Abstract
Acetylcholinesterase (AChE) anchors onto cell membranes by a transmembrane protein PRiMA (proline-rich membrane anchor) as a tetrameric form in vertebrate brain. The assembly of AChE tetramer with PRiMA requires the C-terminal “t-peptide” in AChE catalytic subunit (AChET). Although mature AChE is well known N-glycosylated, the role of glycosylation in forming the physiologically active PRiMA-linked AChE tetramer has not been studied. Here, several lines of evidence indicate that the N-linked glycosylation of AChET plays a major role for acquisition of AChE full enzymatic activity but does not affect its oligomerization. The expression of the AChET mutant, in which all N-glycosylation sites were deleted, together with PRiMA in HEK293T cells produced a glycan-depleted PRiMA-linked AChE tetramer but with a much higher Km value as compared with the wild type. This glycan-depleted enzyme was assembled in endoplasmic reticulum but was not transported to Golgi apparatus or plasma membrane.
Keywords: Acetylcholinesterase, Glycosylation, Membrane Trafficking, Protein Assembly, Subcellular Fractionation, PRiMA, Tetramer
Introduction
Acetylcholinesterase (AChE),2 a heavily N-glycosylated enzyme, is responsible for the hydrolysis of acetylcholine at the cholinergic synapses, which is essential for the control of neurotransmission. In vertebrates, AChE possesses alternative C-terminal R or H or T peptide that defines its post-translational maturation, quaternary association, and anchoring (1). AChER generates soluble monomers (G1), AChEH generates glycolipid-anchored dimers (G2), and AChET, which is largely predominant in the brain and muscle, generates a multitude of oligomers. The functional localization of AChET depends on the association of tetramer(s) with anchoring proteins: the collagen tail (ColQ) and the proline-rich membrane anchor (PRiMA). The ColQ-associated AChE (A12) is attached to the basal lamina at vertebrate neuromuscular junctions (2, 3), and the PRiMA-linked AChE tetramer (G4) is anchored on cell membrane of the brain (4–6) and muscle (7). The association of the catalytic subunits (AChET) with ColQ and PRiMA proteins is mostly based on a tight interaction between the four C-terminal t-peptides of AChET subunits and a proline-rich attachment domain that exists in both ColQ and PRiMA (8, 9).
N-Glycosylation, a form of co-translational or post-translational modification, occurs in a very large proportion of secreted or membrane-bound proteins; pre-assembled carbohydrate chains are co-translationally attached to asparagines and may be post-translationally modified in the endoplasmic reticulum (ER) and in the Golgi apparatus, resulting in chains of various sizes, composition, and complexity. The glycans can affect a host of cellular processes. In vertebrates, AChE molecules were shown to have various numbers of carbohydrate chains. Different glycosylation patterns of AChE have been found in different tissues and in different molecular forms. In human and bovine, G2 AChE from erythrocyte membrane is more heavily glycosylated than G4 AChE in the brain (10, 11). In chicken, AChE from muscle is ∼5 kDa heavier than the enzyme from brain, possibly due to variation of glycosylation (12). In bovine muscle, G4 AChE carries carbohydrate chains of the complex type, whereas G1 appears to possess only the high mannose type (13). These lines of evidence suggest that different post-translational modifications in the carbohydrate chains affect the oligomerization of AChE molecular forms.
Without added oligosaccharides, many proteins remain misfolded, aggregated, and degraded without a proper transportation from ER to the Golgi complex and beyond (14). The N-glycosylation of human AChET plays an important function during the biosynthesis and secretion of G1 and G2 forms of AChE in transfected cells; the elimination of N-glycosylation sites in AChET mutants dramatically reduced the secreted enzyme (15). Post-translational modification of N-glycans was used as a marker for the progression of AChE through the different subcellular compartments in cultured muscles (16). In addition, the accumulation of molecular forms of AChE with an altered pattern of glycosylation has been observed in the brain and cerebrospinal fluid of Alzheimer patients (17). In particular, AChE is associated with the senile plaques and shows a higher degree of glycosylation than in normal tissues (18).
Here we analyzed the role of N-glycosylation on the assembly and trafficking of PRiMA-linked G4 AChE. By expressing AChET mutants, in which single or multiple N-glycosylation sites were removed, we found that N-glycans play important roles for AChE biosynthesis. The depletion of N-glycosylation did not prevent the assembly of AChET subunits with PRiMA in forming a PRiMA-linked G4 tetramer. However, the glycan-depleted PRiMA-linked AChE, which was retained in the ER compartment, was enzymatically less active and stable than that of wild-type G4 AChE. These results suggest that in addition to assisting the folding of AChET protein, the N-glycans are essential for the proper trafficking of PRiMA-linked AChE from ER to Golgi apparatus.
EXPERIMENTAL PROCEDURES
Cell Cultures
The human embryonic kidney fibroblast cell line (HEK293T) was obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum at 37 °C in a water-saturated 5% CO2 incubator. All reagents for cell cultures were purchased from Invitrogen.
DNA Constructs and Transfection
The cDNAs encoding wild-type human AChET and glycosylation mutants (AChETN296Q, AChETN381Q, AChETN495Q, and AChETN296Q/N381Q/N495Q, as described in Ref. 15) were subcloned in a pcDNA4 vector under a CMV promoter. The cDNA encoding full-length mouse PRiMA, tagged with an HA epitope (YPY DVP DYA) at the C terminus, was subcloned in a pEF-BOS vector under the control of an EF-1α promoter (4, 19). The cDNA encoding full-length chicken AChET was subcloned in a pcDNA3 vector under a CMV promoter (20, 21). One day after plating, cultured HZK293T cells were transfected with cDNAs by calcium phosphate precipitation (22). The cells were collected 2 days after the transfection in a low salt lysis buffer (10 mm HEPES, pH 7.5, 1 mm EDTA, 1 mm EGTA, 150 mm NaCl, and 0.5% Triton X-100) with the addition of the following protease inhibitors: 10 μg/ml leupeptin, 10 μg/ml aprotinin, 20 μm pepstatin, and 2.5 mm benzamidine HCl. The homogenization was done by vortexing for 15 min, and the homogenates were clarified by centrifugation for 30 min at 16,000 × g at 4 °C.
Sucrose Density Gradients
Separation of AChE molecular forms was performed by sucrose density gradient analysis as described previously (6, 22). In brief, continuous sucrose gradients (5–20%) in a detergent-containing buffer (10 mm HEPES, pH 7.5, 1 mm EDTA, 1 mm EGTA, 0.2% Brij-97, and 150 mm NaCl) were prepared in 12-ml polyallomer ultracentrifugation tubes with a 0.4-ml cushion of 60% sucrose at the bottom. Samples of cell extracts (0.2 ml) containing equal amounts of protein were mixed with the sedimentation markers, alkaline phosphatase (6.1 S; Roche Applied Science) and β-galactosidase (16 S; Roche Applied Science) and loaded onto the gradients to be centrifuged at 38,000 rpm in a Sorvall TH 641 rotor at 4 °C for 16 h. Approximately 45 fractions were collected and subjected to enzymatic activity assays.
Enzymatic Activity Assay
AChE activity was determined according to the method of Ellman (23) with minor modifications. The cell lysates were incubated with 0.1 mm tetraisopropylpyrophosphoramide (iso-OMPA; Sigma) for 10 min to inhibit butyrylcholinesterase activity. Samples of about 5–20 μl were then added to the reaction mixture with final concentrations of 0.625 mm acetylthiocholine iodide (ATCh; Sigma) and 0.5 mm 5,5′-dithiobis-2-nitrobenzoic acid (Sigma) in 80 mm sodium phosphate, pH 7.4. The increase in absorbance at 410 nm was recorded, and the specific enzyme activity was expressed as absorbance units/min/μg of protein. The effects of substrate concentration on AChE activities were determined under similar assay conditions using increasing ATCh concentrations. Km values were obtained from Lineweaver-Burk plots. The amounts of the various AChE forms were determined by summation of the enzymatic activities corresponding to the peaks of their respective sedimentation profiles. The sedimentation values of the enzymes were calculated from the positions of the markers, alkaline phosphatase and β-galactosidase, as described previously (22).
Western Blot Analysis
For non-reducing SDS-PAGE, G2 fractions obtained from the sucrose density gradients were denatured at 100 °C for 5 min in a buffer containing 2% SDS and separated by electrophoresis in 8% SDS-PAGE. For reducing SDS-PAGE, the protein lysate was denatured in the presence of 2% SDS and 100 mm β-mercaptoethanol. Anti-human AChE antibody E-19 (1:2,000, Santa Cruz Biotechnology, Santa Cruz, CA) (24), anti-chicken AChE antibody 3D10 (1:5,000, purified at 1 mg/ml) (12), or anti-tubulin antibody (1:50,000, Sigma) (25) was used for Western blot analyses. The immune complexes were visualized using the enhanced chemiluminescence (ECL) method (Amersham Biosciences) in strictly standardized conditions. The intensities of the bands were quantified by ImageJ 1.43u analysis software using a calibration plot constructed from a parallel gel with serial dilutions of AChET-expressed cell lysates. The labeling intensities of protein bands were in the non-saturating range of calibration curves.
De-glycosylation
Ninety μl of cell lysate, obtained from the transfected cell cultures, were mixed with 10 μl of 10× incubation buffer (200 mm sodium phosphate, pH 6, 100 mm EDTA,1% SDS, 10% β-mercaptoethanol, and 5% Triton X-100). One mm PMSF was added to inhibit protease activity. The reaction was incubated with 0.5 μl of endoglycosidase H (Endo H; Roche Applied Science) or peptide N-glycosidase F (PNGase F; Roche Applied Science) for 16 h at 37 °C and then processed for Western blot analysis. For de-glycosylation without sample denaturation, SDS and β-mercaptoethanol were excluded from 10× incubation buffer. The experiment was performed without PMSF to preserve AChE enzymatic activity. The reaction was incubated with 2 μl of Endo H or PNGase F for 24 h at 37 °C and then processed for other assays.
Subcellular Fractionation
Forty-eight hours after the transfection, the cultured HEK293T cells, grown in 100-mm dishes, were detached from confluent cultures in 0.5 ml of homogenization buffer (10 mm HEPES, pH 7.4, 1 mm EDTA, 0. 25 m sucrose, supplemented with protease inhibitors as in the low salt lysis buffer). The cells were disrupted using 10 strokes in a Dounce homogenizer on ice followed by 10 passages through a 30-gauge needle with a 1-ml syringe. Nuclei and unbroken cells were pelleted by centrifugation at 500 × g for 10 min. The clarification process was repeated one or more times until no visible pellet was detected. Post-nuclear supernatants from the centrifugation steps were combined and centrifuged at 80,000 × g for 1 h. The vesicle pellet was resuspended in 0.8 ml of homogenization buffer and applied on top of linear iodixanol gradients (1–20%, Sigma). The gradients were centrifuged in a Sorvall TH 641 rotor at 200,000 × g for 3 h at 4 °C. Sequential 1-ml fractions were collected from the top of the gradient, and the distribution of the intracellular markers was examined by Western blotting with different antibodies: calnexin (1:10,000, Sigma) (26) for the ER, Golgi matrix protein 130 (GM130) (1:1,000, BD Biosciences) (27) for the Golgi apparatus, and Na+/K+-ATPase (1:1,000, Abcam, Cambridge, UK) (28) for the plasma membrane (PM).
Immunoprecipitation
Five hundred μl of G2 or G4 fractions, obtained from the sucrose density gradients, were incubated with anti-human AChE antibody E-19 (1:200) or anti-chicken AChE monoclonal antibody (3D10, 1:200) at 4 °C overnight. Neither antibody cross-reacted with the other corresponding antigen (21). Each sample was added with 30 μl of protein G-agarose (Roche Applied Science) and incubated for 4 h at 4 °C. The protein G beads were centrifuged in a microcentrifuge tube and washed three times with ice-cold extraction buffer. The supernatant was discarded by careful aspiration, and the protein G beads were then subjected to Western blotting and probed with anti-human AChE and anti-chicken AChE antibodies.
Immunofluorescence Analysis
The transfected HEK293T cell cultures were fixed by 4% paraformaldehyde in PBS for 10 min followed by 50 mm ammonium chloride (NH4Cl) treatment for 30 min. Samples were blocked by 5% BSA in PBS with or without 0.2% Triton X-100 for 1 h at room temperature. Cultures were stained with anti-human AChE antibody (1:500) or double-stained with anti-chicken AChE (1:500) and anti-human AChE (1:500) antibodies for 16 h at 4 °C followed by the corresponding fluorescence-conjugated secondary antibodies (Alexa 488-conjugated anti-goat. Alexa 555-conjugated anti-mouse) for 2 h at room temperature. Samples were dehydrated serially with ice-cold 50, 75, 95, and 100% ethanol and mounted with fluorescence mounting medium (DAKO, Carpinteria, CA). The samples were then examined using a fluorescence microscopy (DMIRE2, Leica, Wetzlar, Germany). Images were captured by Leica confocal software (Version 2.61) using PL APO 40 × 0.75 DRY objective with excitation at 488 nm/emission at 500–535 nm for green and excitation at 543 nm/emission at 560–620 nm for red.
Other Assays
Protein concentrations were measured by the Bradford method with a kit from Bio-Rad. Statistical tests were performed using one-way analysis of variance; differences from basal or control values were classified as * where p < 0.05, ** where p < 0.01, and *** where p < 0.001.
RESULTS
Glycosylation of AChET
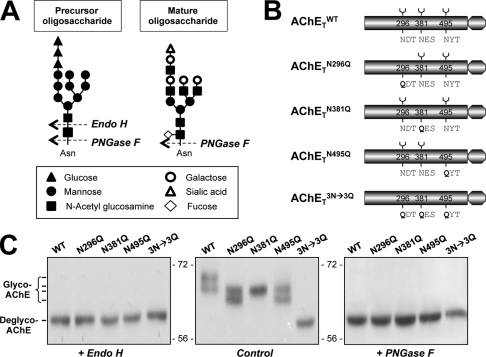
To demonstrate that AChET is post-translationally N-glycosylated in our expression system of HEK293T cells, we used two glycosidases: Endo H and PNGase F. Endo H cleaves only high mannose glycan chains that have not been modified in the Golgi apparatus, whereas PNGase F removes modified complex chains that have been processed in the Golgi apparatus (Fig. 1A). As reported by Velan (15), the wild-type human AChET (AChETWT) sequence possesses three putative N-linked glycosylation sites, located at 296, 381, and 495 (Fig. 1B). We used mutants in which these sites were suppressed by replacing asparagines by glutamines to determine whether they are all actually utilized in the glycosylation of AChET. Each site was suppressed individually in AChETN296Q, AChETN381Q, and AChETN495Q mutants. Additionally, a triple mutant, AChETN296Q/N381Q/N495Q, devoid of all three N-glycosylation sites, was constructed. The cDNAs encoding AChETWT, AChETN296Q, AChETN381Q, AChETN495Q, and AChETN296Q/N381Q/N495Q were separately transfected in HEK293T cells.
FIGURE 1.
Characterization of N-glycosylation of human AChET. A, representative cutting sites of Endo H and PNGase F are shown. N-Linked oligosaccharide chains share a chitobiose core (two N-acetyl glucosamine) linked to one side to an asparagine residue of a potential N-glycosylation site (Asn-X-Ser/Thr) and on the other side to a trimannosyl core (three mannose) that is linked to high mannose structure in precursor oligosaccharide or various complex sugar chains in mature oligosaccharide. Endo H specifically recognizes high mannose oligosaccharides, cleaving between the N-acetylglucosamine residues of the diacetylchitobiose core, whereas PNGase F cleaves all N-linked glycans attached to asparagine. A possible example is shown for illustration. B, shown are schematic representations of human AChET and its glycosylation site mutants. C, HEK293T cells were single-transfected with cDNAs encoding AChETWT, AChETN296Q, AChETN381Q, AChETN495Q, and AChETN296Q/N381Q/N495Q. The cell lysates were incubated with Endo H or PNGase F and analyzed by Western blotting with anti-AChE antibody. Representative gels from three independent experiments are shown (n = 3).
In AChETWT cDNA-transfected cells, G1/G2 form AChE was formed (supplemental Fig. 1). The cell lysates were incubated with Endo H or PNGase F, and the digested proteins were compared by Western blotting; there were at least two species of AChET migrating on the gel at 68∼70 kDa (Fig. 1C). The two species of AChET were shifted to a single band after digestion with Endo H or PNGase F, producing a single band of low molecular size (∼60 kDa) corresponding to the de-glycosylated AChET. The formation of AChE molecular forms (i.e. G1/G2) by the glycosylation mutants was similar to that of the wild type (supplemental Fig. 1). For the glycosylation mutants, different migration patterns of these mutants were observed; double bands of AChET appeared in AChETN296Q- and AChETN495Q-transfected cells but only a single band occurred in AChETN381Q-transfected cells (Fig. 1C). In addition, the molecular mass of AChETN296Q/N381Q/N495Q was at ∼60 kDa (the lowest band), corresponding exactly to totally un-glycosylated AChET. The protein sizes formed by these mutants were in line with the previous study (15). The elimination of glycosylation by Endo H or PNGase F treatment resulted in a decrease in apparent molecular mass of AChET to ∼60 kDa for all the mutants (Fig. 1C). Therefore, the three putative N-linked glycosylation sites of AChET are fully utilized, and AChET contains no other N-glycosylation site.
The cDNA encoding AChET or the glycosylation mutants was transfected together with PRiMA cDNA into HEK293T cells: G4 AChE was formed in all cases (supplemental Fig. 1). In the co-expression of AChETWT and PRiMA, additional species of AChET with higher apparent masses of 68 ∼ 72 kDa were revealed (Fig. 2A, upper panel). In contrast, the co-expression of PRiMA had no significant effect on the glycosylation pattern of the mutants (Fig. 2A, upper panel).
FIGURE 2.
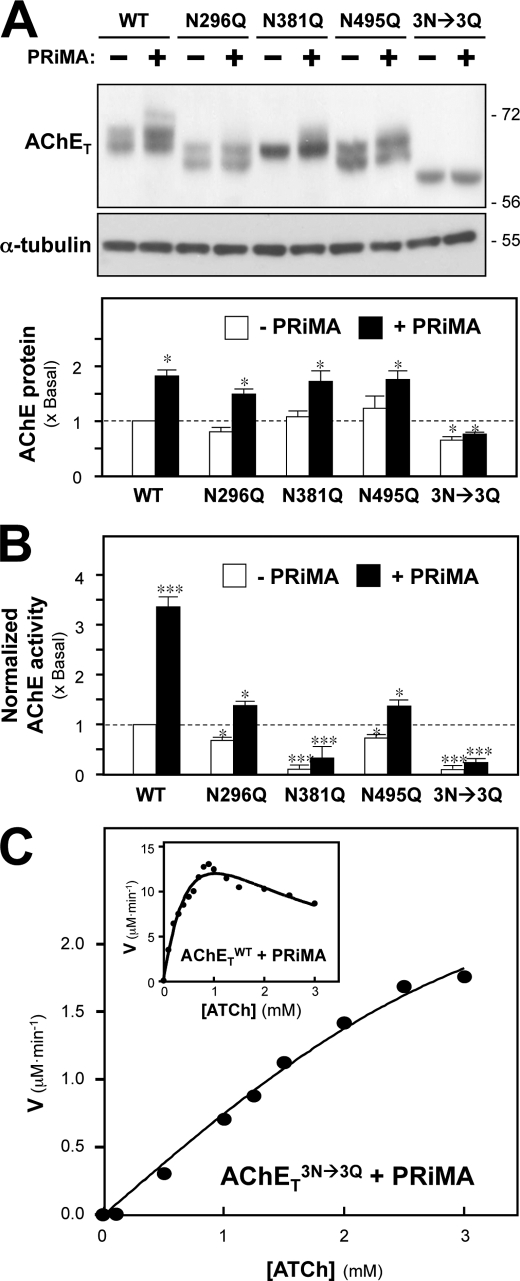
N-Glycosylation of AChET affects the production of catalytic activity. A, HEK293T cells were transfected with cDNAs encoding AChETWT, AChETN296Q, AChETN381Q, AChETN495Q, and AChETN296Q/N381Q/N495Q with or without PRiMA. The proteins were analyzed as in Fig. 1C. α-Tubulin (∼55 kDa) served as a loading control. The lower panel shows quantitation of AChET protein from the blots by calibrated densitometry. B, AChE enzymatic activity in the transfected cells from A was determined by the Ellman assay. The data are normalized to the amount of AChET protein and are expressed as the ratio to the value obtained for cells expressing AChETWT alone, which is arbitrarily set to 1. A similar profile was achieved if the enzymatic activity was normalized to total protein (not shown for clarity). C, AChETWT and PRiMA (inset) or AChETN296Q/N381Q/N495Q and PRiMA co-expressed cell lysates were subjected to Ellman assay using different concentrations of ATCh substrate. The reaction velocity (V) was calculated at each concentration of substrate. Representative profiles from six independent experiments are shown. Values are the means ± S.E. obtained in four independent experiments (n = 4), each with triplicate samples. *, p < 0.05; ***, p < 0.001 compared with AChETWT alone control.
By comparing the protein expression of AChETWT and its glycosylation mutants in transfected HEK293T cells, we then examined the role of PRiMA in directing the expression profile of AChET. The expression levels of AChETWT and its mutants in the absence of PRiMA were comparable, differing by less than 10% (Fig. 2A, lower panel). Co-expression of AChET with PRiMA markedly increased the protein levels of wild-type and single mutants, except for the AChETN296Q/N381Q/N495Q mutant, which remained at a low level of expression (Fig. 2A, lower panel). In addition, the enzymatic activity of AChETWT and its glycosylation mutants in transfected HEK293T cells was compared. In the presence of PRiMA the enzymatic activity was markedly enhanced in all cases, as normalized to AChET protein in each sample (Fig. 2B); the increase was more obvious in AChETWT. On the other hand, the enzymatic activity of the glycosylation mutants was dramatically decreased as compared with that of AChETWT. In the absence of PRiMA, the enzymatic activity of AChETN296Q and AChETN495Q was reduced to 70 ∼ 80% that of AChETWT, whereas the enzymatic activity of AChETN381Q and AChETN296Q/N381Q/N495Q was dramatically reduced to only ∼10% of the control (Fig. 2B). The co-expression with PRiMA did not help that much in terms of AChE activity of those mutants. The rates of hydrolysis of ATCh by AChETWT and AChETN296Q/N381Q/N495Q were measured as a function of ATCh concentration by using AChETWT or AChETN296Q/N381Q/N495Q and PRiMA-co-expressed cell lysates (Fig. 2C). AChETWT exhibited the classical bell-shaped curve of hydrolysis rate versus substrate concentration in which the substrate inhibition started from 1 mm of ATCh. On the other hand, AChETN296Q/N381Q/N495Q showed no inhibition even at a substrate concentration as high as 3 mm. These results suggest a possible collaboration of the three glycosylation sites in directing AChE folding and/or catalytic activity.
We compared the catalytic properties of AChETWT and of its glycosylation mutants. Table 1 shows different Km values of those mutated enzymes. In general, the PRiMA-linked AChE (i.e. G4) showed a lower Km as compared with that of G1/G2 enzyme (no PRiMA). The Km values of AChETN296Q and AChETN495Q enzymes were 15∼30% higher than that of AChETWT enzyme, and those of AChETN381Q and AChETN296Q/N381Q/N495Q enzymes were about 60-fold higher. These data suggest that N-glycans, particularly on Asn-381, are very important for maintaining the conformation of catalytically active AChE. Differences in Km appear to contribute to the activity differences as shown in Fig. 2B. The Lineweaver-Burk plots for the Km value determination were shown in supplemental Fig. 2.
TABLE 1.
N-Glycosylation affects the catalytic function of AChE
Cell lysates from HEK293T cells expressing AChETWT, AChETN296Q, AChETN381Q, AChETN495Q, or AChETN296Q/N381Q/N495Q with (mainly G4) or without (mainly G1/G2) PRiMA were subjected to Ellman assays using different concentrations of ATCh as substrate, which would not cause substrate inhibition on AChE. The reaction velocity was determined at each concentration of substrate. Km values were determined according to a Lineweaver-Burk plot. Values are the means ± S.E. obtained from nine independent experiments (n = 9).
| −PRiMA | +PRiMA | |
|---|---|---|
| μm | μm | |
| AChETWT | 294 ± 82 | 261 ± 73 |
| AChETN296Q | 326 ± 98 | 330 ± 69 |
| AChETN381Q | 17,379 ± 2961 | 16,480 ± 3690 |
| AChETN495Q | 314 ± 71 | 307 ± 94 |
| AChETN296Q/N381Q/N495Q | 19,058 ± 3667 | 17,013 ± 2799 |
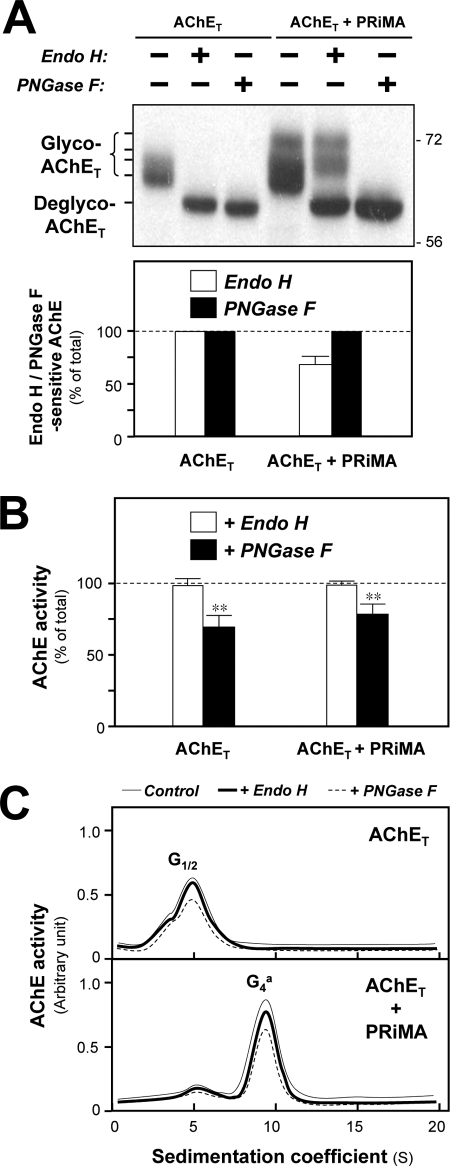
In the co-expression of AChET and PRiMA, several species of AChET migrating on the gel from 68 ∼ 72 kDa were revealed; the higher molecular mass species were distinct as compared with that of singly AChET-expressed cells (Fig. 3A, upper panel). After treatment with Endo H, the 68∼70-kDa bands were shifted to a single band at ∼60 kDa, whereas the migration of the highest molecular mass bands at 70∼72 kDa was unaffected. Treatment with PNGase F resulted in a mobility shift of all AChET, producing the completely deglycosylated species at ∼60 kDa (Fig. 3A, upper panel). These results suggested that an extensive glycosylation with more complex sugar chains was triggered in PRiMA-linked G4 AChE after it was processed from ER to Golgi. In addition, AChET was 100% Endo H-sensitive when expressed alone (Fig. 3A, lower panel) and, therefore, appeared to be retained in the ER; this evidence was also revealed in PRiMA knock-out mice (29). However, AChET was only partially Endo H-sensitive when co-expressed with PRiMA, suggesting that a portion of PRiMA-linked G4 AChE was transported to the Golgi apparatus, as it contained a mixture of high mannose chains and complex oligosaccharides. In that case, a quantification of the Western blotting indicated that about 70% G4 AChE remained Endo H-sensitive (Fig. 3A, lower panel), i.e. never transit to the Golgi apparatus.
FIGURE 3.
N-Glycosylation is not required the catalytic activity of folded AChET protein. A, HEK293T cells were transfected with cDNAs encoding AChET with or without cDNA encoding PRiMA. Equal amounts of protein from the cell lysates were incubated with or without Endo H or PNGase F under the non-denaturing conditions. The cell lysates were analyzed by SDS-PAGE and Western blotting with anti-AChE antibody (upper panel). The amount of the Endo H-sensitive or PNGase F-sensitive AChET was quantified by calibrated densitometry (lower panel). The data are expressed as % of total AChET protein. B, AChE enzymatic activity of the cell lysate from A was determined by Ellman assay. The data are expressed as % of total AChE activity before the treatment. C, the cell lysates from A were subjected to sucrose density gradient analysis. The enzymatic activities are plotted as a function of the S value, estimated from the position of the sedimentation markers. The enzymatic activities are expressed in arbitrary units. Representative gradient profiles and gels from four independent experiments are shown. Values are the means ± S.E. obtained in four independent experiments (n = 4). **, p < 0.01 compared with undigested control.
We also studied the effect of de-glycosylation on active/mature wild-type AChE by Endo H or PNGase F digestion. After the de-glycosylation by PNGase F, the enzymatic activity of AChE, expressed with or without PRiMA, was reduced by ∼30% (Fig. 3B). In contrast, Endo H showed no effect on the enzymatic activity. The digestion of Endo H on the native enzyme showed a dose- and time-dependent manner, as revealed here by the protein pattern under a complete digestion of Endo H (supplemental Fig. 3). We also found that the elimination of glycans did not change the sedimentation properties or proportions of AChE molecular forms (Fig. 3C). This indicates that once the AChE protein has been correctly folded, the glycans are not required to maintain its catalytically active conformation or its oligomeric structure.
Effects of N-Glycosylation on the Assembly of G4 AChE
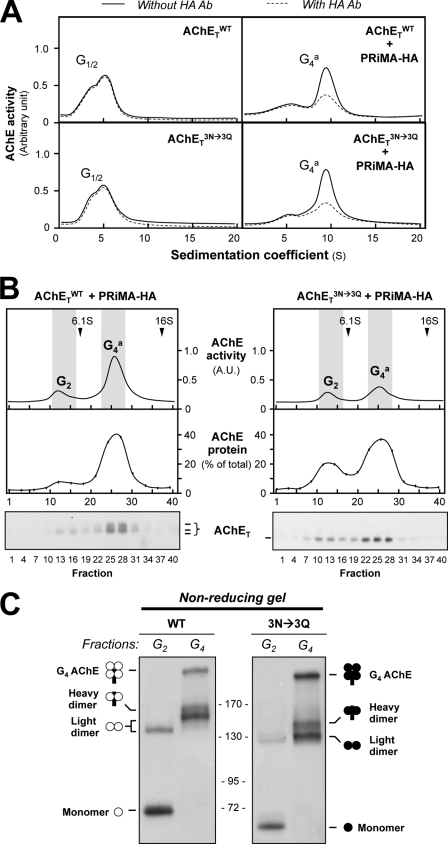
We analyzed the influence of glycosylation on the assembly of PRiMA-linked AChE tetramers. The sedimentation profiles of AChE activity were similar for the wild-type AChETWT and for the un-glycosylated mutant AChETN296Q/N381Q/N495Q, except for the difference in the level of enzymatic activity when expressed alone or with PRiMA (Fig. 4A). Like AChETWT, AChETN296Q/N381Q/N495Q produced only G1 and G2 forms when expressed alone, whereas the G4 form was produced when AChETN296Q/N381Q/N495Q was co-expressed with PRiMA-HA (HA epitope-tagged). To confirm whether the G4 form was PRiMA-linked or not, we performed an immunodepletion assay using anti-HA antibody. The G4 form of AChETN296Q/N381Q/N495Q was depleted by anti-HA antibody by ∼80%, indicating that the G4 enzymes, formed by AChETWT and AChETN296Q/N381Q/N495Q, were associated with PRiMA (Fig. 4A).
FIGURE 4.
N-Glycosylation of AChET is not required for its assembly with PRiMA. A, HEK293T cells were transfected with the cDNAs encoding AChETWT or AChETN296Q/N381Q/N495Q with or without PRiMA-HA. Cell lysates containing equal amounts of AChE activity were incubated with or without an anti-HA antibody (Ab). After precipitation by protein G-agarose, the supernatants were subjected to sucrose density gradient analysis. B, cell lysates containing equal amounts of total protein were subjected to sucrose density gradient analysis. Fractions were assayed for AChE enzymatic activity and AChET protein by Western blotting. The upper panel shows the enzymatic activity, whereas the middle and lower panels indicate the amount of AChET protein in selected fractions. Sedimentation markers are shown. The enzymatic activities are expressed in arbitrary units (A.U.), and the protein is shown as % of total AChET protein. C, G2 and G4 fractions, corresponding to the shaded zones of the gradients in B, were analyzed by non-reducing electrophoresis and Western blotting with the anti-AChE antibody. Representative gradient profiles and gels from three independent experiments are shown (n = 3).
In addition, we determined the level of AChET protein expression in different forms of AChE by quantification of Western blotting (Fig. 4B). Lysates from cells co-expressing AChETWT and PRiMA-HA or co-expressing AChETN296Q/N381Q/N495Q and PRiMA-HA, containing equal amounts of total protein, were subjected to sucrose-density gradient centrifugation. The enzymatic activity and AChETWT protein were determined in each fraction. The amount of AChET protein correlated well with the enzymatic activity in the sucrose-density gradient (Fig. 4B), i.e. G4 enzyme activity matched with the amount of AChET. On the other hand, the un-glycosylated G4 enzyme, formed by AChETN296Q/N381Q/N495Q and PRiMA, shared similarity to that of the wild type. This confirmed that the un-glycosylated AChETN296Q/N381Q/N495Q mutant, like AChETWT, was able to assemble with PRiMA to form an ∼10 S sedimentating G4 form, showing that N-glycosylation was not necessary for the oligomerization.
The subunit composition of the G2 and G4 forms was studied by non-denaturing gel electrophoresis. The G2-enriched fractions, collected from cells expressing AChETWT in the absence of PRiMA, contained only monomers and dimers (Fig. 4C). Light dimers (no PRiMA), heavy dimers (with PRiMA), and tetramers (with PRiMA) were observed in the G4-enriched fractions, as reported previously (21). We obtained identical results in the case of AChETN296Q/N381Q/N495Q, which suggested the oligomerization of AChE dimer or tetramer was not affected by the glycosylation of AChET.
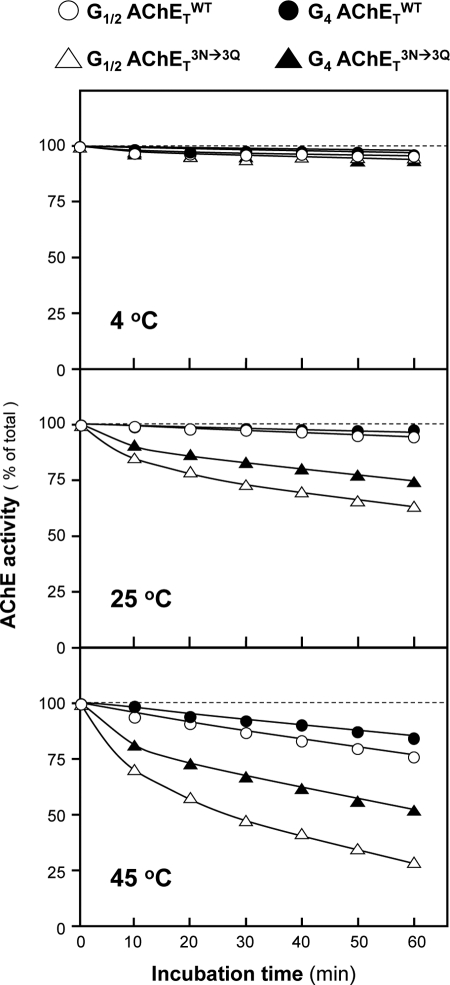
The influence of N-glycosylation on the thermal stability of different forms of AChETWT and AChETN296Q/N381Q/N495Q was determined by comparing the time-dependent loss of enzymatic activity at different temperatures. The G1/2 fractions were obtained from cells expressing AChETWT or AChETN296Q/N381Q/N495Q without PRiMA, and the G4 fractions were obtained from cells expressing AChETWT or AChETN296Q/N381Q/N495Q with PRiMA. At 4 °C, G1/2 and G4 forms of AChETWT or AChETN296Q/N381Q/N495Q were rather stable, i.e. the enzymatic activity did not change over a period of 1 h (Fig. 5). At 25 and 45 °C, AChETN296Q/N381Q/N495Q presented a significant decrease in the enzymatic activity (Fig. 5). The rank order of thermal sensitivity was G4 AChETWT > G1/2 AChETWT > G4 AChETN296Q/N381Q/N495Q > G1/2 AChETN296Q/N381Q/N495Q. This indicates that the N-glycans of AChE contribute to the enzyme stability.
FIGURE 5.
The thermostability of glycan-depleted PRiMA-linked G4 AChE. G1/2 and G4 fractions of AChETWT or AChETN296Q/N381Q/N495Q from Fig. 4 were incubated at 4, 25, and 55 °C. Samples were collected at 10-min intervals from 0 to 60 min and chilled on ice, and their residual enzymatic activity was determined. The data are expressed as the % of the original activity (time 0). Values are the means ± S.E., with triplicate samples (n = 3). The S.E. bars are not shown for clarity (<5% of the means).
Effects of N-Glycosylation on the Trafficking of PRiMA-linked G4 AChE
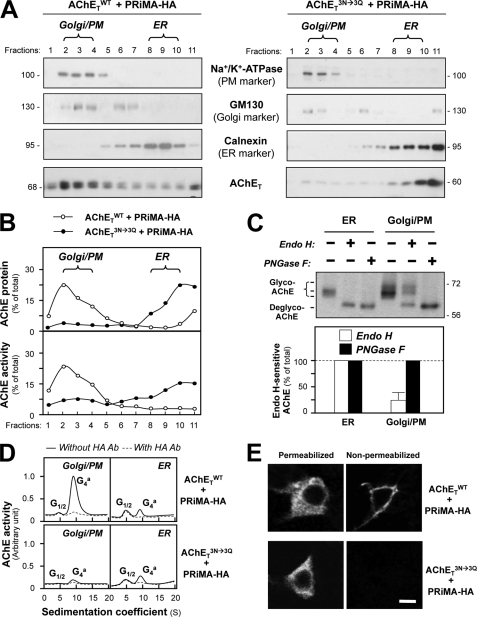
Although the abolishment of N-glycosylation on AChET subunits did not prevent their assembly with PRiMA, it might affect the proper trafficking of the oligomeric protein. To address this question, subcellular fractionation was performed to determine the distribution of AChET in cultures expressing AChETWT or AChETN296Q/N381Q/N495Q together with PRiMA-HA. The subcellular compartments were separated in continuous iodixanol gradients and identified by Western blotting with specific antibodies against different marker proteins. The PM marker (Na+/K+-ATP) and the Golgi marker (GM130) were found at the top of the gradient, i.e. fractions 2–4, whereas the ER marker (calnexin) was enriched at the bottom of the gradient, i.e. fractions 8–10 (Fig. 6A). The last fraction was excluded because of the contamination of aggregated proteins. In cells co-expressing AChETWT and PRiMA-HA, AChET underwent a typical pattern of ER-to-Golgi maturation, with the majority of mature AChET (heavily glycosylated) localized in Golgi/PM fractions (Fig. 6A). In contrast, the glycosylation mutant AChETN296Q/N381Q/N495Q was localized almost exclusively in the ER fractions, with only a very small proportion in the Golgi/PM fractions (Fig. 6A). Similarly, the other glycosylation mutants were predominantly localized in ER fractions; but the extent was not as severe as that of AChETN296Q/N381Q/N495Q mutant (supplemental Fig. 4A). Fig. 6B shows the quantitative distribution of AChET in different subcellular organelles. In the absence of glycosylation, AChETN296Q/N381Q/N495Q protein was retained in the ER and failed to be transported to the Golgi apparatus. In AChETWT expression, the enzymatic activity was in line to the protein expression, i.e. higher activity in the Golgi apparatus. In contrast, the ER-retained AChETN296Q/N381Q/N495Q, although in high abundance, showed very little enzymatic activity (Fig. 6B). These phenomena were also applied to other mutants (supplemental Fig. 4B). This result was in line with the low enzymatic activity of the un-glycosylated AChETN296Q/N381Q/N495Q mutant. In addition, all AChET, within the G4 enzyme formed by AChET and PRiMA, in the ER was sensitive to Endo H or PNGase F digestion, whereas only ∼30% in Golgi/PM fraction was Endo H-sensitive (Fig. 6C).
FIGURE 6.
N-Glycosylation is necessary for the trafficking of PRiMA-linked G4 AChE. A, HEK293T cells were transfected with the cDNAs encoding AChETWT or AChETN296Q/N381Q/N495Q and PRiMA-HA. After 48 h the transfected cells were subjected to subcellular fractionation using a continuous iodixanol density gradient, and 11 fractions were collected. The distribution of ER (fractions 8–10) and Golgi/PM (plasma membrane) (fractions 2–4) subcellular compartments was determined by immunoblotting samples from each fraction with marker proteins, Na+/K+-ATP (PM), GM130 (Golgi) and calnexin (ER). The distribution of AChET protein was analyzed by immunoblotting with the anti-AChE antibody. B, the amount of AChET protein or AChE activity in each fraction was quantified from the blots (A) by calibrated densitometry. The data are expressed as the % of total AChET protein (upper panel) or AChE activity (lower panel). C, samples of ER- and Golgi/PM-enriched fractions from cells co-expressing AChETWT and PRiMA-HA were incubated with Endo H or PNGase F (upper panel). The proportion of Endo H-sensitive or PNGase F-sensitive AChET was quantified by calibrated densitometry (lower panel). The data are expressed as % of the total AChET protein. D, samples from ER- and Golgi/PM-enriched fractions from A were incubated with and without anti-HA antibody (Ab, 1:1000, Sigma). After precipitation by protein G-agarose, the supernatants were subjected to sucrose density gradient analysis. The enzymatic activities are plotted as a function of the S value, estimated from the position of the sedimentation markers. The enzymatic activities are expressed in arbitrary units. E, transfected HEK293T cells as in A were immuno-stained with the anti-AChE antibody without permeabilization, showing AChE exposed at the plasma membrane and with permeabilization by Triton X-100 (0.2%) showing AChE in subcellular compartments. Bar, 20 μm.
Because AChETN296Q/N381Q/N495Q is able to assemble with PRiMA to form a PRiMA-linked G4 enzyme, it is interesting to know whether the ER-retained fraction is PRiMA-linked or not. We, therefore, examined whether G4 AChE from each fraction was associated with the PRiMA-HA construct, identified with an anti-HA antibody (Fig. 6D). The molecular forms of AChE were analyzed by sucrose-density gradient. In both cases of enzymes formed by AChETWT and AChETN296Q/N381Q/N495Q with PRiMA, we found that the ER-enriched fractions contained a G4 form that could be immuno-depleted by the anti-HA antibody (Fig. 6D), indicating that it was PRiMA-linked. In contrast to AChETWT, the glycan-depleted AChE (formed by AChETN296Q/N381Q/N495Q) was not recovered in the Golgi/PM-enriched fractions (Fig. 6D). Thus, in the absence of N-glycosylation, AChET could assemble with PRiMA to form a PRiMA-linked G4 enzyme, but the oligomer failed to be exported from the ER to the Golgi apparatus.
The trafficking of AChE was further analyzed by immunofluorescence staining of transfected cells that expressed AChETWT or AChETN296Q/N381Q/N495Q together with PRiMA. The cells were stained with the anti-AChE antibody with or without permeabilization by Triton X-100. An intense immunoreactivity was observed for both AChETWT and AChETN296Q/N381Q/N495Q in permeabilized cells, indicating that both proteins were expressed at similar levels (Fig. 6E). Without permeabilization, the staining was detected on the plasma membrane of cells expressing AChETWT but not AChETN296Q/N381Q/N495Q (Fig. 6E). This supports our conclusion that N-glycans are required for the proper membrane targeting of PRiMA-linked G4 AChE.
Formation of Interspecies Chicken-Human AChE G4 Hybrids
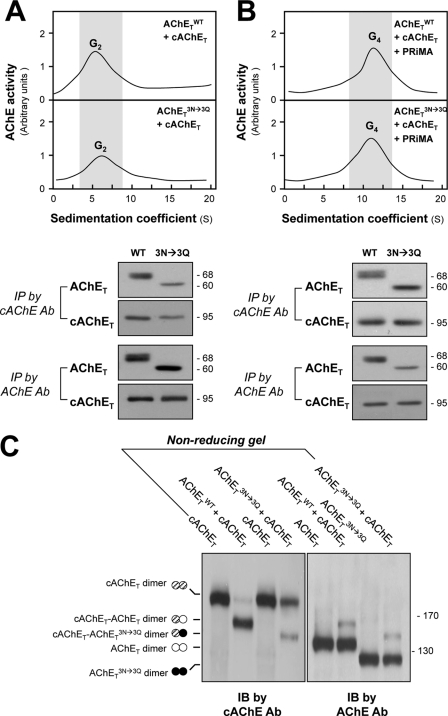
We have shown that mammalian and chicken AChET subunits can form mixed AChET dimers or tetramers, in agreement with the notion that this association depends on the “four-helix-bundle” contact between two catalytic domains (21). To investigate whether glycosylated and un-glycosylated AChET subunits possess the same capacity for dimeric assembly, we co-expressed human AChETWT or its glycosylation mutant AChETN296Q/N381Q/N495Q with chicken AChET (cAChET) in HEK293T cells in the presence or absence of PRiMA. When expressed without PRiMA, the co-expression of AChETWT and cAChET or of AChETN296Q/N381Q/N495Q and cAChET produced mainly G2 dimers (Fig. 7A, upper panel). The formation of mixed, interspecies dimers was examined by immunoprecipitation with anti-human and anti-chicken AChE antibodies using the G2-enriched fractions obtained from sucrose density gradients (Fig. 7A, lower panel). As previously reported (21), AChETWT could be pulled down by the anti-chicken AChE antibody, and this was also the case for AChETN296Q/N381Q/N495Q, showing that it can also associate with cAChET in mixed human-chicken dimers. AChETWT and cAChET as well as AChETN296Q/N381Q/N495Q and cAChET were also co-expressed with PRiMA in HEK293T cells, producing G4 forms of AChE (Fig. 7B, upper panel). Immunoprecipitation experiments confirmed that cAChET could associate with AChETWT or AChETN296Q/N381Q/N495Q in mixed PRiMA-linked tetramers (Fig. 7B, lower panel). Therefore, PRiMA is able to recruit human and chicken AChET subunits into mixed tetramers, even for the un-glycosylated AChETN296Q/N381Q/N495Q subunits.
FIGURE 7.
N-Glycosylation is not required for the formation of human-chicken AChE dimers and tetramers. A, HEK293T cells were transfected with equal amounts of cDNAs encoding human AChET or AChETN296Q/N381Q/N495Q together with chicken AChET (cAChET). Equal amounts of protein from cell lysates were subjected to sucrose density gradient analysis. The enzymatic activities are plotted as a function of the S value, estimated from the position of sedimentation markers (upper panel). G2 fractions were collected for immunoprecipitation (IP) by either anti-human AChE antibody (Ab) or anti-cAChE antibody. The immunoprecipitated complexes were analyzed by SDS-PAGE and Western blotting with anti-AChE and anti-cAChE antibodies, as indicated (lower panel). Both antibodies did not cross-react with other corresponding antigen. B, HEK293T cells were transfected with equal amount of cDNAs encoding AChET or AChETN296Q/N381Q/N495Q together with cAChET and PRiMA. Equal amounts of protein from cell lysates were subjected to sucrose density gradient analysis as in A. G4 fractions were collected for immunoprecipitation as in A. C, extracts from cells expressing AChETWT and cAChET separately or together were analyzed by non-reducing SDS-PAGE and labeled with anti-cAChE and anti-AChE antibodies. The presence of an intermediate band between cAChE and AChETN296Q/N381Q/N495Q dimers, recognized by both antibodies, demonstrates the formation of mixed cAChET-AChETN296Q/N381Q/N495Q dimers. IB, immunoblot.
The formation of a dimer involves a disulfide linkage near the C terminus of the two AChET subunits (21). To further confirm the formation of interspecies dimers between AChETN296Q/N381Q/N495Q and cAChET, we performed non-reducing gel electrophoresis and Western blotting (Fig. 7C). In lysates from cells co-expressing AChETWT and cAChET, we observed the presence of cAChET dimers (∼190 kDa), AChETWT dimers (∼140 kDa), and an intermediate heterodimer (∼165 kDa; AChETWT-cAChET dimer) that was labeled by both anti-chicken and anti-human AChE antibodies. Similarly, co-expression of AChETN296Q/N381Q/N495Q and cAChET produced cAChET dimers, AChETN296Q/N381Q/N495Q dimers (∼125 kDa) and an intermediate heterodimer (∼155 kDa) that could also be labeled by both antibodies. Moreover, the proportion of the heterodimer formed was normally lower that that of the total cAChET dimer and total human AChET dimer (Fig. 7C). This might be caused by the various stabilities of different dimers or the preference of the subunit selection during the dimer formation. Thus, the glycosylated and un-glycosylated AChET could form interspecies dimers, indicating that the contact between two subunits in a dimer does not depend on the glycosylation.
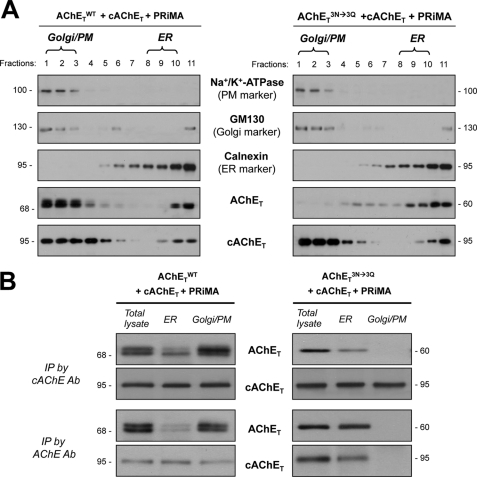
We further analyzed the traffic of mixed PRiMA-linked AChETN296Q/N381Q/N495Q-cAChET tetramers. Does the glycosylated cAChET rescue AChETN296Q/N381Q/N495Q from being retained in the ER? In cultured cells expressing AChETWT or AChETN296Q/N381Q/N495Q with cAChET and PRiMA (as in Fig. 7), the subcellular fractionation was performed to determine the distribution of AChET in the cultures. As expected, the mixed PRiMA-linked tetramer, formed by AChETWT and cAChET, underwent a typical pattern of ER-to-Golgi maturation, with the majority of mature AChET (heavy glycosylated) localized in Golgi/PM fractions (Fig. 8A, left panel). In contrast, the mixed PRiMA-linked tetramer formed by AChETN296Q/N381Q/N495Q and cAChET was extensively localized in the ER fractions (Fig. 8A, right panel), i.e. high level of AChETN296Q/N381Q/N495Q being retained in the ER. The cAChET was still predominantly localized in the Golgi/PM fractions; because of the formation of intraspecies chicken G4 AChE, i.e. PRiMA-linked cAChET. The proper formation of interspecies of G4 AChE was confirmed by the immunoprecipitation in the cell lysates received from different subcellular compartments. As expected, the mixed PRiMA-linked tetramer, formed by AChETWT and cAChET, was precipitated by either anti-human (recognized at ∼68 kDa bands) or anti-chicken AChE (recognized at ∼95 kDa bands) antibodies (Fig. 8B); this mixed enzyme was revealed in both ER and Golgi/PM fractions. In contrast, the G4 AChETN296Q/N381Q/N495Q-cAChET enzyme was only revealed in the ER fractions, which was absent in the Golgi/PM fractions (Fig. 8B). Thus, the rescue of AChETN296Q/N381Q/N495Q-cAChET mixed tetramer being retained in the ER by the glycosylated cAChET was not possible.
FIGURE 8.
N-Glycosylation is required for the trafficking of PRiMA-linked mixed chicken-human AChE tetramer. A, HEK293T cells were transfected with the cDNAs encoding AChETWT or AChETN296Q/N381Q/N495Q together with cAChET and PRiMA. After 48 h the transfected cells were subjected to subcellular fractionation, and the distribution of ER and Golgi/PM subcellular compartments was determined by immunoblotting as in Fig. 6A. The distribution of AChET and cAChET proteins was analyzed by immunoblotting with the anti-AChE antibodies. B, protein extracts from the total lysate and ER-enriched and Golgi/PM-enriched fractions of the transfected cells from A were immunoprecipitated (IP) by either anti-human AChE antibody or anti-chicken AChE antibody (Ab). The immunoprecipitated complexes were analyzed by SDS-PAGE and Western blotting with anti-human AChE and anti-chicken AChE antibodies as indicated. Representative gels from three independent experiments are shown.
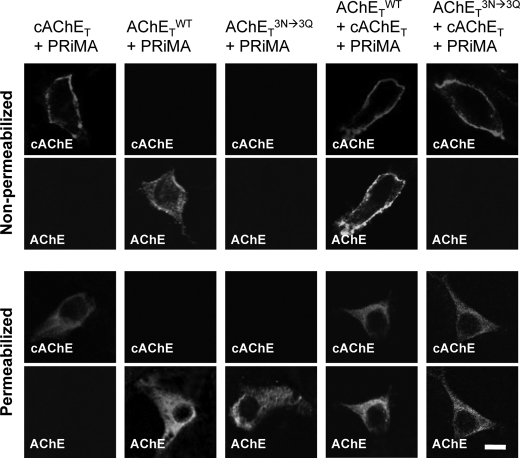
In addition, we immuno-stained surface proteins in transfected HEK293T, expressing PRiMA-linked AChET-cAChET mixed tetramers (see also Fig. 7). Both AChETWT and cAChET could be detected on the surface of cells co-expressing these two subunits with PRiMA (Fig. 9). In contrast, we observed no human AChE on the surface of cells co-expressing the un-glycosylated AChETN296Q/N381Q/N495Q mutant together with cAChE and PRiMA, indicating that the PRiMA-linked cAChET-AChETN296Q/N381Q/N495Q and AChETN296Q/N381Q/N495Q-AChETN296Q/N381Q/N495Q tetramers did not reach the plasma membrane but probably remained trapped in the ER. Therefore, the un-glycosylation on one of the AChET subunits in the tetramer appears to represent efficient the ER retention signals.
FIGURE 9.
N-Glycosylation is required for the plasma membrane targeting of PRiMA-linked mixed chicken-human AChE tetramer. HEK293T cells were transfected with different combinations of cDNAs as indicated. After 48 h, cells were immuno-stained with anti-chicken AChE or anti-human AChE antibody without or with permeabilization by Triton X-100 (0.2%) to visualize AChE on the plasma membrane or total AChE, including subcellular compartments, respectively. Bar, 20 μm.
DISCUSSION
In the present study, we analyzed the effects of N-glycosylation on the enzymatic activity and oligomerization of PRiMA-linked G4 AChE. The enzymatic activity was significantly affected by glycosylation, but this post-translational modification showed no effect on the assembly of PRiMA-linked G4 AChE. The enzymatic activities of G4 AChE, formed by AChETN296Q and AChETN495Q, were reduced to 70∼80% of AChETWT, whereas the enzymatic activity of G4 AChETN381Q was dramatically reduced to ∼10% only. The glycan-depleted tetrameric AChE (AChETN296Q/N381Q/N495Q) possessed less than 5% of the enzymatic activity of wild type. This indicates that the oligosaccharides, carried by the three glycosylation sites, function in a co-operative manner. Kinetic analyses further confirmed an increase of Km values in glycan-depleted AChEs as compared with the wild-type enzyme. On the other hand, the removal of oligosaccharide side chains from mature AChE molecules by Endo H/PNGase F did not seem to alter the overall structure of the protein because most of the AChE activity was retained after the de-glycosylation. Therefore, the N-glycosylation plays an important role in assisting the folding of newly synthesized AChET protein, but the conformation of catalytically active (fully folded) G4 AChE no longer depends on the presence of N-linked carbohydrates.
The glycosylation of AChET did not appear to play a significant role in oligomerization of PRiMA-linked G4 AChE. The glycan-depleted AChETN296Q/N381Q/N495Q mutant could form oligomers, which appeared structurally similar to that of the wild-type enzyme, including (i) identical sedimentation in sucrose density gradients, (ii) identical organization of disulfide linkages in dimers and in PRiMA-linked tetramers, and (iii) identical ability to form interspecies dimers and/or tetramers. Structural studies have shown that the dimerization of AChET depends on the formation of a four-helix-bundle, in which two α-helices of each catalytic domain are closely apposed with the corresponding helices of the other one (30, 31). Indeed, the four-helix-bundle is highly conserved among different species of AChE catalytic subunits (21). On the other hand, the formation of AChE tetramer depends on the presence of a C-terminal t-peptide (21, 31, 32) of AChET. The assembly of a tetramer is efficiently induced by a proline-rich attachment domain-containing protein, ColQ or PRiMA (8, 33), that interacts with the AChET t-peptide. Thus, the proper folding of a catalytically active AChE monomer and the assembly of AChET subunits into a PRiMA-linked tetramer are two independent events.
ER is the entry point into the “classical” secretory pathway for most membrane-bound and secreted proteins (34) and exerts an efficient “quality control” that ensures that proteins are correctly folded before allowing their transit to the Golgi apparatus and beyond. It is generally believed that N-linked glycosylation participates in the control of protein folding and intracellular trafficking and that this post-translational modification also protects proteins from proteolytic degradation (14, 35). Consistent with our current understanding of glycoprotein metabolism, the synthesis and processing of PRiMA-linked G4 AChET follows an orderly sequence of events beginning with the sequestering of the nascent polypeptide chain and the addition of preassembled oligosaccharides in the ER followed by transfer into the Golgi apparatus and modification of the glycan chains. When the sites of N-glycosylation are eliminated, the folding of AChET partially fails, leading to a severe loss of the enzymatic activity. Although the glycan-depleted AChET can still assemble with PRiMA to form PRiMA-linked G4 AChE in the ER, the oligomeric enzyme failed to be exported to the Golgi apparatus. Our results show that the assembly of PRiMA-linked G4 AChE is completed in the ER, not in the Golgi, and that the suppression of N-glycosylation can prevent the trafficking of assembled AChE. The ultimate fate of glycan-depleted and less active AChE is probably degradation, e.g. via quality control chaperons, e.g. calnexin/calreticulin that targets the misfolded proteins for degradation and prevents them proceeding from ER to Golgi. The control of glycosylation, therefore, could be as a means in regulating the level of functional AChE in the brain. Supporting this notion, the alteration in AChE glycosylation has been reported in the brain and cerebrospinal fluid of patients from Alzheimer disease (17).
The biogenesis of ColQ-associated AChE in muscle has been extensively studied by Rotundo and co-workers (36–38). Their results suggest that (i) the assembly of dimers and/or tetramers occurs in the ER, and the association with ColQ is completed in the Golgi apparatus; (ii) about 70–80% of the total newly synthesized Endo H-sensitive AChE enzyme does not transit to Golgi apparatus and undergoes a rapid degradation; (iii) a small fraction of AChE is processed in Golgi apparatus, where it becomes Endo H-insensitive and is exported. By using recombinant DNA in an expression system, our current results accord with the studies by Rotundo and co-workers (36–38). First, the assembly of PRiMA-linked G4 AChE having minimal glycosylation is completed in the ER; the ER-retained enzymes are mainly Endo H-sensitive. Second, the full glycosylated AChET should have a transit to Golgi apparatus and acquire further glycosylation that is Endo H-insensitive. This fully glycosylated enzyme reaches the plasma membrane as a functional enzyme. Third, the glycan-depleted AChE, as generated here by the mutants, could represent the partial glycosylated AChE, i.e. they are retained in ER, enzymatic less active and unstable. Contrarily, the ColQ-linked AChE (A12 form) was proposed to be fully assembled in the Golgi apparatus (16) instead of being assembled in the ER, as revealed here for the PRiMA-linked tetramers. On top of the aforementioned evidences, we would like to propose that the glycan-depleted AChE corresponds to the fraction of enzymatically less active AChE that is retained and degraded in the ER, as described in avian tissues (36, 39). In line with this notion, the specific activity of AChE, normalized to AChE protein, is lower in the ER than that in the Golgi apparatus. Thus the transit from the ER to the Golgi apparatus would depend on the completion or not of N-glycosylation of AChET. Moreover, a glycan-depleted PRiMA-linked AChE was shown here to be enzymatically less active. However, the inactive or less active AChE reported previously in chicken brain (39) or in muscle (36, 40, 41) was restricted to the G1 and G2 forms, not the G4 form. A possible explanation for this discrepancy could be the partially glycosylated and poorly active G4 AChE is processed to degradation rapidly in vivo.
Supplementary Material
Acknowledgments
We thank Prof. Avigdor Shafferman from the Department of Biochemistry and Molecular Genetics, Israel Institute for Biological Research, Ness-Ziona, Israel for the cDNAs encoding the un-glycosylated mutants of human AChET. In addition we are grateful to Drs. Jean Massoulié and Suzanne Bon from the Institut de Biologie de l'Ecole Normale Supérieure, Paris, France, for advice during the study.
This work was supported by grants from the Research Grants Council of Hong Kong (662407, 660409, 662911, F-HK21/06T) and the Croucher Foundation (CAS-CF07/08.SC03) (to K. W. K. T. and R. C. Y. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- AChE
- acetylcholinesterase
- cAChET
- chicken AChET
- PRiMA
- proline-rich membrane anchor
- ColQ
- collagen tail
- Endo H
- endoglycosidase H
- ER
- endoplasmic reticulum
- GM130
- Golgi matrix protein 130
- PM
- plasma membrane
- PNGase F
- peptide N-glycosidase F
- ATCh
- acetylthiocholine iodide
- iso-OMPA
- tetraisopropylpyrophosphoramide
- ER
- endoplasmic reticulum.
REFERENCES
- 1. Massoulié J., Pezzementi L., Bon S., Krejci E., Vallette F. M. (1993) Prog. Neurobiol. 41, 31–91 [DOI] [PubMed] [Google Scholar]
- 2. Krejci E., Thomine S., Boschetti N., Legay C., Sketelj J., Massoulié J. (1997) J. Biol. Chem. 272, 22840–22847 [DOI] [PubMed] [Google Scholar]
- 3. Massoulié J., Millard C. B. (2009) Curr. Opin. Pharmacol. 9, 316–325 [DOI] [PubMed] [Google Scholar]
- 4. Perrier A. L., Massoulié J., Krejci E. (2002) Neuron 33, 275–285 [DOI] [PubMed] [Google Scholar]
- 5. Mok M. K., Leung K. W., Xie H. Q., Guo A. J., Chen V. P., Zhu J. T., Choi R. C., Tsim K. W. (2009) Neurosci. Lett. 461, 202–206 [DOI] [PubMed] [Google Scholar]
- 6. Xie H. Q., Liang D., Leung K. W., Chen V. P., Zhu K. Y., Chan W. K., Choi R. C., Massoulié J., Tsim K. W. (2010) J. Biol. Chem. 285, 11537–11546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie H. Q., Choi R. C., Leung K. W., Siow N. L., Kong L. W., Lau F. T., Peng H. B., Tsim K. W. (2007) J. Biol. Chem. 282, 11765–11775 [DOI] [PubMed] [Google Scholar]
- 8. Bon S., Coussen F., Massoulié J. (1997) J. Biol. Chem. 272, 3016–3021 [DOI] [PubMed] [Google Scholar]
- 9. Dvir H., Harel M., Bon S., Liu W. Q., Vidal M., Garbay C., Sussman J. L., Massoulié J., Silman I. (2004) EMBO J. 23, 4394–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liao J., Heider H., Sun M. C., Brodbeck U. (1992) J. Neurochem. 58, 1230–1238 [DOI] [PubMed] [Google Scholar]
- 11. Liao J., Heider H., Sun M. C., Stieger S., Brodbeck U. (1991) Eur. J. Biochem. 198, 59–65 [DOI] [PubMed] [Google Scholar]
- 12. Tsim K. W., Randall W. R., Barnard E. A. (1988) J. Neurochem. 51, 95–104 [DOI] [PubMed] [Google Scholar]
- 13. Méflah K., Bernard S., Massoulié J. (1984) Biochimie 66, 59–69 [DOI] [PubMed] [Google Scholar]
- 14. Helenius A. (1994) Mol. Biol. Cell 5, 253–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Velan B., Kronman C., Ordentlich A., Flashner Y., Leitner M., Cohen S., Shafferman A. (1993) Biochem. J. 296, 649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rotundo R. L. (1984) Proc. Natl. Acad. Sci. U.S.A. 81, 479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sáez-Valero J., Barquero M. S., Marcos A., McLean C. A., Small D. H. (2000) J. Neurol. Neurosurg. Psychiatry 69, 664–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mimori Y., Nakamura S., Yukawa M. (1997) Behav. Brain Res. 83, 25–30 [DOI] [PubMed] [Google Scholar]
- 19. Noureddine H., Carvalho S., Schmitt C., Massoulié J., Bon S. (2008) J. Biol. Chem. 283, 20722–20732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gough N. R., Randall W. R. (1995) J. Neurochem. 65, 2734–2741 [DOI] [PubMed] [Google Scholar]
- 21. Chen V. P., Xie H. Q., Chan W. K., Leung K. W., Chan G. K., Choi R. C., Bon S., Massoulié J., Tsim K. W. (2010) J. Biol. Chem. 285, 27265–27278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi R. C., Leung P. W., Dong T. T., Wan D. C., Tsim K. W. (1996) Neurosci. Lett. 217, 165–168 [PubMed] [Google Scholar]
- 23. Ellman G. L., Courtney K. D., Andres V., Jr., Feather-Stone R. M. (1961) Biochem. Pharmacol. 7, 88–95 [DOI] [PubMed] [Google Scholar]
- 24. Santos S. C., Vala I., Miguel C., Barata J. T., Garção P., Agostinho P., Mendes M., Coelho A. V., Calado A., Oliveira C. R., e Silva J. M., Saldanha C. (2007) J. Biol. Chem. 282, 25597–25603 [DOI] [PubMed] [Google Scholar]
- 25. Tung E. K., Choi R. C., Siow N. L., Jiang J. X., Ling K. K., Simon J., Barnard E. A., Tsim K. W. (2004) Mol. Pharmacol. 66, 794–806 [DOI] [PubMed] [Google Scholar]
- 26. Holden P., Horton W. A. (2009) BMC Res. Notes 2, 243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marra P., Maffucci T., Daniele T., Tullio G. D., Ikehara Y., Chan E. K., Luini A., Beznoussenko G., Mironov A., De Matteis M. A. (2001) Nat. Cell Biol. 3, 1101–1113 [DOI] [PubMed] [Google Scholar]
- 28. Meyer-Schaller N., Chou Y. C., Sumara I., Martin D. D., Kurz T., Katheder N., Hofmann K., Berthiaume L. G., Sicheri F., Peter M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 12365–12370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dobbertin A., Hrabovska A., Dembele K., Camp S., Taylor P., Krejci E., Bernard V. (2009) J. Neurosci. 29, 4519–4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. (1991) Science 253, 872–879 [DOI] [PubMed] [Google Scholar]
- 31. Morel N., Leroy J., Ayon A., Massoulié J., Bon S. (2001) J. Biol. Chem. 276, 37379–37389 [DOI] [PubMed] [Google Scholar]
- 32. Liang D., Blouet J. P., Borrega F., Bon S., Massoulié J. (2009) FEBS J. 276, 94–108 [DOI] [PubMed] [Google Scholar]
- 33. Noureddine H., Schmitt C., Liu W., Garbay C., Massoulié J., Bon S. (2007) J. Biol. Chem. 282, 3487–3497 [DOI] [PubMed] [Google Scholar]
- 34. Palade G. (1975) Science 189, 347–358 [DOI] [PubMed] [Google Scholar]
- 35. Luzikov V. N. (2002) Biochemistry 67, 171–183 [DOI] [PubMed] [Google Scholar]
- 36. Rotundo R. L. (1988) J. Biol. Chem. 263, 19398–19406 [PubMed] [Google Scholar]
- 37. Rotundo R. L., Thomas K., Porter-Jordan K., Benson R. J., Fernandez-Valle C., Fine R. E. (1989) J. Biol. Chem. 264, 3146–3152 [PubMed] [Google Scholar]
- 38. Ruiz C. A., Rotundo R. L. (2009) J. Biol. Chem. 284, 21488–21495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chatel J. M., Grassi J., Frobert Y., Massoulié J., Vallette F. M. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 2476–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choi R. C., Yung L. Y., Dong T. T., Wan D. C., Wong Y. H., Tsim K. W. (1998) J. Neurochem. 71, 152–160 [DOI] [PubMed] [Google Scholar]
- 41. Choi R. C., Siow N. L., Zhu S. Q., Wan D. C., Wong Y. H., Tsim K. W. (2001) Mol. Cell. Neurosci. 17, 732–745 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.