Background: We have investigated which portion of thyroglobulin is engaged in its final oxidative maturation.
Results: We demonstrate that terminal oxidation of thyroglobulin is linked to region I of the protein.
Conclusion: Final acquisition of secretory competence includes conformational maturation in the interval between linker and hinge segments of region I.
Significance: The results can explain human goitrous hypothyroidism, especially that caused by Tg-C1245R.
Keywords: Endoplasmic Reticulum (ER), Intracellular Trafficking, Protein Folding, Secretion, Thyroid
Abstract
In vertebrates, the thyroglobulin (Tg) gene product must be exported to the lumen of thyroid follicles for thyroid hormone synthesis. In toto, Tg is composed of multiple type-1 repeats connected by linker and hinge (altogether considered as “region I,” nearly 1,200 residues); regions II-III (∼720 residues); and cholinesterase-like (ChEL) domain (∼570 residues). Regions II-III and ChEL rapidly acquire competence for secretion, yet regions I-II-III require 20 min to become a partially mature disulfide isomer; stabilization of a fully oxidized form requires ChEL. Transition from partially mature to mature Tg occurs as a discrete “jump” in mobility by nonreducing SDS-PAGE, suggesting formation of at most a few final pairings of Cys residues that may be separated by significant intervening primary sequence. Using two independent approaches, we have investigated which portion of Tg is engaged in this late stage of its maturation. First, we demonstrate that this event is linked to oxidation involving region I. Introduction of the Tg-C1245R mutation in the hinge (identical to that causing human goitrous hypothyroidism) inhibits this maturation, although the Cys-1245 partner remains unidentified. Second, we find that Tg truncated after its fourth type-1 repeat is a fully independent secretory protein. Together, the data indicate that final acquisition of secretory competence includes conformational maturation in the interval between linker and hinge segments of region I.
Introduction
In vertebrates, thyroid hormone is synthesized in the apical secretory cavity of follicles of the thyroid gland. In this process, secreted thyroglobulin (Tg)3 (1, 2) must undergo iodination by the activity of thyroid peroxidase (3). Tg structure, rather than the specificity thyroid peroxidase (4, 5), is responsible for coupling of di-iodotyrosyl residues 5 and 130 to form thyroxine within the Tg molecule (6, 7).
In general, Tg mutants causing genetic hypothyroidism and/or goiter are defective for secretion and thus fail to be delivered to the site of iodination (8). Rate-limiting in the overall process of intracellular transport is Tg export from the endoplasmic reticulum (ER) (9), which in turn is dependent on folding of the newly synthesized Tg protein (10–13).
The normal folding process for Tg begins with transient formation of mixed disulfide bonds (14) with endogenous ER oxidoreductases in complexes called “A,” “B,” and “C,” as visualized by nonreducing SDS-PAGE (15). Concomitant with resolution of these complexes, and thereafter, there is extensive intramolecular disulfide bond maturation within internal repeat domains of the molecule (16). The oxidation of Tg monomers culminates in a transition from the “D” to oxidized “E” isoform that precedes homodimerization (17), which helps to satisfy “ER quality control” requirements that control efficiency and rate of Tg export from the ER (18).
The primary sequence of Tg (∼2,746 residues in mouse Tg after signal peptide cleavage) is composed of a disulfide-rich contiguous region I (multiple type-1 repeats (19) plus linker and hinge segments); regions II-III (type-2 repeats that are distant members of the GCC2/GCC3 domain superfamily (20–22) and one final type-1 repeat followed by type-3 repeats that exhibit only internal homology); and the cholinesterase-like (ChEL) domain (23). The majority of described pathogenic Tg mutations tend to fall within region I or the ChEL domain (24). Region I mutants or a truncated region I-II-III cannot fold properly (25). Intriguingly, ChEL domain mutants similarly impair upstream Tg folding because ChEL serves as an intramolecular chaperone for I-II-III (26). The mechanism involves stabilization of the oxidized E isoform of I-II-III, but other than requiring ChEL (26), nothing further is known about the region of Tg involved in this critical D-to-E transition.
In this study, we have used two lines of approach to narrow the candidate segment involved in late Tg oxidative maturation, including selective cysteine deletion and Tg truncation mutagenesis. Taken together, these approaches point to a sequence spanning the linker and hinge segments of region I as critical for terminal Tg folding for export from the ER.
EXPERIMENTAL PROCEDURES
Materials
Lipofectamine 2000, Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, penicillin, and streptomycin were from Invitrogen; 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS) was from Molecular Probes; Zysorbin was from Zymed Laboratories Inc.; Complete protease inhibitor mixture was from Roche Applied Science; brefeldin A and protein A-agarose were from Sigma; peptide-N-glycosidase F (PNGase F) and endoglycosidase H were from New England Biolabs (Beverly, MA); Trans35S-Label was from MP Biomedicals (Irvine, CA). Rabbit polyclonal anti-Tg (containing antibodies against epitopes at both N-terminal and C-terminal regions of the protein) has been previously described (27).
Mutagenesis of Mouse Tg
cDNAs encoding secretory ChEL and truncated Tg regions I-II-III and secretory ChEL and ChEL-Myc have been described previously (17, 25, 26). Other constructs described in this study were made using the QuikChange site-directed mutagenesis kit (Agilent Technologies, Inc.) using following the mutagenic primers paired with their complements: Tg-C175X (5′-GCCTGTCCAGGAACAGAAGCTCATTTCCGAAGAGGACCTGTGATTTGATCTGATCC-3′); Tg-R277X (Myc-tagged, 5′-CTGGCAGATTTGAGCAGAAGCTTATCTCTGAAGAAGACCTGTAGGCTGCCACCAGATTTG-3′); Tg-P279X (Myc-tagged, 5′-GGCAGATTTCGGTGCGAGCAGAAGTTGATATCAGAAGAGGACCTTTAGACCAGATTTGG-3′); Tg-G293X (Myc-tagged, 5′-GCCACCAGATTTGAGCAGAAGCTTATCTCTGAAGAAGACCTGTAGGCTGCCACCAG-3′); Tg-A340X (5′-GGGCAGCCTCCATCTTGCTAGTGAGATCAGTCATGTGCCTTGG-3′); Tg-C388S (5′-GGTGTCAGATCGGACACATCCTCCCCACCCAGAATCAAGG-3′); Tg-C1245R (5′-TGCTGACTCTCCAGGCTACGAATCAGTGGCCC-3′); Tg-C1489S (5′-GGAGCTTTCAGCAAAACCCATTCTGTCACTGACTGTCAGAAGAATGAGG-3′); Tg-C1973S (5′-CCAATGGGTTCTTTGAGAGTGAGCGGCTCTGTGACAGGG-3′); and Tg region I (Tg-A1435X, 5′-CCCAACCACCAGACAGGATTGACTGGGCTGTGTGAAATGCCC-3′). Tg-C345S,C388S was made from Tg-C388S using the following primer and its complement (5′-GCGCTGCAGATCAGTCATCTGCCTTGGAAAGGCAGCAGGCC-3′). To make the secretory region II-III-HA, a SalI restriction site was introduced into 5′ end of the first type-2 repeat by using a pair of mutagenesis primer and its complement (5′-GGTTCTACAGAGTCCCAACCACCAGTCGACATGCTCTGGGCTGTGTGAAATGC-3′). The prolactin signal peptide was fused in-frame at the 5′ end of the first type-2 repeat using an engineered SalI site as described previously (28), and HA tag was added using the following primer and its complement (5′-CCGGAAGTCTTACCCCTATGACGTCCCAGATTATGCATGATCCACACCTTCTGTACGC-3′). Tg regions I-II-III lacking the third type-1 repeat were made as follows. Two separate PCRs were done using two sets of primers and I-II-III as a template (PCR 1, forward primer, 5′-AGACCCAAGCTGGCTAGCGTTTAAACGGG-3′, and reverse primer, 5′-TACTTCACACTTAGTGGGACACCGAGTTGGCCTGCCCTGC-3′; PCR 2, forward primer, 5′-GGCCAACTCGGTGTCCCACTAAGTGTGAAGTAGAACAGTTTGC-3′, and reverse primer, 5′-TTCTCGAGCCATCAAGTTCTTTGCCTCGGG-3′). Two amplified products were purified and combined as templates for the final PCR. The final PCR was done using the forward primer of PCR 1 and the reverse primer of PCR 2. The amplified DNA was digested with NheI and XhoI and transferred into I-II-III. Each final product was confirmed by direct DNA sequencing.
Cell Culture and Transfection
HEK293 cells (simply called 293 cells) were cultured in DMEM with 10% fetal bovine serum in 6-well plates at 37 °C in a humidified 5% CO2 incubator. Plasmids were transiently transfected using Lipofectamine 2000 transfection reagent following the manufacturer's instructions.
Metabolic Labeling and Immunoprecipitation
Transfected 293 cells were starved for 30 min in Met/Cys-free DMEM and then pulse-labeled with 180 μCi/ml 35S-amino acids. The cells were then washed with an excess of cold Met/Cys and chased in complete DMEM plus serum. At each time point, cells were lysed in buffer containing 1% Nonidet P-40, 0.1% SDS, 0.1 m NaCl, 2 mm EDTA, 25 mm Tris, pH 7.4, and a protease inhibitor mixture. For immunoprecipitation, anti-Tg antibody was incubated with cell or media samples overnight at 4 °C, and the immunoprecipitate was recovered with protein A-agarose and washed three times in immunoprecipitation buffer. For alkylation, the immunoprecipitates were incubated with 5 mm AMS for 30 min at 30 °C as described previously (26). All samples were finally boiled in SDS sample buffer with or without reducing agent (0.1 m dithiothreitol) as indicated, resolved by SDS-PAGE, and analyzed by fluorography or phosphorimaging.
Endoglycosidase H and PNGase F Digestion
Immunoprecipitates were boiled for 10 min in 0.5% SDS containing 1% 2-mercaptoethanol in 20 mm Tris, pH 7.4 (or without reducing agent for nonreducing condition). Denatured samples were digested with 250 units of endoglycosidase H in 50 mm sodium citrate, pH 5.5, for 1 h at 37 °C or PNGase F in 50 mm sodium phosphate, pH 7.5, for 1 h at 37 °C.
RESULTS
I-II-III Oxidative Folding
Early Tg oxidative maturation includes mixed disulfide complexes called bands A, B, and C (17) that engage ER oxidoreductases, such as Tg-ERp57 and Tg-protein-disulfide isomerase (Tg-PDI) adducts (15). After dissociation from ER oxidoreductases, Tg monomer folding proceeds during its residence in the ER, en route to final formation of roughly 60 intrachain disulfide bonds (16). This conformational maturation is easily perturbed (9) and is temperature-sensitive, tending to arrest at an immature folding intermediate known as the D-isoform (immature monomer) (26). Indeed, I-II-III contains the information needed to mature to the monomeric E-isoform (26), but features in ChEL (and not other cholinesterase superfamily members) are required to produce a stable E-isoform that is competent for export from the ER (29).
To understand how far along I-II-III oxidation progresses in the absence of ChEL (i.e. prior to acquisition of secretion competence), we used nonreducing SDS-PAGE to examine the maturation of the polypeptide during its first 50 min after synthesis. At the zero chase time, a broad band of immature I-II-III was already apparent (Fig. 1, lane 2). Within 20 min of chase, there was sharpening of this D-isoform (lane 3), which did not appear to mature further in the absence of ChEL (lane 4). To examine whether most disulfide bonds in I-II-III (containing 116 of the 122 cysteines of Tg) had already formed during these first 20 min, the samples were alkylated with AMS and thereafter fully reduced and analyzed by SDS-PAGE and fluorography. Alkylation adds only ∼500 daltons of molecular mass for each cysteine modified. Upon alkylation, newly synthesized I-II-III at the zero chase time exposed sufficient cysteine thiols to gain significant molecular mass beyond that predicted by the ∼240-kDa polypeptide (Fig. 1, lane 6); by 20 min after synthesis, alkylation was markedly decreased (lane 7), and this did not change further as a function of time (lane 8). The mobilities of these bands at the later chase times were nearly identical to those of fully reduced I-II-III in the absence of alkylation (Fig. 1, lanes 10–12); thus, most oxidative maturation of Tg has already occurred within the D-isoform of I-II-III prior to its final maturation for export.
FIGURE 1.
Oxidative maturation of I-II-III in the absence of ChEL. 293 cells transiently transfected to express I-II-III were pulse-labeled for 10 min with 35S-amino acids and then chased in complete medium for the times indicated before immunoprecipitation with anti-Tg. The immunoprecipitates were either analyzed directly by nonreducing SDS-PAGE (first panel) or alkylated with AMS (see “Experimental Procedures”) and then analyzed by reducing SDS-PAGE (middle panel) or analyzed directly by reducing SDS-PAGE (last panel) (a nonspecific band was recovered even from untransfected control cells shown in lanes 1, 5, and 9). In the absence of ChEL within 20 min of chase, I-II-III is arrested at the maturation state of the D-isoform (26), but oxidation to this form correlates with loss of the ability to add molecular mass upon alkylation with AMS. The position of the 181-kDa molecular mass marker is indicated. Con = empty vector.
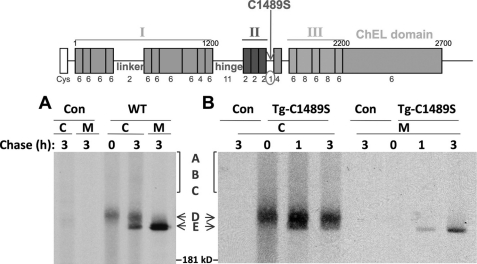
The D-to-E transition is a late oxidative event seen as a discrete “jump down” in the nonreducing SDS-PAGE mobility of Tg (Fig. 2A). This sudden diminution suggests formation of one or more disulfide bonds engaging Cys partners at a sufficient distance within the primary sequence so as to decrease the hydrodynamic radius of the denatured protein. The (nine) type-1A repeats of region I employ a pattern of disulfide pairing engaging Cys-1-Cys-2, Cys-3-Cys-4, and Cys-5-Cys-6, and the internal folding of the (two) type-1B repeats also consumes all of their internal thiols (30). Similarly, Cys residues in type-2 and -3 repeats and in the ChEL domain are all consumed in internal disulfide bonds, and collectively, these actually represent the vast majority of total Cys residues in Tg (31). We therefore scanned the Tg sequence looking for additional Cys residues that might reside at a greater distance from one another and that were unpaired, so as to be candidates that might participate in the D-to-E maturation step (Fig. 2, schematic diagram). A short segment after region II bears a single Cys-1489 residue that seemed a very plausible candidate (Fig. 2, schematic diagram); for this reason, we prepared a Tg-C1489S mutant. Tg-C1489S did appear less efficient for secretion, and only little intracellular mature E-isoform (awaiting secretion) remained at 3 h of chase; nevertheless essentially all of the few secreted molecules did achieve the mature E conformation (Fig. 2B).
FIGURE 2.
Oxidative maturation of wild-type Tg and Tg-C1489S. The schematic diagram above the data indicates the primary structure of Tg. The single Cys residue following region II was mutated (C1489S), as indicated in the diagram. In the experiment, 293 cells were transiently transfected to express either wild-type Tg (WT) or the point mutant. Con = empty vector. The cells were then pulse-labeled for 10 min with 35S-amino acids and chased in complete medium for the times indicated. Cell lysates (C) and chase media (M) were collected before immunoprecipitation with anti-Tg. Immunoprecipitates were deglycosylated with PNGase F, analyzed by nonreducing SDS-PAGE, and imaged by fluorography.
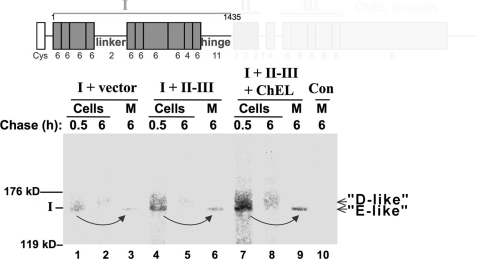
In contrast to export of full-length Tg, which is a slow process (10, 16, 18), regions II-III and ChEL are each competent for rapid export as independent secretory proteins and together assist in export of region I (28). To test whether region I was the source of the D-to-E transition, we followed the oxidative maturation of region I (Tg-A1435X) by nonreducing SDS-PAGE after deglycosylation with PNGase F. When co-transfected along with empty vector, the region I band was barely perceptible, suggesting instability (Fig. 3, lanes 1–3). When co-transfected with both secretory II-III (Fig. 3, lanes 4–6) plus secretory ChEL (lanes 7–9), formation of an “E-like” isomer (and secretion) was increasingly apparent. Although these data indicate stabilization of region I by II-III and ChEL (28), they also strongly suggest that region I directly participates in D-to-E maturation.
FIGURE 3.
Oxidative maturation of Tg region I. Above the data, the schematic diagram shows the region I construct (Tg-A1435X) used in this experiment. In the experiment, 293 cells were either untransfected controls (Con) or transfected either with Tg region I or region I plus secretory II-III ± secretory ChEL. The cells were pulse-labeled for 15 min with 35S-amino acids and chased in complete medium for the times indicated. Cell lysates (Cells) and chase media (M) were then collected before immunoprecipitation with anti-Tg, deglycosylation with PNGase F, and analysis by nonreducing SDS-PAGE and fluorography. The positions of the 119- and 176-kDa molecular mass markers are indicated.
To continue the search for “free” Cys residues within region I that could be engaged in the D-to-E transition, we explored the hinge segment following the 10th Tg type-1 repeat, as well as the linker sequence between the fourth and fifth type-1 repeat. Hishinuma et al. (32, 33) described two missense mutations in the hinge segment in which cysteine substitution is linked to thyroid pathology: a mild defect associated with adenomatous goiter in patients expressing Tg-C1977S and more severe congenital hypothyroid goiter caused by Tg-C1245R. Upon neonatal screening, both cause elevation of serum thyroid-stimulating hormone, and based upon analysis of goiter tissue, both activate the thyroidal ER stress response, with the Tg-C1245R mutant inducing more ER molecular chaperones than Tg-C1977S (34). We therefore examined the equivalent of these two mutations engineered into the mouse Tg cDNA.
Transfected 293 cells were metabolically labeled with 35S-amino acids and chased in the presence of brefeldin A (BFA) to accumulate secretory proteins within the ER, thereby simplifying the sample analysis. Newly synthesized Tg was immunoprecipitated and deglycosylated with PNGase F prior to analysis by nonreducing SDS-PAGE and fluorography. Immediately after synthesis, some disulfide-linked Tg complexes were detected for both mutants (although distinct bands A, B, and C were not individually resolved; Fig. 4A, lanes 2 and 5). After chase, Tg-C1973S (equivalent to human Tg-C1977S) achieved maturation to the E-isoform (albeit inefficiently), whereas Tg-C1245R appeared blocked in progression beyond the D-isoform (Fig. 4A, lanes 3 and 4). Although these data implicate Cys-1245 in a late stage of Tg oxidative maturation, a caveat is that blockade of protein export with BFA induces ER stress (35); thus, we cannot exclude indirect pharmacological effects that might promote misfolding of Tg-C1245R in the ER.
FIGURE 4.
Oxidative maturation of Tg bearing selective point mutations in the hinge and linker sequences of region I. Above the data, the schematic diagram indicates the primary structure of Tg; the positions of the point mutants studied are indicated. A, 293 cells were transiently transfected to express either empty vector (Con) or the Tg constructs indicated above. The cells were then pulse-labeled for 10 min with 35S-amino acids and chased in complete medium in the presence of BFA to block labeled protein secretion. At the chase times indicated, cell lysates were collected before immunoprecipitation with anti-Tg, deglycosylation with PNGase F, and analysis by nonreducing SDS-PAGE and fluorography. B, cells as in panel A were pulse-labeled and chased for the times indicated in the absence of BFA. Cell lysates (C) and chase media (M) were collected before immunoprecipitation with anti-Tg and analysis as in panel A.
We therefore repeated the pulse-chase experiment like that in Fig. 4A, but this time in the absence of BFA. Although at least some mutant Tg protein was recovered in the medium, it was apparent that only a small fraction of secreted Tg-C1245R molecules migrated as a band comparable with the mature E-isoform (the lowest band of Fig. 4B, lane 3), whereas other molecules escaped from cells despite imperfect oxidation (lane 3), and there was little or no intracellular mature E-isoform awaiting secretion at 3 h of chase (lane 2). By contrast, there are two Cys residues in the linker sequence between the fourth and fifth type-1 repeats of region I (Fig. 4, schematic diagram), and neither a single Tg-C388S nor double Tg-C345S,C388S mutant exhibited a blockade in oxidation to the mature E-isoform, excluding these residues as a disulfide bonding partner with Cys-1245. Together, the data in Fig. 4, A and B, suggest that C1245R in the hinge segment is impaired in a late stage of Tg oxidation that is linked to neonatal hypothyroidism (32, 33).
Dissection of Secretion Competence within Region I
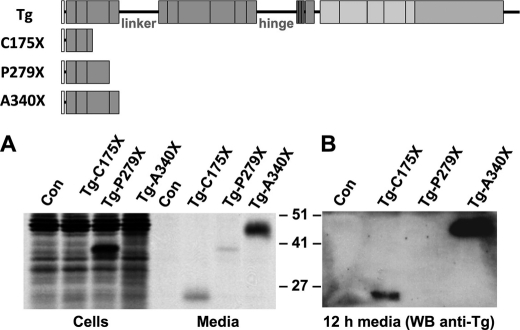
Growing evidence supports the hypothesis that region I is the most difficult-to-fold portion of Tg (28). Other than the linker and hinge segments, this region is composed exclusively of type-1 repeating domains that have an evolutionarily programmed internally folded structure (30). An early study suggested that a patient homozygous for the Tg-R277X truncation exhibited only mild disease and proposed that a foreshortened Tg-R277X would be secretion-competent (36). However, more recent studies have linked the same genotype to severe goitrous hypothyroidism (37). Such a mutant is interesting in terms of folding requirements for secretion because this naturally occurring mutation deletes Cys-278, disrupting the last disulfide bond of the third type-1 repeat. In contrast, we suggested that a recombinant Tg truncation Tg-C175X, which leaves an even number of Cys residues forming the first disulfide bond of the third type-1 repeat, is secretable (38). Because the third type-1 repeat actually terminates at Cys-278, in the current study, we truncated Tg at P279X, ending cleanly after this last cysteine. To further encourage formation of this last disulfide bond of the third repeat, we also created a construct that included an additional sequence initiating the fourth type-1 repeat by generating Tg-G293X. Each of these constructs was metabolically labeled in cells.
Within 5 h after synthesis, a majority of full-length Tg, as well as Tg-C175X, was recovered in the medium rather than in the cells (Fig. 5A; the lower number of Cys residues in Tg-C175X results in decreased band intensity because of decreased incorporation of 35S-amino acids). However, the truncated Tg-R277X constructs with an incomplete third type-1 repeat, a complete third repeat (Tg-P279X), or extra sequence entering the fourth type-1 repeat (Tg-G293X) were each hardly secreted (Fig. 5A). Specifically, secretion of the naturally occurring Tg-R277X mutant was essentially undetectable. Although secretion of Tg-P279X was minimally improved, the presence of that final Cys residue might correlate with slightly greater protein stability inside cells (Fig. 5A). By contrast, the behavior of Tg-G293X is very similar to that reported in severely hypothyroid Dutch goats (39) that express Tg-Y296X (40), which creates a much less stable protein (Fig. 5A). When examined after 5 h of chase by endoglycosidase H digest, Tg-C175X recovered in the medium was endoglycosidase H-resistant (Fig. 5B, left), whereas most Tg-R277X (recovered in cells) was endoglycosidase H-sensitive (Fig. 5B, right). Because each of our constructs, Tg-C175X, Tg-R277X, Tg-P279X, and Tg-G293X, were Myc epitope-tagged at the C terminus, we also examined Tg-R277X lacking a Myc tag to ensure that the tag did not play a role in the result, but untagged Tg-R277X also was not secreted (Fig. 6, lane 5).
FIGURE 5.
Expression and secretion of truncation mutants in Tg region I. To the left of the data, the schematic diagram indicates the primary structure of the Tg truncation constructs used in this experiment. A, 293 cells were either untransfected (Con) or transiently transfected to express the Tg constructs indicated above. Each construct was engineered to include a C-terminal Myc tag to ensure that immunoreactivity was identical between the proteins. The cells were then pulse-labeled for 30 min with 35S-amino acids and chased in complete medium for 5 h. At that time, cell lysates were collected before immunoprecipitation with anti-Myc and analysis by reducing SDS-PAGE and fluorography (a nonspecific band was recovered in the media even from untransfected control cells). B, cells transfected with the constructs indicated were pulse-labeled and chased as in panel A. Cell lysates (C) and chase media (M) were collected before immunoprecipitation, digestion with or without endoglycosidase H (± Endo H), and analysis as in panel A. The positions of molecular mass marker proteins are shown.
FIGURE 6.
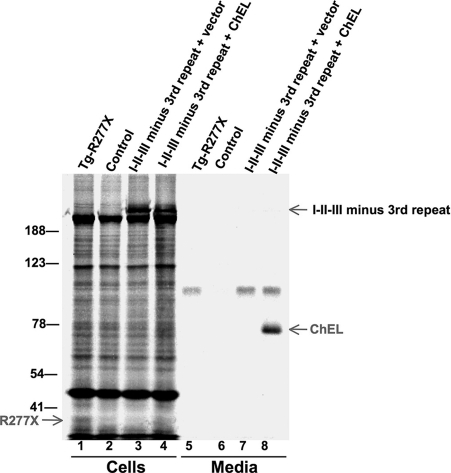
Deletion of the third type-1 repeat does not improve I-II-III secretion. 293 cells were either untransfected controls or transfected to express I-II-III lacking the third type-1 repeat ± secretory ChEL. The cells were then pulse-labeled with 35S-amino acids for 30 min and chased in complete medium for 4 h. Cell lysates and collected chase media were immunoprecipitated with anti-Tg and analyzed by reducing SDS-PAGE and fluorography (a nonspecific band was recovered in the media even from untransfected control cells). The positions of molecular mass marker proteins are shown.
Altogether, the data support that Tg termination just before, at, or just after the third type-1 repeat results in a protein with features that are recognized as immature/misfolded within the ER. We considered that the third Tg type-1 repeat might be a candidate site for assistance by ChEL in the stabilization of I-II-III. Indeed, when we deleted the third Tg type-1 repeat from I-II-III, the well established secretion enhancement of I-II-III by ChEL (26) was no longer observed; however, this did not reflect specificity because I-II-III lacking its third Tg type-1 repeat remained incompetent for secretion with or without ChEL (Fig. 6).
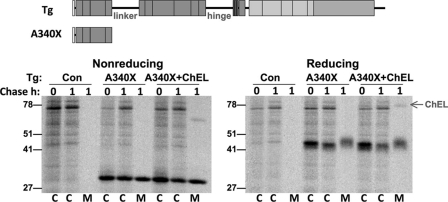
Importantly, when we extended the truncated Tg to the end of the fourth repeat (prior to the linker segment), Tg-A340X was extremely well expressed and well secreted, as measured either by a pulse-chase-immunoprecipitation analysis (Fig. 7A) or by immunoblotting of culture media collected for 12 h (Fig. 7B). Indeed, Tg-A340X exhibited equivalent disulfide bond formation, glycosylation, and secretion, regardless of the presence or absence of secretory ChEL (Fig. 8). Because completion of the fourth type-1 repeat efficiently converts truncated Tg into an independent secretion-competent protein not requiring assistance from other Tg regions, ChEL stabilization of mature Tg structure is likely to involve a part of Tg region I residing beyond these first four repeating units.
FIGURE 7.
A truncated Tg containing the first four type-1 repeats is secreted efficiently. Above the data, the schematic diagram shows the construct(s) used in this figure. A, 293 cells were transiently transfected to express either empty vector (Con) or the Tg constructs indicated above. The cells were then pulse-labeled for 30 min with 35S-amino acids and chased in complete medium for 5 h. At that time, cell lysates and media were collected before immunoprecipitation with anti-Tg and analysis by reducing SDS-PAGE and fluorography. B, the media bathing cells transfected as in panel A were collected for 12 h and analyzed by immunoblotting with anti-Tg (WB anti-Tg). The positions of molecular mass marker proteins are shown.
FIGURE 8.
A truncated Tg containing the first four type-1 repeats is secreted rapidly and independently of ChEL. Above the data, the schematic diagram shows the construct(s) used in this figure. In the experiment, 293 cells were transiently transfected to express either empty vector (Con) or Tg-A340X ± secretory ChEL. The cells were then pulse-labeled for 30 min with 35S-amino acids and chased in complete medium for 1 h. At that time, cell lysates (C) and media (M) were collected before immunoprecipitation with anti-Tg and analysis by SDS-PAGE under nonreducing (left panel) or reducing (right panel) conditions. Note that for Tg-A340X, disulfide oxidation (as measured by mobility difference between reduced and nonreduced gel bands), glycosylation of secreted protein, and extent of secretion were unaffected by the presence of the ChEL domain. The positions of molecular mass marker proteins are shown.
DISCUSSION
The ChEL domain serves as an intramolecular chaperone for I-II-III, stabilizing the E-isoform that is competent for homodimerization and export (Fig. 2A) (17, 26). Growing evidence points increasingly to the idea that the folding of region I, comprising its 10 type-1 repeats plus linker and hinge segments, is likely to be rate-limiting for Tg maturation. First, most disulfide oxidation of Tg occurs before the D-to-E transition (Fig. 1), which is a property that can be attributed to region I (Fig. 3). Second, both II-III and ChEL can function as fully independent secretory proteins, pointing to Tg region I as the most challenging portion to fold (28).
Based on the finding that the naturally occurring Tg-R277X is extremely inefficient in export (Figs. 5 and 6), consistent with a more severe form of goitrous hypothyroidism (37) than had previously been recognized (36), we considered the possibility that ChEL provides chaperone assistance primarily to the third type-1 repeat of Tg. Indeed, this third type-1 repeat is an unfavorable stopping point for the Tg protein, whether it is at the precise end of the repeat (Tg-P279X), or shortly thereafter as has been reported in Dutch goats (39) that express Tg-Y296X (40) or in the recombinant Tg-G293X described herein. However, we could not find evidence that I-II-III could acquire ChEL-independent secretory competence merely by deleting the third type-1 repeat; rather, the protein became dysfunctional in a manner that could not be rescued by ChEL (Fig. 6). Quite possibly, the milder hypothyroidism phenotype described in some homozygous patients bearing Tg-R277X point to other genetic and environmental factors that may mitigate the hypothyroidism (41, 42) rather than direct secretory rescue of the protein. Moreover, we now find that Tg-A340X, comprising precisely the first through fourth type-1 repeats, functions as an independent folding unit, exhibiting rapid and efficient secretion that does not require assistance from other Tg regions for its transport (Figs. 7 and 8).
We hypothesize that the protracted and complex oxidative folding (Fig. 2A) involves a portion of Tg in which the linker and hinge segments figure prominently (Fig. 9). Herein, we provide evidence that region I derives benefit from the presence of other Tg regions (Fig. 3), consistent with intramolecular assistance within Tg (26, 28). Disulfide pairing of the solitary Cys-1489, a cysteine residing just beyond region II (Fig. 2, schematic diagram), is unlikely to be important within the context of region II-III, which is readily secreted in the absence of other Tg regions (Fig. 9). However, Cys-1489 is likely to participate in the efficiency of oxidative maturation within region I because Tg-C1489S is inefficient in formation and secretion of the E-isoform (Fig. 2B).
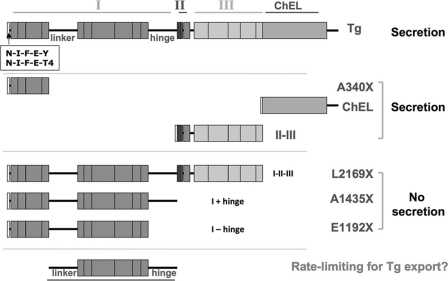
FIGURE 9.
Summary of truncated Tg constructs and their secretory behavior. The schematic illustrates the results of secretion of distinct Tg truncation mutations presented in this study and in Ref. 28. From this we propose that folding the segment shown at bottom, including both linker and hinge regions, may be rate-limiting for Tg folding and export.
Most interesting from these studies is that the Tg-C1245R point mutation in the hinge segment, responsible for human congenital hypothyroid goiter (32, 33), directly impairs efficient D-to-E maturation (Fig. 4). Thus, either the Cys-1245 residue is engaged in a penultimate bond needed to promote terminal native disulfide pairing or this residue normally participates directly in terminal disulfide pairing but can be replaced (albeit inefficiently) by an alternate nearby cysteine. Further work is still needed to identify the structural roles played by the 10 other Cys residues in the hinge segment; additionally, at this time, the Cys residue that partners with Cys-1245 remains to be discovered.
In summary, evidence presented in this study supports a growing view that region I folding may be rate-limiting in the process of normal Tg maturation for thyroid hormonogenesis and that this is linked to a requirement for other Tg regions (28). As we clarify the regional interactions, narrowing the possibilities for how Tg obtains intramolecular stabilization by ChEL and II-III (Fig. 3), we gain increasing insight into the molecular pathogenesis of congenital hypothyroidism caused by mutations that alter the Tg coding sequence.
Acknowledgments
We gratefully acknowledge the University of Michigan Diabetes Research and Training Center Cell and Molecular Biology Core (supported by Grant NIH5P60 DK20572) and the University of Michigan Cancer Center Core laboratories (supported by Grant P30 CA46592).
This work was supported, in whole or in part, by National Institutes of Health Grant R01-DK40344 (to P. A.).
- Tg
- thyroglobulin
- ChEL
- cholinesterase-like
- ER
- endoplasmic reticulum
- AMS
- 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid
- PNGase
- peptide-N-glycosidase F
- BFA
- brefeldin A.
REFERENCES
- 1. Taurog A. (1999) Biochimie 81, 557–562 [DOI] [PubMed] [Google Scholar]
- 2. Arvan P., Di Jeso B. (2004) in The Thyroid (Braverman L. E., Utiger R. eds), 9 Ed., pp. 77–95, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 3. Lamas L., Taurog A. (1977) Endocrinology 100, 1129–1136 [DOI] [PubMed] [Google Scholar]
- 4. Turakulov IaKh., Saatov T., Babaev T. A., Rasuleva GKh., Makhmudov VKh. (1976) Biokhimiia 41, 1004–1007 [PubMed] [Google Scholar]
- 5. Xiao S., Dorris M. L., Rawitch A. B., Taurog A. (1996) Arch. Biochem. Biophys. 334, 284–294 [DOI] [PubMed] [Google Scholar]
- 6. Marriq C., Lejeune P. J., Venot N., Vinet L. (1991) Mol. Cell. Endocrinol. 81, 155–164 [DOI] [PubMed] [Google Scholar]
- 7. Dunn A. D., Corsi C. M., Myers H. E., Dunn J. T. (1998) J. Biol. Chem. 273, 25223–25229 [DOI] [PubMed] [Google Scholar]
- 8. Vono-Toniolo J., Rivolta C. M., Targovnik H. M., Medeiros-Neto G., Kopp P. (2005) Thyroid 15, 1021–1033 [DOI] [PubMed] [Google Scholar]
- 9. Arvan P., Kim P. S., Kuliawat R., Prabakaran D., Muresan Z., Yoo S. E., Abu Hossain S. (1997) Thyroid 7, 89–105 [DOI] [PubMed] [Google Scholar]
- 10. Kim P. S., Arvan P. (1991) J. Biol. Chem. 266, 12412–12418 [PubMed] [Google Scholar]
- 11. Kim P. S., Arvan P. (1995) J. Cell Biol. 128, 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muresan Z., Arvan P. (1997) J. Biol. Chem. 272, 26095–26102 [DOI] [PubMed] [Google Scholar]
- 13. Muresan Z., Arvan P. (1998) Mol. Endocrinol. 12, 458–467 [DOI] [PubMed] [Google Scholar]
- 14. Kim P. S., Kim K. R., Arvan P. (1993) Am. J. Physiol. 265, C704–C711 [DOI] [PubMed] [Google Scholar]
- 15. Di Jeso B., Park Y. N., Ulianich L., Treglia A. S., Urbanas M. L., High S., Arvan P. (2005) Mol. Cell. Biol. 25, 9793–9805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Jeso B., Ulianich L., Pacifico F., Leonardi A., Vito P., Consiglio E., Formisano S., Arvan P. (2003) Biochem. J. 370, 449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee J., Wang X., Di Jeso B., Arvan P. (2009) J. Biol. Chem. 284, 12752–12761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim P. S., Bole D., Arvan P. (1992) J. Cell Biol. 118, 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mihelic M., Turk D. (2007) Biol. Chem. 388, 1123–1130 [DOI] [PubMed] [Google Scholar]
- 20. Putnam N. H., Butts T., Ferrier D. E., Furlong R. F., Hellsten U., Kawashima T., Robinson-Rechavi M., Shoguchi E., Terry A., Yu J. K., Benito-Gutiérrez E. L., Dubchak I., Garcia-Fernàndez J., Gibson-Brown J. J., Grigoriev I. V., Horton A. C., de Jong P. J., Jurka J., Kapitonov V. V., Kohara Y., Kuroki Y., Lindquist E., Lucas S., Osoegawa K., Pennacchio L. A., Salamov A. A., Satou Y., Sauka-Spengler T., Schmutz J., Shin-I T., Toyoda A., Bronner-Fraser M., Fujiyama A., Holland L. Z., Holland P. W., Satoh N., Rokhsar D. S. (2008) Nature 453, 1064–1071 [DOI] [PubMed] [Google Scholar]
- 21. Ghedin E., Wang S., Spiro D., Caler E., Zhao Q., Crabtree J., Allen J. E., Delcher A. L., Guiliano D. B., Miranda-Saavedra D., Angiuoli S. V., Creasy T., Amedeo P., Haas B., El-Sayed N. M., Wortman J. R., Feldblyum T., Tallon L., Schatz M., Shumway M., Koo H., Salzberg S. L., Schobel S., Pertea M., Pop M., White O., Barton G. J., Carlow C. K., Crawford M. J., Daub J., Dimmic M. W., Estes C. F., Foster J. M., Ganatra M., Gregory W. F., Johnson N. M., Jin J., Komuniecki R., Korf I., Kumar S., Laney S., Li B. W., Li W., Lindblom T. H., Lustigman S., Ma D., Maina C. V., Martin D. M., McCarter J. P., McReynolds L., Mitreva M., Nutman T. B., Parkinson J., Peregrín-Alvarez J. M., Poole C., Ren Q., Saunders L., Sluder A. E., Smith K., Stanke M., Unnasch T. R., Ware J., Wei A. D., Weil G., Williams D. J., Zhang Y., Williams S. A., Fraser-Liggett C., Slatko B., Blaxter M. L., Scott A. L. (2007) Science 317, 1756–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hall N., Pain A., Berriman M., Churcher C., Harris B., Harris D., Mungall K., Bowman S., Atkin R., Baker S., Barron A., Brooks K., Buckee C. O., Burrows C., Cherevach I., Chillingworth C., Chillingworth T., Christodoulou Z., Clark L., Clark R., Corton C., Cronin A., Davies R., Davis P., Dear P., Dearden F., Doggett J., Feltwell T., Goble A., Goodhead I., Gwilliam R., Hamlin N., Hance Z., Harper D., Hauser H., Hornsby T., Holroyd S., Horrocks P., Humphray S., Jagels K., James K. D., Johnson D., Kerhornou A., Knights A., Konfortov B., Kyes S., Larke N., Lawson D., Lennard N., Line A., Maddison M., McLean J., Mooney P., Moule S., Murphy L., Oliver K., Ormond D., Price C., Quail M. A., Rabbinowitsch E., Rajandream M. A., Rutter S., Rutherford K. M., Sanders M., Simmonds M., Seeger K., Sharp S., Smith R., Squares R., Squares S., Stevens K., Taylor K., Tivey A., Unwin L., Whitehead S., Woodward J., Sulston J. E., Craig A., Newbold C., Barrell B. G. (2002) Nature 419, 527–531 [DOI] [PubMed] [Google Scholar]
- 23. Christophe D., Vassart G. (1990) Trends Endocrinol. Metab. 1, 351–356 [DOI] [PubMed] [Google Scholar]
- 24. Targovnik H. M., Esperante S. A., Rivolta C. M. (2010) Mol. Cell. Endocrinol. 322, 44–55 [DOI] [PubMed] [Google Scholar]
- 25. Park Y. N., Arvan P. (2004) J. Biol. Chem. 279, 17085–17089 [DOI] [PubMed] [Google Scholar]
- 26. Lee J., Di Jeso B., Arvan P. (2008) J. Clin. Invest. 118, 2950–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim P. S., Kwon O. Y., Arvan P. (1996) J. Cell Biol. 133, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee J., Arvan P. (2011) J. Biol. Chem. 286, 26327–26333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang X., Lee J., Di Jeso B., Treglia A. S., Comoletti D., Dubi N., Taylor P., Arvan P. (2010) J. Biol. Chem. 285, 17564–17573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Molina F., Bouanani M., Pau B., Granier C. (1996) Eur. J. Biochem. 240, 125–133 [DOI] [PubMed] [Google Scholar]
- 31. Mercken L., Simons M. J., Swillens S., Massaer M., Vassart G. (1985) Nature 316, 647–651 [DOI] [PubMed] [Google Scholar]
- 32. Hishinuma A., Takamatsu J., Ohyama Y., Yokozawa T., Kanno Y., Kuma K., Yoshida S., Matsuura N., Ieiri T. (1999) J. Clin. Endocrinol. Metab. 84, 1438–1444 [DOI] [PubMed] [Google Scholar]
- 33. Hishinuma A., Fukata S., Nishiyama S., Nishi Y., Oh-Ishi M., Murata Y., Ohyama Y., Matsuura N., Kasai K., Harada S., Kitanaka S., Takamatsu J., Kiwaki K., Ohye H., Uruno T., Tomoda C., Tajima T., Kuma K., Miyauchi A., Ieiri T. (2006) J. Clin. Endocrinol. Metab. 91, 3100–3104 [DOI] [PubMed] [Google Scholar]
- 34. Baryshev M., Sargsyan E., Wallin G., Lejnieks A., Furudate S., Hishinuma A., Mkrtchian S. (2004) J. Mol. Endocrinol. 32, 903–920 [DOI] [PubMed] [Google Scholar]
- 35. Fujikawa T., Munakata T., Kondo S., Satoh N., Wada S. (2010) Cell Stress Chaperones 15, 193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van de Graaf S. A., Ris-Stalpers C., Veenboer G. J., Cammenga M., Santos C., Targovnik H. M., de Vijlder J. J., Medeiros-Neto G. (1999) J. Clin. Endocrinol. Metab. 84, 2537–2542 [DOI] [PubMed] [Google Scholar]
- 37. Citterio C. E., Coutant R., Rouleau S., Miralles García J. M., Gonzalez-Sarmiento R., Rivolta C. M., Targovnik H. M. (2010) Clin. Endocrinol. (Oxf.) 74, 533–535 [DOI] [PubMed] [Google Scholar]
- 38. Kim P. S., Lee J., Jongsamak P., Menon S., Li B., Hossain S. A., Bae J. H., Panijpan B., Arvan P. (2008) Mol. Endocrinol. 22, 477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Vijlder J. J., van Voorthuizen W. F., van Dijk J. E., Rijnberk A., Tegelaers W. H. (1978) Endocrinology 102, 1214–1222 [DOI] [PubMed] [Google Scholar]
- 40. Veenboer G. J., de Vijlder J. J. (1993) Endocrinology 132, 377–381 [DOI] [PubMed] [Google Scholar]
- 41. van Voorthuizen W. F., de Vijlder J. J., van Dijk J. E., Tegelaers W. H. (1978) Endocrinology 103, 2105–2111 [DOI] [PubMed] [Google Scholar]
- 42. Vono J., Lima N., Knobel M., Medeiros-Neto G. (1996) Thyroid 6, 11–15 [DOI] [PubMed] [Google Scholar]