Abstract
Activation of bone morphogenetic protein (BMP) receptor II (BMPRII) promotes pulmonary artery endothelial cell (PAEC) survival, proliferation, and migration. Mutations to BMPRII are associated with the development of pulmonary arterial hypertension (PAH). Endothelial dysfunction, including decreased endothelial nitric-oxide synthase (eNOS) activity and loss of bioactive nitric oxide (NO), plays a prominent role in the development of PAH. We hypothesized that stimulation of BMPRII promotes normal PAEC function by activating eNOS. We report that BMPRII ligands, BMP2 and BMP4, (i) stimulate eNOS phosphorylation at a critical regulatory site, (ii) increase eNOS activity, and (iii) result in canonical changes in eNOS protein-protein interactions. The stimulation of eNOS activity by BMPRII ligands was largely dependent on protein kinase A (PKA) activation, as demonstrated using the PKA inhibitors H89 and myristoylated PKI(6–22) amide. PAEC migration stimulated by BMP2 and BMP4 was inhibited by the NOS inhibitor l-nitroarginine methyl ester, providing functional evidence of eNOS activation. Furthermore, BMP2 and BMP4 failed to stimulate eNOS phosphorylation when BMPRII was knocked down by siRNA. Most important to the pathophysiology of the disease, BMP2 and BMP4 failed to stimulate eNOS phosphorylation in PAECs isolated from patients with mutations in the BMPR2 gene. These data demonstrate a new action of BMPs/BMPRII in the pulmonary endothelium and provide novel mechanistic insight into the pathogenesis of PAH.
Keywords: Bone Morphogenetic Protein (BMP), Endothelial Cell, Nitric-oxide Synthase, Protein Kinase A (PKA), Pulmonary Hypertension, BMPRII, eNOS
Introduction
Pulmonary arterial hypertension (PAH)2 is a devastating disease, characterized by increasing pulmonary arterial pressures, leading to right heart failure and eventually, death (1, 2). Recent studies demonstrate an important role for BMPRII in the pathogenesis of the disease when mutations to the BMPR2 gene were identified as a major underlying cause of heritable or familial PAH (FPAH) (3–5). Approximately 144 different mutations in 210 patients with FPAH have now been identified (6–8). Additionally, it is estimated that 20% of patients with idiopathic PAH (IPAH) harbor mutations to the BMPR2 gene. These mutations may be a previously undetected inherited mutation or arise from sporadic mutation of the gene.
BMPRII is widely expressed in normal tissues. In the lung vasculature, BMPRII is most highly expressed on endothelial cells (9) and at lower levels in smooth muscle cells and fibroblasts. Expression of BMPRII is markedly reduced in the pulmonary vasculature of patients with FPAH and IPAH, regardless of whether they harbor mutations in the BMPR2 gene (9).
Pulmonary artery smooth muscle cells (PASMCs) from patients with mutations in BMPRII have a reduced capacity to activate Smad1/5. This is coupled with a reduced ability of BMPs to inhibit proliferation of PASMCs (10). In contrast, the BMPRII ligands BMP2 and BMP4 promote proliferation, migration, and survival in PAECs (11, 12). The opposite effects of BMPs on PAECs and PASMCs provide a convincing model for pulmonary vascular damage in PAH, where a loss of BMPRII function in PAECs may promote increased endothelial apoptosis, which compromises the integrity of the endothelial barrier and contributes to endothelial dysfunction. This scenario would allow for increased access of serum factors to the underlying smooth muscle cells, thus promoting vascular remodeling.
Endothelial cells (ECs) are recognized as major regulators of vascular function, and endothelial dysfunction is defined by a complex imbalance in EC production of vasoconstrictors, smooth muscle cell mitogens, and prothrombotic stimuli versus vasodilators, inhibitors of smooth muscle cell proliferation, and antithrombotic mediators. EC dysfunction in PAH is characterized by an imbalance between the vasodilators NO and prostacyclin and vasoconstrictors such as endothelin-1 and thromboxane A2.
In this study we tested the hypothesis that BMPs, via BMPRII, stimulate eNOS activity, increasing bioavailable NO. In this way, loss of BMPRII could contribute to endothelial dysfunction, a critical step in the pathogenesis of PAH.
EXPERIMENTAL PROCEDURES
Materials
BMP4 and vascular endothelial growth factor (VEGF) were from R&D Systems (Minneapolis, MN). BMP2 and mPKI were from Enzo Life Sciences (Plymouth Meeting, PA). H89 and LY294002 were from Sigma. Antibodies against eNOS, phospho-eNOS, and BMPRII and Hsp90 were from BD Biosciences (Franklin Lakes, NJ). Anti-caveolin-1 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin antibody was from Cell Signaling Technologies (Danvers, MA). All other chemicals and reagents were from Sigma unless otherwise noted.
Cell Culture
Bovine pulmonary artery endothelial cells (bPAECs) and human PAECs (hPAECs) were purchased from Lonza (Basel, Switzerland). Mutant hPAECs were isolated from patient lungs at the time of transplant as described previously (13, 14). Mutant #1 has a deletion in exons 1–8 whereas mutant #2 has a deletion in exons 4–5. bPAECs were cultured in microvascular endothelial growth medium (EGM2-MV; Lonza). Normal and mutant hPAECs were cultured in endothelial cell growth medium (EGM2; Lonza) on plates precoated with fibronectin (1 μg/cm2). All cells were maintained in a sterile, humidified cell culture incubator at 37 °C with 5% CO2. Cells were passaged at 60–80% confluence by dissociation from plates with 0.25% trypsin-EDTA (Invitrogen). Primary cultures of passage 5–8 were used in the experiments.
Immunoprecipitation
Cells were washed twice with PBS and lysed in modified radioimmune precipitation assay buffer (100 mm Tris-HCl, pH 7.4, 1% v/v Nonidet P-40, 10 mm NaF, 1 mm vanadate, 10 μg/ml aprotinin, 10 μg/ml leupeptin). Insoluble material was removed by centrifugation and protein concentrations determined using the Bio-Rad DC protein assay. Antibodies were immobilized to protein A-conjugated Dynabeads (Invitrogen). The antibody-conjugated beads were washed by magnetic separation, and equal amounts of protein samples were added to the washed beads. After 10 min of incubation, the immune complexes were isolated by magnetic separation, washed, and eluted at 75 °C in 2× SDS-PAGE sample buffer for 5 min. Immunoprecipitated proteins were detected by Western blot analysis.
NOS Activity Assay
The activity of eNOS was determined in detergent-solubilized lysates of bPAECs by measuring the conversion of [14C]arginine (PerkinElmer Life Sciences) to [14C]citrulline under Vmax conditions as described previously (15).
Migration Assay
bPAECs were grown to confluence and serum-starved with 0.2% BSA for 24 h. The cells were treated with VEGF (50 ng/ml), BMP2 (30 ng/ml), or BMP4 (30 ng/ml). l-NAME (100 μm) treatment was given 30 min before the treatment with BMP2 or BMP4. The cell monolayers were then wounded by scratching lengthwise with a sterile pipette tip. Images were obtained at time 0 and 18 h after treatments. Wound area was determined using Photoshop CS3 extended software (Adobe, San Jose, CA), and migration was calculated as percent decrease in wound area.
Confocal Microscopy
hPAECs plated onto gelatin-coated coverslips were washed three times with PBS and fixed in 2% paraformaldehyde for 15 min. The cells were washed three times with PBS and blocked and permeabilized in 2% bovine serum albumin, 0.1% Triton X-100in PBS for 20. The cells were then washed and incubated for 60 min with caveolin-1 and BMPRII antibody. Bound antibody was detected using Alexa Fluor 488 or 594 (Molecular Probes), or Cy5 (Jackson ImmunoResearch Laboratories, West Grove, PA)-conjugated secondary antibody. Images were collected on an Olympus Fluoview 1000 confocal microscope.
BMPRII siRNA
All siRNAs were designed and synthesized by Dharmacon (Chicago, IL). siRNA transfection was performed using a Lipofectamine reagent (Invitrogen) according to the manufacturer's instructions. Nontargeting siRNA was used as a negative control. To achieve optimal transfection efficiency, transfection reagent, siRNA, cell density, and time of transfection were optimized.
RESULTS
BMP2 and BMP4 Stimulate eNOS Phosphorylation and Activity in bPAECs
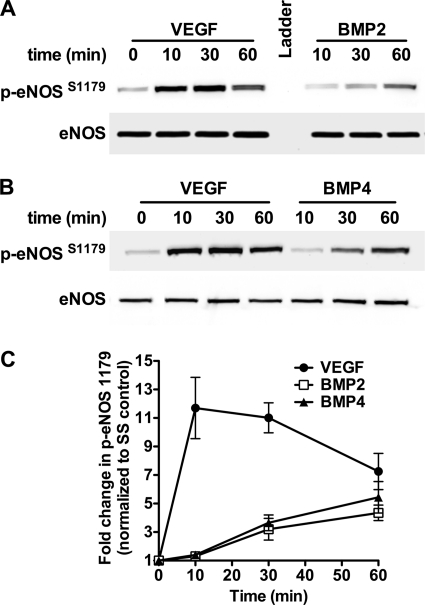
To determine the effect of BMPs on eNOS phosphorylation in PAECs, serum-starved bPAECs were stimulated for various times with BMP2 (30 ng/ml) or BMP4 (30 ng/ml) or with VEGF (50 ng/ml) as a positive control. BMP2 (Fig. 1A) and BMP4 (Fig. 1B) elicited an approximate 5-fold increase in eNOS phosphorylation at serine 1179 (serine 1177 in human) by 60 min (Fig. 1C). The effect of BMP2 and BMP4 on eNOS phosphorylation was similar but was of lower magnitude and delayed compared with that of VEGF.
FIGURE 1.
BMP2 and BMP4 stimulate eNOS phosphorylation in PAECs. A and B, representative Western blots of bPAECs stimulated with BMP2 (A) or BMP4 (B) for various times and analyzed for eNOS phosphorylation at serine 1179. VEGF was used as a positive control. C, results of densitometric analysis of Western blots from four individual experiments. Data show the -fold change in eNOS phosphorylation and are the mean ± S.D. (error bars).
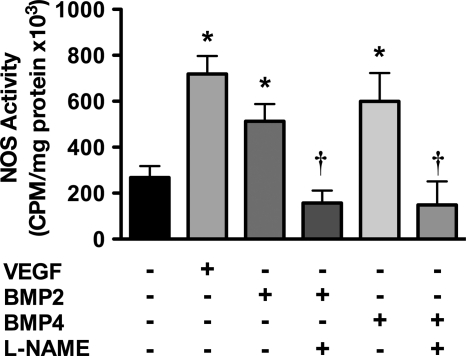
The increase in eNOS phosphorylation correlated with increased eNOS activity. Serum-starved bPAECs were stimulated with BMP2, BMP4, or VEGF for 30 min and assayed for eNOS activity under Vmax conditions. Treatment of bPAECs with BMP2 and BMP4 resulted in a significant increase in eNOS activity (Fig. 2). Importantly, the eNOS activity stimulated by BMP2 and BMP4 was inhibited by the NOS inhibitor l-NAME.
FIGURE 2.
BMP2 and BMP4 stimulate eNOS enzymatic activity in PAECs. bPAECs were stimulated with BMP2, BMP4, or VEGF for 30 min, and lysates were analyzed for eNOS activity by measuring the conversion of arginine to citrulline. l-NAME added to the enzyme activity assay was used to confirm that the conversion of arginine to citrulline was due to NOS activity. Data show the mean ± S.D. (error bars) of three individual experiments. *, p < 0.05 versus serum-starved control; †, p < 0.05 versus corresponding treatment with l-NAME.
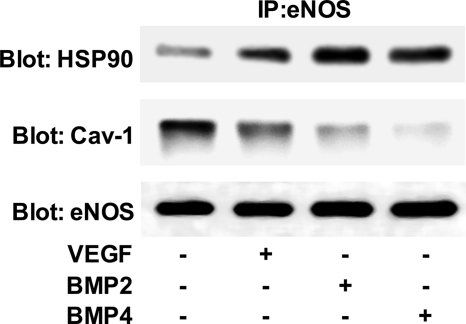
BMPs Elicit Canonical Changes in eNOS Protein-Protein Interactions
Co-immunoprecipitation experiments were used to determine whether BMP2 and BMP4 could elicit changes in eNOS protein-protein interactions, similar to VEGF. Serum-starved bPAECs were stimulated with BMP2, BMP4, or VEGF for 30 min. The cells were lysed in modified radioimmune precipitation assay buffer and eNOS immunoprecipitated. The immunoprecipitates were analyzed for the presence of caveolin-1, Hsp90, and eNOS by SDS-PAGE, followed by Western blot analysis. Fig. 3 shows that similar to VEGF, BMP2 and BMP4 cause the dissociation of eNOS from caveolin-1 and increased the eNOS-Hsp90 interaction.
FIGURE 3.
BMP2 and BMP4 elicit canonical changes in eNOS protein-protein interactions. eNOS was immunoprecipitated (IP) from bPAECs stimulated for 30 min with BMP2, BMP4, or VEGF. Immunoprecipitates were subjected to SDS-PAGE and analyzed by Western blotting for co-immunoprecipitation of Hsp90 and caveolin-1 (Cav-1). The blots shown are representative of three individual experiments.
Dependence of BMP-mediated eNOS Phosphorylation on AKT and PKA
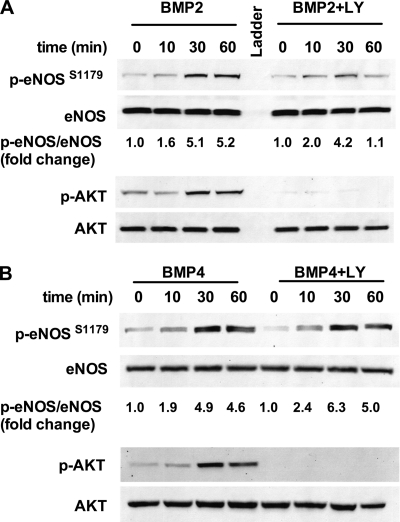
Based on previous studies, we explored a potential role for the protein kinase AKT in the phosphorylation of eNOS in response to BMP2 and BMP4. Serum-starved bPAECs were stimulated for various times in the presence or absence of the PI3K inhibitor LY294002. BMP2 and BMP4 elicited AKT phosphorylation, which was abrogated by LY294002. Interestingly, inhibition of AKT phosphorylation led to divergent effects on BMP2- versus BMP4-mediated eNOS phosphorylation. LY204002 showed a moderate effect on the amplitude of eNOS phosphorylation and a more robust effect on the duration of eNOS phosphorylation in response to BMP2 (Fig. 4A). In contrast, LY294002 had no effect on the ability of BMP4 to stimulate eNOS phosphorylation (Fig. 4B).
FIGURE 4.
BMP2- and BMP4-stimulated eNOS phosphorylation is largely independent of AKT activation. bPAECs were incubated with or without the PI3K inhibitor LY294002 (10 μm) 15 min prior to stimulation with BMP2 (A) or BMP4 (B). Western blots shown are representative of four individual experiments.
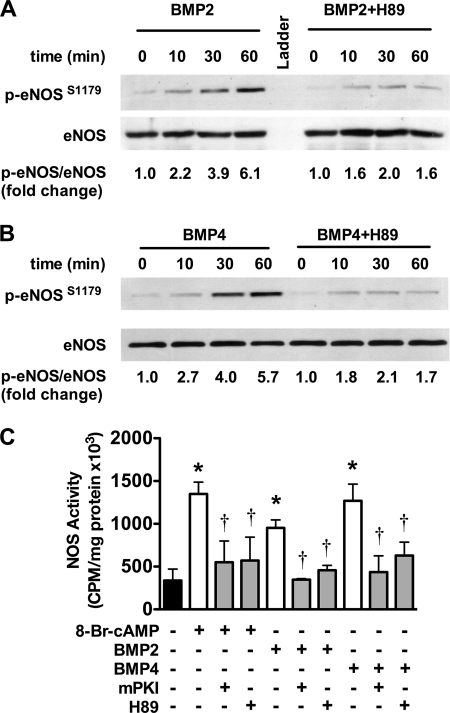
Because inhibition of PI3K/AKT had no effect on BMP4-mediated eNOS phosphorylation we explored a role for PKA in phosphorylating eNOS at this site. Unlike PI3K inhibition, inhibition of PKA with H89 had a profound effect on BMP-mediated eNOS phosphorylation in response to BMP2 (Fig. 5A) and BMP4 (Fig. 5B), attenuating both the amplitude and duration of eNOS phosphorylation. The effect of H89 was not due to inhibition of AKT phosphorylation because AKT phosphorylation was enhanced by H89 (data not shown). In addition, mPKI or H89 abrogated 8-Br-cAMP-, BMP2-, and BMP4-stimulated eNOS activity (Fig. 5C). These data implicate PKA as the primary kinase involved in eNOS phosphorylation in response to BMPs.
FIGURE 5.
BMP2- and BMP4-stimulated eNOS phosphorylation is PKA-dependent. A and B, PAECs were incubated with or without the PKA inhibitor H89 (10 μm) 15 min prior to stimulation with BMP2 (A) or BMP4 (B) and assessed for eNOS phosphorylation. Western blots shown are representative of four individual experiments. C, PAECs were incubated with or without the PKA inhibitors H89 (10 μm) or mPKI (5 μm) 15 min prior to stimulation with 8-Br-cAMP, BMP2, or BMP4. After 60 min, the cells were lysed and assayed for the conversion of [3H]arginine to [3H]citrulline. Data are representative of three individual experiments. Error bars, S.D. *, p < 0.05 versus unstimulated control; †, p < 0.05 versus corresponding treatment without inhibitors.
BMP-stimulated Migration Is eNOS-dependent
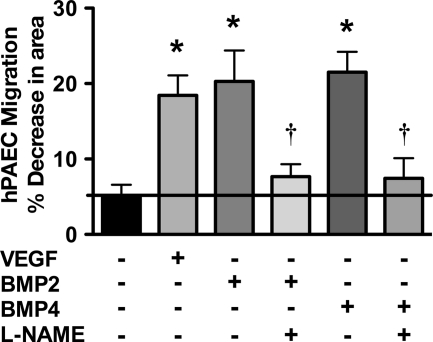
In healthy blood vessels, endothelial cell migration is an important means of repair in response to vessel injury. Endothelial cell migration in response to VEGF is eNOS-dependent. We explored whether migration in response to BMP2/4 is also eNOS-dependent. Serum-starved bPAECs were stimulated with BMP2 or BMP4 in the presence or absence of l-NAME, or with VEGF. The cell monolayers were then wounded, and the migration of the cells into the wounded area was monitored for 18 h. BMP2, BMP4, and VEGF stimulated bPAEC migration by a nearly 4-fold increase in migration compared with unstimulated controls (Fig. 6). The migration, due to BMP2/4, was inhibited by l-NAME, demonstrating that BMP-stimulated migration is eNOS-dependent.
FIGURE 6.
BMP2 and BMP4 stimulate eNOS-dependent PAEC migration. PAECs were stimulated with VEGF, BMP2, or BMP4 with or without l-NAME (100 μm) and assayed for migration using the monolayer wound assay. Data represent the mean ± S.D. (error bars) of three individual experiments. *, p < 0.05 versus serum-starved control; †, p < 0.05 versus corresponding treatment without l-NAME.
BMPRII Mediates eNOS Activation by BMPs
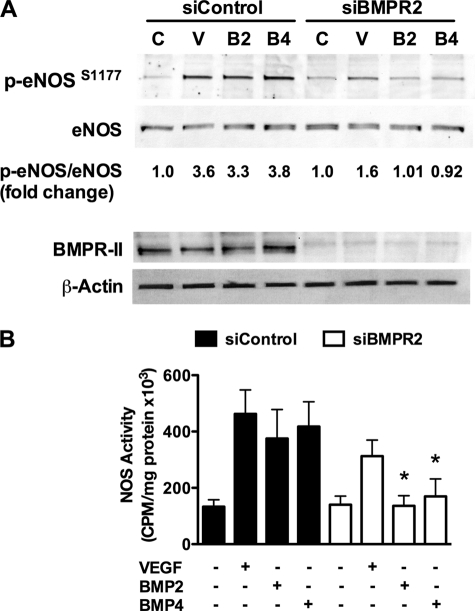
In addition to BMPRII, there are other type II receptors that bind to and are activated by BMP2/4, including the activin type II receptors (ActR-II and ActR-IIB). To determine a specific role of BMPRII in the activation of eNOS by BMPs, we knocked down BMPRII using siRNA. BMPRII siRNA, but not control siRNA, prevented BMP2/4-stimulated eNOS phosphorylation and activity (Fig. 7, A and B). Knockdown of BMPRII also had a significant inhibitory effect on VEGF-induced eNOS phosphorylation (Fig. 7A), and VEGF-induced activity trended toward inhibition but did not reach significance (Fig. 7B). Interestingly, BMPRII siRNA had no effect on acetylcholine-induced eNOS phosphorylation or activity, suggesting that BMPRII did not cause global eNOS defects (data not shown). These data demonstrate a specific role for BMPRII in mediating eNOS activation by BMP2/4.
FIGURE 7.
BMP2- and BMP4-stimulated eNOS phosphorylation is dependent on BMPRII. Serum-starved hPAECs transfected with control or BMPRII siRNA were stimulated for various times with BMP2, BMP4, or VEGF and then assayed for eNOS phosphorylation by Western blot analysis (A) or eNOS activity (B). The blots shown are representative of three individual experiments. Error bars, S.D. *, p < 0.05.
BMPRII and eNOS Interact in hPAECs
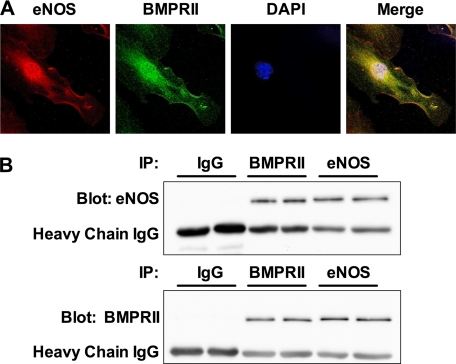
Similar to eNOS, BMPRII was recently shown to reside within caveolae and interact with caveolin-1 in PAECs. This suggested that the stimulation of eNOS by BMP2 and BMP4 might be facilitated by the interaction of BMPRII with eNOS. Initially, we explored this possibility by co-immunofluorescence confocal microscopy. Fig. 8A shows representative photomicrographs of hPAECs stained against eNOS (red) and BMPRII (green). Nuclei are counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). Both proteins show similar distribution in both the perinuclear region and at the plasma membrane. Merging the two images reveals that there is significant co-localization of the two proteins in both the perinuclear region and at the plasma membrane.
FIGURE 8.
BMPRII interacts with eNOS in PAECs. A, confocal images of hPAECs co-stained with antibodies against eNOS (red) and BMPRII (green). B, BMPRII or eNOS was immunoprecipitated (IP) from hPAEC lysates. Nonspecific IgG served as a control. Immunoprecipitates were then analyzed by Western blotting for the presence of BMPRII and eNOS. The blots show two experiments run in parallel and are representative of a total of four experiments.
We further explored the interaction of BMPRII with eNOS in hPAECs by co-immunoprecipitation experiments. Lysates from hPAECs were immunoprecipitated with anti-eNOS antibody, anti-BMPRII antibody, or control IgG and then analyzed for BMPRII and eNOS in the immunoprecipitate. Fig. 8B shows that pulldown with α-BMPRII or α-eNOS antibody, but not control IgG, resulted in the co-immunoprecipitation of the other protein. This reciprocal co-immunoprecipitation of the two proteins provides strong evidence of their interaction in hPAECs.
BMPRII Mutations Prevent eNOS Phosphorylation
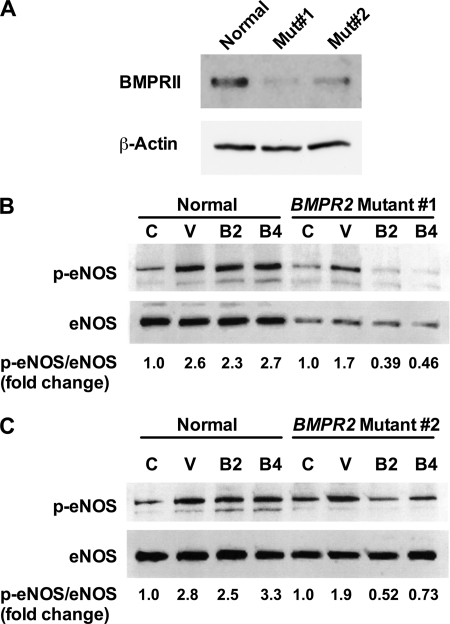
To establish further a role for BMPRII in BMP-mediated eNOS phosphorylation and explore relevance to PAH, we next tested the ability of BMP2 and BMP4 to phosphorylate eNOS in PAECs from two PAH patients with BMPR2 mutations in the coding region. These mutations result in haploinsufficiency and decreased BMPRII expression (Fig. 9A). Mutant PAECs or PAECs from a non-PAH lung were stimulated with BMP2, BMP4, or VEGF for 45 min. Phosphorylation of eNOS was determined by Western blot analysis. Stimulation of mutant PAECs with BMP2 and BMP4 led to a decrease in eNOS phosphorylation compared with the untreated control, whereas normal PAECs showed a robust increase in eNOS phosphorylation (Fig. 9, B and C).
FIGURE 9.
Defective eNOS phosphorylation in hPAECs from patients harboring BMPR2 mutations. A, Western blot analysis of hPAECs from a control patient or two patients with mutations in the BMPR2 gene. B and C, serum-starved hPAECs from two patients with mutations in the BMPR2 gene or from a non-PAH lung were stimulated for 45 min with VEGF, BMP2, or BMP4, and lysates were analyzed for eNOS phosphorylation at serine 1177. The blots shown are representative of three individual experiments.
DISCUSSION
The major finding from this study is that BMP2/4 stimulate eNOS phosphorylation and activity in PAECs via BMPRII. We further demonstrate that the intracellular signaling leading to eNOS activation is largely dependent on PKA. Migration studies demonstrate that BMP-stimulated PAEC migration requires eNOS activity because the NOS inhibitor l-NAME blocked BMP-dependent migration. Finally, we demonstrate that stimulation of PAECs from PAH patients with mutations in the BMPR2 gene leads to decreased eNOS phosphorylation at serine 1177.
Phosphorylation of eNOS at Serine 1177 has been shown to be primarily mediated by activation of AKT (16, 17). In this study we observed BMP-mediated AKT phosphorylation, which is indicative of increased AKT activity. Unexpectedly, inhibition of AKT activation using the PI3K inhibitor LY294002 had only a moderate effect on BMP2-mediated eNOS phosphorylation and no effect on BMP4-mediated eNOS phosphorylation. In addition to AKT, PKA has also been shown to phosphorylate eNOS at serine 1177 (18). This led us to explore whether PKA is involved in BMP-stimulated eNOS phosphorylation. Consistent with this hypothesis, the PKA inhibitor H89 reduced BMP2 and BMP4 stimulated eNOS phosphorylation by ∼70%. H89 had no effect on AKT phosphorylation. In addition, H89 and mPKI, another PKA inhibitor, inhibited 8-Br-cAMP- and BMP-stimulated eNOS activity. Thus, we conclude that PKA plays a prominent role in eNOS phosphorylation in response to BMPs. It is interesting to note that there is little evidence of BMP/BMPRII-mediated PKA activation in the literature, although BMP2 has been shown to activate PKA in chondrocytes (19).
In addition to phosphorylation, eNOS is regulated by protein-protein interactions. In its basal, inactive state, eNOS interacts with caveolin-1 (20), the main structural protein of caveolae. Upon stimulation of endothelial cells with agonists such as VEGF, eNOS dissociates with caveolin-1 and increases its association with Hsp90, which acts to facilitate electron transfer through eNOS (21, 22). Consistent with this current view of agonist-dependent eNOS activation, stimulation of PAECs with BMP2 or BMP4 elicited these canonical changes in eNOS interaction with caveolin-1 and Hsp90.
This study did not address the calcium dependence of eNOS activation mediated by BMP2/4 and BMPRII. Regardless of whether activation of BMPRII leads to calcium influx there is sufficient evidence in the literature demonstrating eNOS activation with or without increases in intracellular calcium. A prime example of this is eNOS activation by insulin. Takahashi and Mendelsohn demonstrated that insulin is capable of stimulating eNOS phosphorylation and activity at basal levels of calcium via recruitment of Hsp90 (23).
Recent studies have identified the interaction of BMPRII with caveolin-1 in various cell types, including PAECs (24–26). This led us to question whether eNOS and BMPRII might interact. Confocal microscopy and reciprocal co-immunoprecipitation experiments provide strong evidence of such an interaction. These data suggest that such an interaction may facilitate the activation of eNOS by BMPRII. Also, siRNA data show that knockdown of BMPRII affects not only BMP-mediated but also, to an extent, VEGF-mediated eNOS activation. This suggests that loss of BMPRII may partially disrupt a larger complex of proteins interacting with eNOS, leading to inefficient signaling. Alternatively, these data may indicate that downstream BMPRII signaling positively regulates VEGF signaling in PAECs. These observations may provide fertile ground for future studies.
Endothelial cell migration is important for reendothelialization in response to arterial injury and endothelial apoptosis (27). A lack of endothelial repair due to endothelial dysfunction exposes the underlying smooth muscle and adventitial layer to serum growth factors, contributing to vascular remodeling. Thus, impaired endothelial cell migration due to endothelial dysfunction early in the pathogenesis of PAH could contribute to vascular remodeling in PAH. The finding that BMPs stimulate PAEC migration via an eNOS-dependent mechanism provides functional significance for BMP-stimulated eNOS activity.
In addition to eNOS, recent studies demonstrate the involvement of Wnt/β-catenin signaling in BMP-2 stimulated PAEC migration, which in the healthy pulmonary endothelium is mediated by BMPRII (28, 29). Although there are few studies examining cross-talk between NO and Wnt/β-catenin signaling, there is some evidence of it in the literature. NO was shown to mediate the activation of Wnt/β-catenin signaling in osteocytes exposed to shear stress (30), and eNOS-mediated vascular permeability was shown to involve S-nitrosylation of β-catenin (31). Thus, it is not unreasonable to speculate that BMP-induced PAEC migration may involve a NO/Wnt/β-catenin signaling mechanism.
The discovery of a role for BMPRII in BMP-mediated eNOS activation in PAECs is likely the most important finding in terms of the pathogenesis of PAH. Loss of BMPRII expression or mutations in the BMPR2 gene are known to play an important contributory role in the development of IPAH and FPAH (9). In the current study, PAECs from two patients with heterozygous mutations to the coding region of BMPR2 were examined for the ability of BMP2/4 to stimulate eNOS phosphorylation. Previous studies demonstrate that BMPRII immunostaining in PAH lung tissue is markedly reduced below the 50% level expected in patients with heterozygous BMPR2 mutations, which was confirmed by Western blot analysis in these cell lines. However, because BMPRII expression has been found to be decreased in PAH patients harboring no mutations in BMPRII it is unclear whether the down-regulation of BMPRII in these cells is related to the mutation.
In terms of the effect of BMPR2 mutations on eNOS activity, data demonstrating decreased eNOS phosphorylation in BMPR2 mutant hPAECs provide a plausible mechanism by which loss of BMPRII-dependent signaling might contribute to endothelial dysfunction in PAH. The decrease in eNOS-phosphorylation compared with serum-starved controls in PAECs from patients with BMPRII mutations has further implications for the pathogenesis of PAH. These data suggest that in patients with such mutations, BMPRII-independent BMP signaling may play an active role in endothelial dysfunction by suppressing eNOS activation.
In summary, we have identified a new role for BMPRII and its ligands, BMP2 and BMP4, as novel mediators of eNOS activation. Considering the important role of eNOS in vascular health, these findings suggest that BMPRII plays a role in maintaining pulmonary vascular homeostasis and that loss of BMPRII may directly contribute to endothelial dysfunction in PAH.
This work was supported, in whole or in part, by National Institutes of Health Grants HL085134 (to P. M. B.) and HL60917 (to S. C. E.).
- PAH
- pulmonary arterial hypertension
- BMP
- bone morphogenetic protein
- BMPRII
- BMP type II receptor
- bPAEC
- bovine PAEC
- 8-Br-cAMP
- 8-bromo-cAMP
- eNOS
- endothelial NOS
- FPAH
- familial PAH
- hPAEC
- human PAEC
- Hsp90
- 90-kDa heat shock protein
- IPAH
- idiopathic PAH
- l-NAME
- l-nitroarginine methyl ester
- PAEC
- pulmonary artery endothelial cell
- mPKI
- myristoylated PKI(6–22)-amide
- PASMC
- pulmonary artery smooth muscle cell.
REFERENCES
- 1. D'Alonzo G. E., Barst R. J., Ayres S. M., Bergofsky E. H., Brundage B. H., Detre K. M., Fishman A. P., Goldring R. M., Groves B. M., Kernis J. T., Levy P. S., Pietra G. G., Lynne M., Reid, Reeves J. T., Rich S., Vreim C. E., Williams G. W., Wu M. (1991) Ann. Intern. Med. 115, 343–349 [DOI] [PubMed] [Google Scholar]
- 2. Sandoval J., Bauerle O., Palomar A., Gómez A., Martínez-Guerra M. L., Beltrán M., Guerrero M. L. (1994) Circulation 89, 1733–1744 [DOI] [PubMed] [Google Scholar]
- 3. Thomson J. R., Machado R. D., Pauciulo M. W., Morgan N. V., Humbert M., Elliott G. C., Ward K., Yacoub M., Mikhail G., Rogers P., Newman J., Wheeler L., Higenbottam T., Gibbs J. S., Egan J., Crozier A., Peacock A., Allcock R., Corris P., Loyd J. E., Trembath R. C., Nichols W. C. (2000) J. Med. Genet. 37, 741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lane K. B., Machado R. D., Pauciulo M. W., Thomson J. R., Phillips J. A., 3rd, Loyd J. E., Nichols W. C., Trembath R. C. (2000) Nat. Genet. 26, 81–84 [DOI] [PubMed] [Google Scholar]
- 5. Deng Z., Morse J. H., Slager S. L., Cuervo N., Moore K. J., Venetos G., Kalachikov S., Cayanis E., Fischer S. G., Barst R. J., Hodge S. E., Knowles J. A. (2000) Am. J. Hum. Genet. 67, 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aldred M. A., Vijayakrishnan J., James V., Soubrier F., Gomez-Sanchez M. A., Martensson G., Galie N., Manes A., Corris P., Simonneau G., Humbert M., Morrell N. W., Trembath R. C. (2006) Hum. Mutat. 27, 212–213 [DOI] [PubMed] [Google Scholar]
- 7. Cogan J. D., Pauciulo M. W., Batchman A. P., Prince M. A., Robbins I. M., Hedges L. K., Stanton K. C., Wheeler L. A., Phillips J. A., 3rd, Loyd J. E., Nichols W. C. (2006) Am. J. Respir. Crit. Care Med. 174, 590–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cogan J. D., Vnencak-Jones C. L., Phillips J. A., 3rd, Lane K. B., Wheeler L. A., Robbins I. M., Garrison G., Hedges L. K., Loyd J. E. (2005) Genet. Med. 7, 169–174 [DOI] [PubMed] [Google Scholar]
- 9. Atkinson C., Stewart S., Upton P. D., Machado R., Thomson J. R., Trembath R. C., Morrell N. W. (2002) Circulation 105, 1672–1678 [DOI] [PubMed] [Google Scholar]
- 10. Yang X., Long L., Southwood M., Rudarakanchana N., Upton P. D., Jeffery T. K., Atkinson C., Chen H., Trembath R. C., Morrell N. W. (2005) Circ. Res. 96, 1053–1063 [DOI] [PubMed] [Google Scholar]
- 11. Teichert-Kuliszewska K., Kutryk M. J., Kuliszewski M. A., Karoubi G., Courtman D. W., Zucco L., Granton J., Stewart D. J. (2006) Circ. Res. 98, 209–217 [DOI] [PubMed] [Google Scholar]
- 12. Valdimarsdottir G., Goumans M. J., Rosendahl A., Brugman M., Itoh S., Lebrin F., Sideras P., ten Dijke P. (2002) Circulation 106, 2263–2270 [DOI] [PubMed] [Google Scholar]
- 13. Xu W., Koeck T., Lara A. R., Neumann D., DiFilippo F. P., Koo M., Janocha A. J., Masri F. A., Arroliga A. C., Jennings C., Dweik R. A., Tuder R. M., Stuehr D. J., Erzurum S. C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1342–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masri F. A., Xu W., Comhair S. A., Asosingh K., Koo M., Vasanji A., Drazba J., Anand-Apte B., Erzurum S. C. (2007) Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L548–554 [DOI] [PubMed] [Google Scholar]
- 15. Bauer P. M., Fulton D., Boo Y. C., Sorescu G. P., Kemp B. E., Jo H., Sessa W. C. (2003) J. Biol. Chem. 278, 14841–14849 [DOI] [PubMed] [Google Scholar]
- 16. Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. (1999) Nature 399, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schleicher M., Yu J., Murata T., Derakhshan B., Atochin D., Qian L., Kashiwagi S., Di Lorenzo A., Harrison K. D., Huang P. L., Sessa W. C. (2009) Sci. Signal. 2, ra41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boo Y. C., Sorescu G., Boyd N., Shiojima I., Walsh K., Du J., Jo H. (2002) J. Biol. Chem. 277, 3388–3396 [DOI] [PubMed] [Google Scholar]
- 19. Lee Y. S., Chuong C. M. (1997) J. Cell. Physiol. 170, 153–165 [DOI] [PubMed] [Google Scholar]
- 20. García-Cardeña G., Fan R., Stern D. F., Liu J., Sessa W. C. (1996) J. Biol. Chem. 271, 27237–27240 [DOI] [PubMed] [Google Scholar]
- 21. Gratton J. P., Fontana J., O'Connor D. S., Garcia-Cardena G., McCabe T. J., Sessa W. C. (2000) J. Biol. Chem. 275, 22268–22272 [DOI] [PubMed] [Google Scholar]
- 22. Pritchard K. A., Jr., Ackerman A. W., Gross E. R., Stepp D. W., Shi Y., Fontana J. T., Baker J. E., Sessa W. C. (2001) J. Biol. Chem. 276, 17621–17624 [DOI] [PubMed] [Google Scholar]
- 23. Takahashi S., Mendelsohn M. E. (2003) J. Biol. Chem. 278, 30821–30827 [DOI] [PubMed] [Google Scholar]
- 24. Nohe A., Keating E., Underhill T. M., Knaus P., Petersen N. O. (2005) J. Cell Sci. 118, 643–650 [DOI] [PubMed] [Google Scholar]
- 25. Ramos M., Lamé M. W., Segall H. J., Wilson D. W. (2006) Vascul. Pharmacol. 44, 50–59 [DOI] [PubMed] [Google Scholar]
- 26. Wertz J. W., Bauer P. M. (2008) Biochem. Biophys. Res. Commun. 375, 557–561 [DOI] [PubMed] [Google Scholar]
- 27. Hirsch E. Z., Chisolm G. M., 3rd, White H. M. (1983) Atherosclerosis 46, 287–307 [DOI] [PubMed] [Google Scholar]
- 28. de Jesus Perez V. A., Alastalo T. P., Wu J. C., Axelrod J. D., Cooke J. P., Amieva M., Rabinovitch M. (2009) J. Cell Biol. 184, 83–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hansmann G., de Jesus Perez V. A., Alastalo T. P., Alvira C. M., Guignabert C., Bekker J. M., Schellong S., Urashima T., Wang L., Morrell N. W., Rabinovitch M. (2008) J. Clin. Invest. 118, 1846–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santos A., Bakker A. D., Zandieh-Doulabi B., de Blieck-Hogervorst J. M., Klein-Nulend J. (2010) Biochem. Biophys. Res. Commun. 391, 364–369 [DOI] [PubMed] [Google Scholar]
- 31. Thibeault S., Rautureau Y., Oubaha M., Faubert D., Wilkes B. C., Delisle C., Gratton J. P. (2010) Mol. Cell. 39, 468–476 [DOI] [PubMed] [Google Scholar]