Abstract
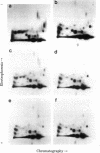
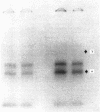
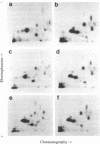
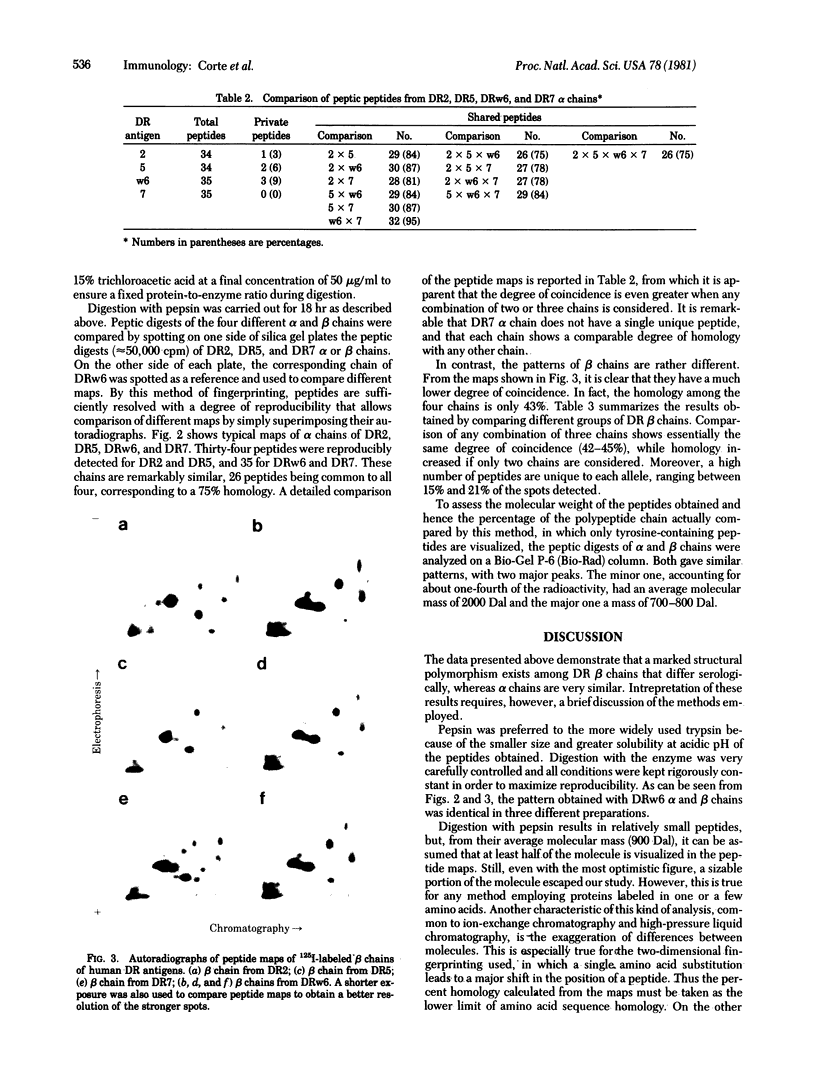
Two-dimensional peptide mapping was used to study the polymorphism of DR antigens, membrane glycoproteins composed of two chains, alpha and beta, and encoded by the human major histocompatibility complex (MHC). Four DR antigens were purified by immunoabsorption from four human lymphoblastoid cell lines homozygous at the DR locus. After labeling with 125I, alpha and beta chains were separated by polyacrylamide gel electrophoresis in sodium dodecyl sulfate and digested with pepsin. Comparison of the peptide maps showed a marked degree of polymorphism among beta chains: only 43% of peptides were common to all four chains and 15-21% of the spots were unique to a given chain. By contrast, only a limited variability was observed among alpha chains. Homology was 75% for the four chains and the percentage of unique peptides was very low. DR7 did not possess even a single unique peptide. The limited variability among alpha chains and the lack of "private" peptides in one of them point to the conclusion that the beta chain is the unique carrier of the alloantigenic specificities. Higher homology within the known crossreactive groups was not observed, suggesting that the determinants responsible for crossreactivity are on different molecules. From a genetic point of view, because beta chains show allele-associated polymorphism, they are likely to be MHC encoded, whereas the minor differences among alpha chains do not allow a similar conclusion. The available data point to an analogy between these DR antigens and the mouse I-E/C antigens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. P., Walker L. E., Russell W. A., Pellegrino M. A., Ferrone S., Reisfeld R. A., Frelinger J. A., Silver J. Murine Ia and human DR antigens: homology of amino-terminal sequences. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3953–3956. doi: 10.1073/pnas.75.8.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Jones E. A., Bodmer W. F., Bodmer J. G., Arce-Gomez B., Snary D., Crumpton M. J. Genetics and serology of HL-A-linked human Ia antigens. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):443–455. doi: 10.1101/sqb.1977.041.01.052. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Jones E. A., Crumpton M. J. Isolation, structure and genetics of HLA-A, -B, -C and -DRw (Ia) antigens. Br Med Bull. 1978 Sep;34(3):241–246. doi: 10.1093/oxfordjournals.bmb.a071504. [DOI] [PubMed] [Google Scholar]
- Bodmer J. G. Ia antigens: definition of the HLA-DRw specificities. Br Med Bull. 1978 Sep;34(3):233–240. doi: 10.1093/oxfordjournals.bmb.a071503. [DOI] [PubMed] [Google Scholar]
- Brown J. L., Kato K., Silver J., Nathenson S. G. Notable diversity in peptide composition of murine H-2K and H-2D alloantigens. Biochemistry. 1974 Jul 16;13(15):3174–3178. doi: 10.1021/bi00712a027. [DOI] [PubMed] [Google Scholar]
- Corte G., Tonda P., Cosulich E., Milstein C. P., Bargellesi A., Ferrarini M. Characterization of IgD. I. Isolation of two molecular forms from human serum. Scand J Immunol. 1979;9(2):141–149. doi: 10.1111/j.1365-3083.1979.tb02716.x. [DOI] [PubMed] [Google Scholar]
- Evans R. L., Faldetta T. J., Humphreys R. E., Pratt D. M., Yunis E. J., Schlossman S. F. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978 Nov 1;148(5):1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrone S., Allison J. P., Pellegrino M. A. Human DR (Ia-like) antigens: biological and molecular profile. Contemp Top Mol Immunol. 1978;7:239–281. doi: 10.1007/978-1-4757-0779-3_8. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., McDevitt H. O. Two-gene control of the expression of a murine Ia antigen. J Exp Med. 1978 Oct 1;148(4):925–939. doi: 10.1084/jem.148.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., Rask L., Fohlman J., Peterson P. A. Heavy HLA-DR (Ia) antigen chain is controlled by the MHC region. Nature. 1978 Oct 26;275(5682):762–764. doi: 10.1038/275762a0. [DOI] [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole L. R. A genetic marker in the variable region of rabbit immunoglobulin heavy chain. Biochem J. 1975 Nov;151(2):351–359. doi: 10.1042/bj1510351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman S. F., Chess L., Humphreys R. E., Strominger J. L. Distribution of Ia-like molecules on the surface of normal and leukemic human cells. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1288–1292. doi: 10.1073/pnas.73.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. A., Strominger J. L. Demonstration of structural polymorphism among HLA-DR light chains by two-dimensional gel electrophoresis. J Exp Med. 1980 Jan 1;151(1):144–165. doi: 10.1084/jem.151.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Ferrone S. Structural polymorphism of human DR antigens. Nature. 1979 May 31;279(5712):436–437. doi: 10.1038/279436a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Kaufman J. F., Terhorst C., Strominger J. L. Purification and structural characterisation of human HLA-linked B-cell antigens. Nature. 1977 Jul 21;268(5617):213–218. doi: 10.1038/268213a0. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Humphreys R. E., McCune J. M., Parham P., Robb R., Springer T., Terhorst C. The immunoglobulin-like structure of human histocompatibility antigens. Fed Proc. 1976 Apr;35(5):1177–1182. [PubMed] [Google Scholar]
- Tanigaki N., Tosi R., Pressman D., Ferrara G. B. Molecular identification of human Ia antigens coded for by a gene locus closely linked to HLA-DR locus. Immunogenetics. 1980;10(2):151–167. doi: 10.1007/BF01561564. [DOI] [PubMed] [Google Scholar]
- Tosi R., Tanigaki N., Centis D., Ferrara G. B., Pressman D. Immunological dissection of human Ia molecules. J Exp Med. 1978 Dec 1;148(6):1592–1611. doi: 10.1084/jem.148.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco M. M., Garotta G., Stocker J. W., Ceppellini R. Murine monoclonal antibodies against HLA structures. Immunol Rev. 1979;47:219–252. doi: 10.1111/j.1600-065x.1979.tb00295.x. [DOI] [PubMed] [Google Scholar]
- van Rood J. J., van Leeuwen A., Jonker M., Termijtelen A., Bradley B. A. Polymorphic B-cell determinants in man. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):417–427. doi: 10.1101/sqb.1977.041.01.050. [DOI] [PubMed] [Google Scholar]