Abstract
Deregulation of the mitotic spindle has been implicated in genomic instability, an important aspect of tumorigenesis and malignant transformation. To ensure the fidelity of chromosome transmission, the mitotic spindle is assembled by exquisite mechanisms and orchestrated by centrosomes in animal cells. Centrosomal proteins especially are thought to act coordinately to ensure accurate spindle formation, but the molecular details remain to be investigated. In this study, we report the molecular characterization and functional analysis of a novel centrosomal protein, Cep70. Our data show that Cep70 localizes to the centrosome throughout the cell cycle and binds to the key centrosomal component, γ-tubulin, through the peptide fragments that contain the coiled-coil domains. Our data further reveal that the centrosomal localization pattern of Cep70 is dependent on its interaction with γ-tubulin. Strikingly, Cep70 plays a significant role in the organization of both preexisting and nascent microtubules in interphase cells. In addition, Cep70 is necessary for the organization and orientation of the bipolar spindle during mitosis. These results thus report for the first time the identification of Cep70 as an important centrosomal protein that interacts with γ-tubulin and underscore its critical role in the regulation of mitotic spindle assembly.
Keywords: Centrosome, Microtubules, Mitosis, Mitotic Spindle, Tubulin, Cep70, γ-Tubulin
Introduction
The centrosome is a small, cytoplasmic non-membranous organelle that acts as the major microtubule organizing center in animal cells (1). In addition to its role in the nucleation and organization of microtubules, the centrosome is critical for the orchestration of cell cycle progression (2, 3). Especially, this organelle dictates the formation of a radial microtubule array during interphase and the establishment of a bipolar spindle during mitosis (3, 4). Centrosomal activity is also indispensable for the execution of cytokinesis and progression from G1 phase to S phase of the cell cycle (2). The consequences of centrosomal aberrations may be grim and range from cell death in extreme cases to other repercussions (5–7). For example, abnormalities in the centrosome may result in disordered cell cycle progression, including chromosome missegregation and aneuploidy, which are associated with tumorigenesis and the acquisition of malignant phenotypes (2, 6–9).
To ensure bipolar organization and geometric precision of the mitotic spindle, the centrosome serves as a cellular hub where several key regulators convene to mediate vital interactions that control spindle assembly (3, 4). The centrosome may also serve to regulate other fundamental cellular functions, such as cell polarity and motility (1). Much progress has been made in the past decade in the identification of novel centrosomal proteins (10, 11), but their interplay with the well established centrosome/spindle pole-resident molecules, such as γ-tubulin, to mediate the organization and assembly of the mitotic spindle remains elusive. In addition, the functional significance of proteins that reside in the centrosomal compartment is still a subject of intense research.
Cep70, a candidate centrosomal protein of 70 kDa, was originally discovered in a proteomic study of the human centrosome, suggesting a potential role in centrosome structure and function (11). The zebrafish homolog of Cep70 has been shown to contribute to the assembly of cilia, hair-like extensions important for cell signaling and development, by determination of the length of the axoneme (12). However, the localization and functions of Cep70 in mammalian cells remain unknown. In this study, we describe the characterization of Cep70 as a genuine centrosomal protein and demonstrate its localization to the centrosome throughout the cell cycle via interaction with γ-tubulin. We also present evidence that Cep70 is a critical regulator for the organization of radial microtubule arrays and the spindle apparatus.
EXPERIMENTAL PROCEDURES
Materials
Nocodazole, DAPI, and antibodies against α-tubulin, γ-tubulin, and β-actin were purchased from Sigma-Aldrich. Cep70 antibody was generated by immunizing mice with maltose-binding protein (MBP)3-tagged Cep70. Pericentrin antibody was from Covance, and horseradish peroxidase-conjugated secondary antibody was from Amersham Biosciences. Fluorescein- or rhodamine-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories. Cep70 siRNAs were synthesized by Dharmacon, and γ-tubulin and pericentrin siRNAs were from Invitrogen.
Plasmids and Proteins
To generate Cep70 cDNA, total RNA was extracted from 293T cells with the TRIzol reagent (Invitrogen), and cDNA was produced with the M-MLV reverse transcriptase (Promega). Cep70 cDNA was first cloned into the pMD18-T vector (TaKaRa) with the following primers: forward, 5′-GGATCCATGTTTCCGGTAGCCC-3′ and reverse, 5′-GTCGACATGATTTGATTTGTTTTCAGTAT-3′). The sequence was confirmed to be identical to the NCBI reference sequence of human Cep70 (NM_024491.2). To generate mammalian expression plasmids for GFP-Cep70 and GST-Cep70, Cep70 cDNA was subcloned into the pEGFPC1 and pEBG vectors, respectively. For the pEGFPC1-Cep70 plasmid, Cep70 was expressed as a fusion to the C terminus of GFP with the insertion of five linker amino acids (SGLRS). Similarly, a series of plasmids that express various truncated forms of Cep70 were constructed using the pEGFPC1 vector. To purify MBP-Cep70, Cep70 cDNA was cloned into the pMALp2T vector to generate the pMALp2T-Cep70 plasmid. Cep70 was expressed as a fusion to the C terminus of MBP. MBP-Cep70 was purified with the amylose resin according to the manufacturer's instructions (New England Biolabs). In vitro transcription and translation of γ-tubulin were performed using the TNT SP6 high-yield wheat germ protein expression system (Promega) as described previously (13).
Cell Culture and Transfection
HeLa and 293T cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum at 37 °C in a humidified atmosphere with 5% CO2. Plasmids were transfected into cells with the Entranster-D reagent (Engreen Biosystem), and siRNAs were transfected with the DharmaFect1 reagent (Dharmacon).
Immunofluorescence Microscopy
HeLa cells grown on glass coverslips were fixed with methanol at −20 °C and blocked with 2% bovine serine albumin in PBS. Cells were incubated with primary antibodies and then with fluorescein- or rhodamine-conjugated secondary antibodies followed by staining with DAPI. Coverslips were then examined with an Axio Observer A1 fluorescence microscope (Carl Zeiss, Inc.). A TCS SP5 confocal microscope (Leica) was used to examine spindle morphology.
Measurement of Cep70 Intensity at the Centrosome
Cep70 intensity at the centrosome was measured as described previously (14). In brief, two computer-generated squares were centered outside of each centrosome stained with the anti-Cep70 antibody (supplemental Fig. S1). The fluorescence within each square was measured, and the data were transferred to Microsoft Excel. The measured fluorescence within the inner square included the Cep70 fluorescence and the background fluorescence, whereas the fluorescence within the region between perimeter of outer and inner squares included mostly the background fluorescence. The real fluorescence of Cep70 at the centrosome was obtained by subtraction of the background fluorescence within the inner square from the measured fluorescence within the inner square (supplemental Fig. S1).
Analysis of Spindle Orientation
Images were obtained with a TCS SP5 confocal microscope (Leica) equipped with the LASAF software, and various parameters for spindle orientation were determined as described previously (15, 16) and shown in the diagram of Fig. 5D. In brief, the vertical distance (v) and the horizontal distance (h) between the two spindle poles were measured directly with the LASAF software. The corrected pole-to-pole distance (d) of the spindle was obtained based on the pythagorean theorem. The angle (α) between the spindle axis and the growth plane was determined with the inverse trigonometric function (Fig. 5D).
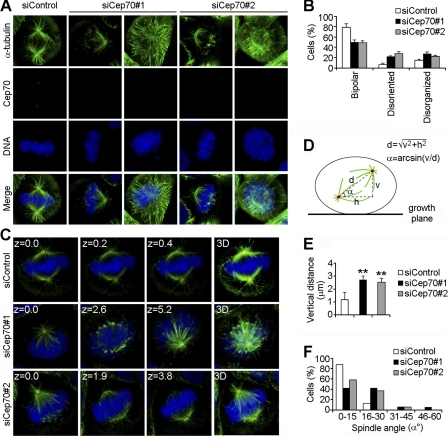
FIGURE 5.
Cep70 is necessary for the organization and orientation of the mitotic spindle. A, HeLa cells transfected with control or Cep70 siRNAs for 72 h were stained with anti-α-tubulin and anti-Cep70 antibodies and DAPI. Representative images of different spindle phenotypes are shown. B, experiments were performed as in A, and the percentages of cells with normal bipolar spindles, disoriented spindles, or disorganized spindles were quantified. Cells with disorganized spindles refer to those without clear spindle poles. Cells with disoriented spindles refer to those exhibiting two spindle poles but at different focal planes. C, HeLa cells transfected with control or Cep70 siRNAs for 72 h were stained with anti-α-tubulin (green) and anti-γ-tubulin (red) antibodies and DAPI (blue). The position of the z stage of the mitotic spindle is indicated in μm, and 3D refers to the projected image. D, a diagram describing the method used for analysis of various parameters of the mitotic spindle. The vertical distance (v) and the horizontal distance (h) between the two spindle poles were measured directly with the LASAF software. The corrected pole-to-pole distance (d) of the spindle was calculated based on the pythagorean theorem. The angle (α) between the spindle axis and the growth plane was determined with the inverse trigonometric function. E, experiments were performed as in C, and the vertical distance between the two spindle poles was measured as described in D. **, p < 0.01 versus control. F, experiments were performed as in C, the spindle angle (α) was determined as described in D, and the percentages of cells with different spindle angles were quantified.
Immunoblotting
Cells were lysed in a buffer containing 1% Triton X-100, 150 mm NaCl, and 50 mm Tris (pH 7.5). Proteins were resolved by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Millipore) as described (17). The membranes were blocked in Tris-buffered saline containing 0.2% Tween 20 and 5% fat-free dry milk and incubated with primary antibody and then with horseradish peroxidase-conjugated secondary antibody. Specific proteins were visualized with enhanced chemiluminescence detection reagent (Pierce).
GST/MBP Pull-down and Immunoprecipitation
GST/MBP pull-down and immunoprecipitation were performed as described previously (18). For GST pull-down, the cell lysate was incubated with glutathione-conjugated agarose beads at 4 °C for 2 h. For MBP pull-down, in vitro translated γ-tubulin was incubated with bacterially purified MBP or MBP-Cep70 immobilized on amylose-conjugated agarose beads at 4 °C for 2 h. The pull-down preparations were analyzed by immunoblotting. For immunoprecipitation, the cell lysate was incubated with anti-γ-tubulin antibody at 4 °C for 2 h, and protein A/G-agarose beads were then added to the solution for another 3 h. The precipitated proteins were then examined by immunoblotting.
Microtubule Regrowth Assay
Cells were treated with 10 μg/ml nocodazole for 1 h to depolymerize microtubules. Nocodazole was then washed out, and microtubule regrowth was examined 1 or 5 min later by immunofluorescent staining with anti-α-tubulin antibody and DAPI as described previously (13). For better visualization of microtubules, cells were treated with a buffer containing 100 mm PIPES, 1 mm EGTA, 0.5 mm MgCl2, and 0.5% Triton X-100 (pH 6.9) before immunofluorescent staining.
RESULTS
Cep70 Is Targeted to the Centrosome throughout the Cell Cycle
To characterize the molecular details of Cep70, we generated an anti-Cep70 antibody by immunizing mice with bacterially purified MBP-Cep70. To examine whether this antibody is specific for Cep70, HeLa cells were transfected with GST or GST-Cep70, and the cell lysate was examined by immunoblotting with anti-GST or anti-Cep70 antibodies. The anti-GST antibody recognized both GST and GST-Cep70, whereas the anti-Cep70 antibody recognized GST-Cep70 and endogenous Cep70 but not GST (Fig. 1A). This result demonstrated the specificity of the anti-Cep70 antibody. Immunoblotting with the anti-Cep70 antibody was unable to detect endogenous Cep70 in 293T cells (supplemental Fig. S2), probably because of the low abundance of this protein in 293T cells.
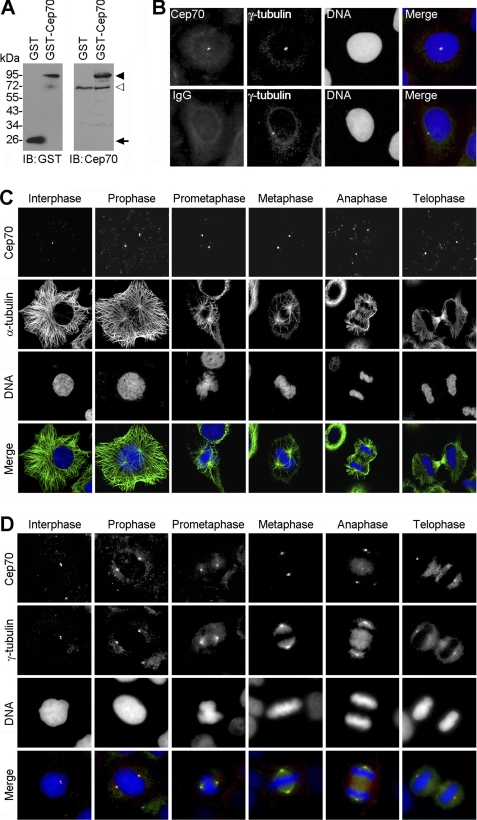
FIGURE 1.
Cep70 is targeted to the centrosome throughout the cell cycle. A, HeLa cells were transfected with GST or GST-Cep70 for 24 h, and the cell lysate was examined by immunoblotting (IB) with anti-GST or anti-Cep70 antibodies. The arrowhead, open arrowhead, and arrow indicate GST-Cep70, endogenous Cep70, and GST, respectively. B, immunofluorescent images of HeLa cells stained with anti-Cep70 (or IgG control) and anti-γ-tubulin antibodies and the DNA dye DAPI. C, HeLa cells were stained with anti-Cep70 and anti-α-tubulin antibodies and DAPI. D, HeLa cells were stained with anti-Cep70 and anti-γ-tubulin antibodies and DAPI.
Next, we investigated the subcellular localization pattern of Cep70 using this specific antibody. Immunofluorescence microscopy revealed that Cep70 colocalized with the centrosomal marker γ-tubulin in HeLa cells (Fig. 1B). By immunofluorescence microscopy, we further found that Cep70 localized at the center of the radial microtubule array in interphase and at the spindle poles during various stages of mitosis (Fig. 1C). Cep70 also colocalized with γ-tubulin in both interphase and mitotic cells (Fig. 1D). These data thus reveal that Cep70 is a centrosomal protein that resides within the centrosome throughout the cell cycle.
Cep70 Interacts with γ-Tubulin and Localizes at the Centrosome through the Peptide Fragments That Contain the Coiled-coil Domains
Given the apparent colocalization of Cep70 with γ-tubulin at the centrosome, we sought to investigate whether Cep70 physically interacts with γ-tubulin. To this end, 293T cells were transfected with GST or GST-Cep70, and a GST pull-down assay was then performed. As shown in Fig. 2A, γ-tubulin was detected in the pull-down preparation of GST-Cep70, indicating their interaction in cells. To study whether Cep70 and γ-tubulin interact directly, in vitro-translated γ-tubulin was incubated with bacterially purified MBP or MBP-Cep70. By immunoblotting, we found the presence of γ-tubulin in the pull-down preparation of MBP-Cep70 (Fig. 2B), suggesting a direct interaction between Cep70 and γ-tubulin.
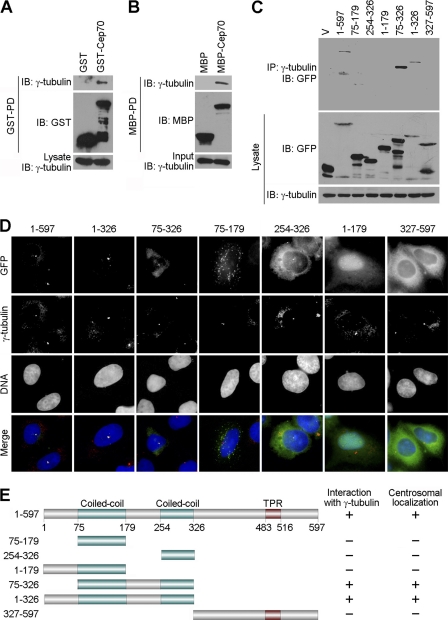
FIGURE 2.
Cep70 interacts with γ-tubulin and localizes at the centrosome through the peptide fragments that contain the coiled-coil domains. A, 293T cells were transfected with GST or GST-Cep70. A GST pull-down (PD) assay was then performed to examine the interaction between Cep70 and γ-tubulin. IB, immunoblot. B, in vitro translated γ-tubulin was incubated with bacterially purified MBP or MBP-Cep70 immobilized on agarose beads. The presence of γ-tubulin in the MBP pull-down preparation was examined by immunoblotting. C, 293T cells were transfected with GFP alone (V) or GFP-Cep70 (1–597 full-length or various truncated forms). Cell lysates and anti-γ-tubulin immunoprecipitates (IP) were immunoblotted with anti-GFP and anti-γ-tubulin antibodies. D, HeLa cells were transfected with GFP-Cep70 (1–597 full-length or various truncated forms) and immunostained with anti-γ-tubulin antibody and DAPI. E, a schematic summarizing the interactions of various forms of Cep70 with γ-tubulin and their centrosomal localization patterns. The domain structure of Cep70 was determined by the UniProt Knowledgebase (UniProtKB). TPR, tetratricopeptide repeat.
To identify the γ-tubulin interaction domain on Cep70, cells were transfected with plasmids that express various truncated forms of Cep70 tagged with GFP. Immunoprecipitation assays revealed that amino acid sequences 1–597 (full-length), 1–326, and 75–326 of Cep70 were able to interact with γ-tubulin but that sequences 75–179, 254–326, 1–179, and 327–597 were unable to interact (Fig. 2, C and E), indicating the importance of the peptide fragments containing the two coiled-coil domains in mediating the interaction of Cep70 with γ-tubulin. Immunofluorescence microscopy using various truncated forms of Cep70 further revealed that the peptide fragments containing the coiled-coil domains of Cep70 were critical for its localization at the centrosome (Fig. 2, D and E).
The Centrosomal Localization of Cep70 Is Mediated by γ-Tubulin
The finding that both the interaction of Cep70 with γ-tubulin and its centrosomal localization were mediated by the peptide fragments containing the coiled-coil domains led us to hypothesize that Cep70 may localize at the centrosome through its interaction with γ-tubulin. To test this hypothesis, cells were transfected with control or γ-tubulin siRNAs and immunostained with anti-Cep70 and anti-γ-tubulin antibodies. We found that siRNA-mediated depletion of γ-tubulin resulted in a significant reduction of Cep70 at the centrosome in both interphase and mitotic cells (Fig. 3, A and B). In contrast, siRNA-mediated depletion of pericentrin (19), another key centrosomal component, had no obvious effect on the centrosomal localization of Cep70 (Fig. 3, A and B).
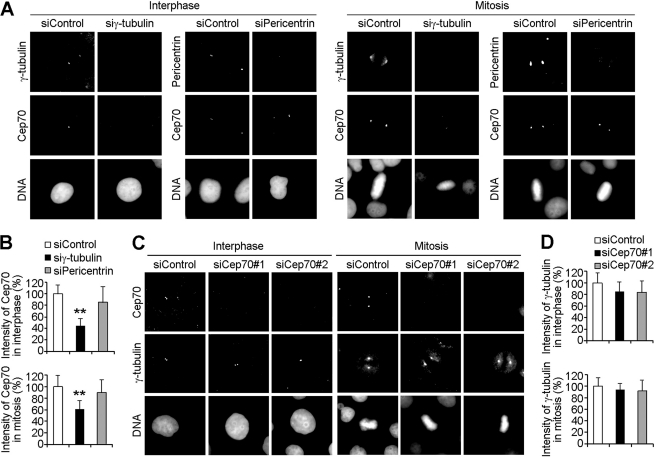
FIGURE 3.
The centrosomal localization of Cep70 is mediated by γ-tubulin. A, HeLa cells transfected with control, γ-tubulin, or pericentrin siRNAs for 72 h were stained with anti-Cep70 and anti-γ-tubulin (or pericentrin) antibodies and DAPI. B, experiments were performed as in A, and the intensity of Cep70 at the centrosome in interphase and mitotic cells was measured. **, p < 0.01 versus control. C, HeLa cells transfected with control or two different Cep70 siRNAs for 72 h were stained with anti-Cep70 and anti-γ-tubulin antibodies and DAPI. D, experiments were performed as in C, and the intensity of γ-tubulin at the centrosome in interphase and mitotic cells was measured.
We then examined whether the depletion of Cep70 affects the centrosomal localization of γ-tubulin. We used two different siRNAs to inhibit Cep70 expression in HeLa cells. Immunoblot analysis revealed that the Cep70 siRNAs could reduce its expression in a time-dependent manner. After 72 h of transfection, the two Cep70 siRNAs reduced its expression by 82 and 88%, respectively (supplemental Fig. S3). Immunofluorescence microscopy also showed that both of the Cep70 siRNAs could effectively inhibit its expression after 72 h of transfection (Fig. 3C). We found that siRNA-mediated depletion of Cep70 did not significantly affect γ-tubulin intensity at the centrosome (Fig. 3, C and D). Taken together, these results demonstrated that the centrosomal localization of Cep70 was critically dependent on γ-tubulin.
Cep70 Plays a Significant Role in the Organization of Both Preexisting and Nascent Microtubules
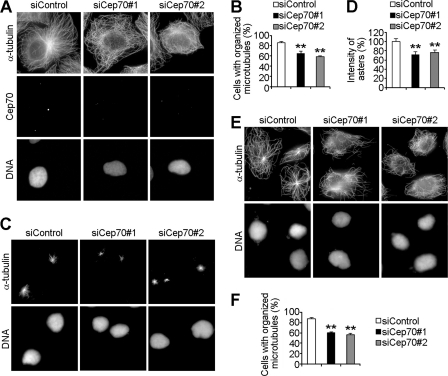
On the basis of the fact that the centrosome is the major microtubule organizing center, we speculated that as a centrosomal protein, Cep70 might play a role in regulating microtubule organization. To investigate this possibility, cells were transfected with control or Cep70 siRNAs and immunostained with anti-α-tubulin to visualize microtubules. A majority of control cells exhibited classic radial microtubule arrays, whereas in cells transfected with Cep70 siRNAs, a significant proportion of cells had disorganized microtubules without clearly defined microtubule organizing centers (Fig. 4, A and B).
FIGURE 4.
Cep70 plays a significant role in the organization of both preexisting and nascent microtubules. A, HeLa cells transfected with control or Cep70 siRNAs for 72 h were stained with anti-α-tubulin and anti-Cep70 antibodies and DAPI. B, experiments were performed as in A, and the percentage of cells with organized microtubules was measured. **, p < 0.01 versus control. C and E, HeLa cells transfected with control or Cep70 siRNAs for 72 h were treated with nocodazole to depolymerize microtubules. Nocodazole was then removed to allow microtubules to regrow for 1 min (C) or 5 min (E). Cells were stained with anti-α-tubulin antibody and DAPI. D, experiments were performed as in C, and the intensity of asters was measured with the analysis of 100 cells for each group. **, p < 0.01 versus control. F, experiments were performed as in E, and the percentage of cells with organized microtubules was measured. **, p < 0.01 versus control.
To examine whether Cep70 also regulates the organization of nascent microtubules, cells transfected with control or Cep70 siRNAs were treated with nocodazole to depolymerize microtubules. Nocodazole was then removed to allow microtubules to regrow for 1 or 5 min. We found that Cep70 siRNAs affected microtubule nucleation, as evidenced by the formation of smaller asters in the Cep70 siRNA groups compared with those in the control group after microtubules regrew for 1 min (Fig. 4, C and D). After microtubules regrew for 5 min, radial microtubule arrays were clearly seen in control cells. In contrast, the reassembled microtubules were disorganized, without clear microtubule organizing centers in a great number of cells transfected with Cep70 siRNAs (Fig. 4, E and F). Thus, these data indicate that Cep70 plays an important role in the organization of both preexisting and nascent microtubules.
Cep70 Is Necessary for the Organization and Orientation of the Mitotic Spindle
The finding that knockdown of Cep70 expression affects microtubule organization prompted us to study its function in mitotic spindle assembly, which is orchestrated by centrosomes in animal cells (3, 4). Cells were transfected with control or Cep70 siRNAs, and spindle morphology was then examined by immunofluorescence microscopy. In the control group, the majority of mitotic cells exhibited normal bipolar spindles, with both spindle poles on the same focal plane (Fig. 5, A and B). However, in the Cep70 siRNA groups, approximately 50% of mitotic cells showed disorganized spindles (without clear spindle poles) or disoriented spindles (exhibiting two spindle poles but at different focal planes) (Fig. 5, A and B).
To investigate the potential role of Cep70 in regulating spindle orientation, cells transfected with control or Cep70 siRNAs were immunostained to visualize spindle microtubules and spindle poles, and the vertical distance between the two spindle poles was measured by confocal microscopy (Fig. 5, C and D). We found that the vertical distance between the two spindle poles was 1.2 μm in control cells and increased to 2.7 and 2.5 μm, respectively, in cells transfected with the two different Cep70 siRNAs (Fig. 5E). To further analyze the function of Cep70 in spindle orientation, we measured the angle between the mitotic spindle axis and the growth plane (Fig. 5D). As shown in Fig. 5F, the majority of control cells assembled bipolar spindles parallel to the growth plane with a spindle angle of 0–15°. In contrast, Cep70 siRNAs resulted in severe defects in spindle orientation, as evidenced by the much wider distribution of spindle angles (Fig. 5F). Taken together, these data revealed that Cep70 was necessary for the organization and orientation of the mitotic spindle.
DISCUSSION
As the major microtubule organizing center of animal cells, the centrosome is involved in a variety of microtubule-associated cellular activities, including the establishment of cell shape and polarity and the regulation of cell migration (1). Through its effect on microtubule organization, the centrosome also plays an essential role in cell cycle progression (2, 3). Abnormal expression of centrosomal proteins can lead to centrosome dysfunction, which is linked to the deregulation of cell cycle progression and the development of cancer (5–9). Despite the importance of the centrosome, its structure and function are still not fully understood. By protein correlation profiling, a mass spectrometry-based proteomic study has identified Cep70 and dozens of other candidate centrosomal proteins (11). However, their subcellular localization patterns and functions are unknown. In this study, we describe the molecular characterization of Cep70 as a genuine centrosomal protein that localizes to the centrosome throughout the cell cycle. Cep70 thus joins the growing list of centrosomal proteins involved in the regulation of centrosome structure and function (10).
Studies in the past decades have improved our understanding of the functional complexity and importance of the centrosome (1, 10). However, the precise interaction and functional coordination of centrosomal proteins with key centrosomal components, such as γ-tubulin, remains enigmatic. By serial deletion analysis, we show in this study that Cep70 physically interacts with γ-tubulin at the centrosome through the peptide fragments that contain the coiled-coil domains. Importantly, our data demonstrate that the centrosomal localization pattern of Cep70 is critically dependent on γ-tubulin but not another centrosomal protein, pericentrin (19). At present, it is unknown how Cep70 synthesized in the cytoplasm is recruited to the centrosome. It is possible that the microtubule minus end-directed motor protein, dynein (20), may transport Cep70 along microtubules toward the centrosome, where it is recognized by γ-tubulin and, consequently, resides at the centrosome. It is also possible that Cep70 binds γ-tubulin in the cytoplasm and that then the Cep70/γ-tubulin complex is transported to the centrosome.
Considering the important role of the centrosome in microtubule organization (1), it is not difficult to understand that siRNA-mediated depletion of Cep70 results in severe defects in the organization of both preexisting and nascent microtubules. It will be important to investigate how Cep70 acts in concert with γ-tubulin and other centrosomal proteins in the regulation of microtubule organization. Our data also show that Cep70 is necessary for the organization and orientation of the mitotic spindle, which is consistent with the function of the centrosome as the driving force for bipolar spindle assembly in animal cells (3, 4). Given that deregulation of the mitotic spindle is associated with genomic instability, which may lead to tumorigenesis and malignant transformation (21), further studies are warranted to examine whether abnormal expression of Cep70 is involved in the development of cancer.
At present, the precise mechanism of how Cep70 is involved in spindle orientation is unknown. There is accumulating evidence that astral microtubules are key players in the regulation of spindle orientation, mainly through their interaction with the cell cortex (22, 23). In addition, the dynein-dynactin complex has been shown to exert pulling forces on astral microtubules, ensuring correct orientation of the spindle (22, 23). Thus, it is conceivable that Cep70 may participate in spindle orientation through its exquisite regulation of astral microtubule behavior. It is also possible that Cep70 may coordinate with other centrosomal components and the dynein-dynactin complex to modulate the pulling forces on astral microtubules that are critical for proper spindle orientation.
In addition to cancer, centrosomal proteins have been implicated in ciliary diseases such as cystic kidney and liver disease and retinal degeneration (5, 7). It is worth noting that the centrosome is both structurally and functionally linked to the basal body and the cilium, and a number of centrosomal proteins also normally localize at the basal body and the ciliary axoneme during different phases of the cell cycle (7). Interestingly, Cep70 has recently been reported to play a role in ciliogenesis in many tissues in the zebrafish embryo (12). Centrosome dysfunction has also been linked to many other human diseases that do not have apparent cell cycle defects, such as Alstrom syndrome and oro-facial-digital syndrome (5). Therefore, it will be interesting to study whether Cep70 is involved in the pathogenesis of ciliary diseases and other disorders.
Supplementary Material
This work was supported by National Natural Science Foundation of China Grants 30825022, 90913021, and 30801407 and National Basic Research Program of China Grants 2007CB914503 and 2010CB912204.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- MBP
- maltose-binding protein
- PIPES
- 1,4-piperazinediethanesulfonic acid.
REFERENCES
- 1. Lüders J., Stearns T. (2007) Nat. Rev. Mol. Cell Biol. 8, 161–167 [DOI] [PubMed] [Google Scholar]
- 2. Doxsey S., Zimmerman W., Mikule K. (2005) Trends Cell Biol. 15, 303–311 [DOI] [PubMed] [Google Scholar]
- 3. Tanenbaum M. E., Medema R. H. (2010) Dev. Cell 19, 797–806 [DOI] [PubMed] [Google Scholar]
- 4. O'Connell C. B., Khodjakov A. L. (2007) J. Cell Sci. 120, 1717–1722 [DOI] [PubMed] [Google Scholar]
- 5. Badano J. L., Teslovich T. M., Katsanis N. (2005) Nat. Rev. Genet. 6, 194–205 [DOI] [PubMed] [Google Scholar]
- 6. Nigg E. A. (2002) Nat. Rev. Cancer 2, 815–825 [DOI] [PubMed] [Google Scholar]
- 7. Nigg E. A., Raff J. W. (2009) Cell 139, 663–678 [DOI] [PubMed] [Google Scholar]
- 8. Ganem N. J., Godinho S. A., Pellman D. (2009) Nature 460, 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Godinho S. A., Kwon M., Pellman D. (2009) Cancer Metastasis Rev 28, 85–98 [DOI] [PubMed] [Google Scholar]
- 10. Azimzadeh J., Bornens M. (2007) J. Cell Sci. 120, 2139–2142 [DOI] [PubMed] [Google Scholar]
- 11. Andersen J. S., Wilkinson C. J., Mayor T., Mortensen P., Nigg E. A., Mann M. (2003) Nature 426, 570–574 [DOI] [PubMed] [Google Scholar]
- 12. Wilkinson C. J., Carl M., Harris W. A. (2009) BMC Cell Biol. 10, 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao J., Huo L., Sun X., Liu M., Li D., Dong J. T., Zhou J. (2008) J. Biol. Chem. 283, 8802–8809 [DOI] [PubMed] [Google Scholar]
- 14. Zhou J., Panda D., Landen J. W., Wilson L., Joshi H. C. (2002) J. Biol. Chem. 277, 17200–17208 [DOI] [PubMed] [Google Scholar]
- 15. Thoma C. R., Toso A., Gutbrodt K. L., Reggi S. P., Frew I. J., Schraml P., Hergovich A., Moch H., Meraldi P., Krek W. (2009) Nat. Cell Biol. 11, 994–1001 [DOI] [PubMed] [Google Scholar]
- 16. Godin J. D., Colombo K., Molina-Calavita M., Keryer G., Zala D., Charrin B. C., Dietrich P., Volvert M. L., Guillemot F., Dragatsis I., Bellaiche Y., Saudou F., Nguyen L., Humbert S. (2010) Neuron 67, 392–406 [DOI] [PubMed] [Google Scholar]
- 17. Liu M., Wang X., Yang Y., Li D., Ren H., Zhu Q., Chen Q., Han S., Hao J., Zhou J. (2010) J. Pathol. 221, 221–228 [DOI] [PubMed] [Google Scholar]
- 18. Sun L., Gao J., Huo L., Sun X., Shi X., Liu M., Li D., Zhang C., Zhou J. (2010) J. Pathol. 221, 425–432 [DOI] [PubMed] [Google Scholar]
- 19. Delaval B., Doxsey S. J. (2010) J. Cell Biol. 188, 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kardon J. R., Vale R. D. (2009) Nat. Rev. Mol. Cell Biol. 10, 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thompson S. L., Bakhoum S. F., Compton D. A. (2010) Curr. Biol. 20, R285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siller K. H., Doe C. Q. (2009) Nat. Cell Biol. 11, 365–374 [DOI] [PubMed] [Google Scholar]
- 23. Pease J. C., Tirnauer J. S. (2011) J. Cell Sci. 124, 1007–1016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.