number of limitations. In the original EPAP vs sham study, there were a large number of exclusion criteria, including patients with severe arterial oxygen desaturation, those who had prior upper airway surgery, or those who had been treated with CPAP. The top four reasons for exclusions were prior CPAP treatment, other serious uncontrolled medical conditions, other sleep disorders, and medications affecting neurocognitive function. The exclusions were designed so that 3 months of sham treatment would not impose a significant health risk and that treatment-naïve patients would be studied. Thus, the results of the study may not generalize to a more heterogeneous population that may contain CPAP failures, prior upper airway surgery, or severe arterial oxygen desaturation.
Another possible limitation of our study was that determination of adherence depended on patient report rather than an objective measure. However, adherence was similar on both the sham and active device in the EPAP vs sham study.
No baseline predictors of treatment success were identified by post hoc analysis. Objective confirmation of efficacy using home sleep studies or in-lab polysomnography and follow-up with the physician to assure adequate adherence is consistent with recommendations for other treatments for OSA.2
In summary, a large, randomized, double-blind, sham-controlled study documented that the nasal EPAP device effectively reduced the AHI and improved oxygenation at month 3; and the 12-month open label extension study showed continued efficacy at 12 months, with significant improvement in subjective sleepiness with good adherence. Thus, nasal EPAP is an effective treatment option for many OSA patients.
DISCLOSURE STATEMENT
This study was funded by Ventus Medical, Inc. Dr. Kryger has received research funding from Respironics, ResMed, Ventus Medical, and Cephalon. Dr. Berry has received Research funding from Respironics, ResMed, and Ventus Medical. Dr. Massie has research funding from Ventus Medical.
ACKNOWLEDGMENTS
Study Investigators: James Andry, Sleep Therapy and Research Center, San Antonio, Texas; Safwan Badr, Wayne State University, Detroit, Michigan; Richard Berry, University of Florida, Gainesville, Florida; Sean Caples, Mayo Clinic, Rochester, Minnesota; Mark Goetting, Sleep Health, Portage, Michigan; Douglas Kirsch, Sleep Health Centers, LLC, Brighton, Massachusetts; Meir Kryger, Gaylord Sleep Medicine, Wallingford, Connecticut; D. Alan Lankford, Sleep Disorders Center of Georgia, Atlanta, Georgia; Clifford Massie, Chicago Sleep Group, Suburban Lung Associates, Elk Grove Village, Illinois; Mark Reploeg, The Corvallis Clinic, Corvallis, Oregon; Leon Rosenthal, Sleep Medicine Associates of Texas, Dallas, Texas; Paula Schweitzer, Sleep Medicine & Research Center, St. Luke's Hospital, Chesterfield, Missouri; David Winslow, Kentucky Research Group, Louisville, Kentucky.
Ventus Medical: Michael Favet and Connie Rey supported the facilitation of statistical analyses with Advance Research Associates and provided some editorial support in processing the manuscript.
ABBREVIATIONS
- AHI
apnea hypopnea index
- AE
adverse event
- BMI
body mass index
- CPAP
continuous positive airway pressure
- EPAP
expiratory positive airway pressure
- ESS
Epworth Sleepiness Scale
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- SpO2
arterial oxygen saturation measured by pulse oximetry
Table S1.
Inclusion and exclusion criteria
Inclusion Criteria
|
Exclusion Criteria
|
Table S2.
Sleep architecture and effects of position and sleep stage
| Week 1 Device Off | Month 12 Device On | p Value (Absolute Change) | |
|---|---|---|---|
|
N = 34 |
|||
| TST | 365.0 ± 61.6 | 349.3 ± 79.2 | 0.28 |
| Wake After Sleep Onset | 50.9 ± 34.7 | 48.6 ± 31.2 | 0.91 |
| Stage N1 | 15.9 ± 10.1 | 14.8 ± 9.9 | 0.46 |
| Stage N2 | 57.3 ± 9.5 | 60.4 ± 12.1 | 0.027 |
| Stage N3 | 10.2 ± 8.8 | 8.6 ± 8.2 | 0.47 |
| Stage REM | 16.6 ± 6.3 | 16.3 ± 6.6 | 0.67 |
|
N = 30 |
|||
| AHI NREM1 | 12.6 (6.0, 22.2 ) | 2.9 (1.2, 8.3) | < 0.001 |
| AHI REM1 | 16.8 (5.7, 53.0) | 3.7 (0.9, 14.4) | < 0.001 |
|
N = 21 |
|||
| AHI Supine1 | 22.0 (9.3, 41.9) | 6.5 (2.5, 20.1) | < 0.001 |
| AHI Non-Supine1 | 3.2 (1.8, 10.3) | 0.8 (0.0, 1.7) | = 0.001 |
Values are median (25, 75 quartile) or, mean ± standard deviation
Patients with > 20 minutes in each position and state
REFERENCES
- 1.Young T, Palta M, Dempsey J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Epstein LJ, Kristo D, Strollo P, et al. Clinical guideline for the evaluation, management, and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Augusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Colrain IM, Brooks S, Black J. A pilot evaluation of a nasal expiratory resistance device for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2008;4:426–33. [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenthal L, Massie CA, Dolan DC, Loomas B, Kram J, Hart RW. A multicenter, prospective study of a novel nasal EPAP device in the treatment of obstructive sleep apnea: efficacy and 30-day adherence. J Clin Sleep Med. 2009;5:532–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh JK, Griffin KS, Schweitzer PK, et al. A convenient expiratory positive airway pressure nasal device for the treatment of sleep apnea in patients non-adherent with continuous positive airway pressure. Sleep Med. 2011;12:147–52. doi: 10.1016/j.sleep.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Patel AV, Hwang D, Rapoport DM, et al. Predictors of response to a nasal expiratory resistor device and its potential mechanisms of action for treatment of obstructive sleep apnea (OSA) J Clin Sleep Med. 2011;7:13–22. [PMC free article] [PubMed] [Google Scholar]
- 8.Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep. 2011;34:479–85. doi: 10.1093/sleep/34.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 11.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;15:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15:365–9. doi: 10.1155/2008/534372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Inclusion and exclusion criteria
Inclusion Criteria
|
Exclusion Criteria
|
Table S2.
Sleep architecture and effects of position and sleep stage
| Week 1 Device Off | Month 12 Device On | p Value (Absolute Change) | |
|---|---|---|---|
|
N = 34 |
|||
| TST | 365.0 ± 61.6 | 349.3 ± 79.2 | 0.28 |
| Wake After Sleep Onset | 50.9 ± 34.7 | 48.6 ± 31.2 | 0.91 |
| Stage N1 | 15.9 ± 10.1 | 14.8 ± 9.9 | 0.46 |
| Stage N2 | 57.3 ± 9.5 | 60.4 ± 12.1 | 0.027 |
| Stage N3 | 10.2 ± 8.8 | 8.6 ± 8.2 | 0.47 |
| Stage REM | 16.6 ± 6.3 | 16.3 ± 6.6 | 0.67 |
|
N = 30 |
|||
| AHI NREM1 | 12.6 (6.0, 22.2 ) | 2.9 (1.2, 8.3) | < 0.001 |
| AHI REM1 | 16.8 (5.7, 53.0) | 3.7 (0.9, 14.4) | < 0.001 |
|
N = 21 |
|||
| AHI Supine1 | 22.0 (9.3, 41.9) | 6.5 (2.5, 20.1) | < 0.001 |
| AHI Non-Supine1 | 3.2 (1.8, 10.3) | 0.8 (0.0, 1.7) | = 0.001 |
Values are median (25, 75 quartile) or, mean ± standard deviation
Patients with > 20 minutes in each position and state