Abstract
Current therapy for asthma is highly effective. β2-Adrenergic receptor (β2AR) agonists are the most effective bronchodilators and relax airway smooth muscle cells through increased cAMP concentrations and directly opening large conductance Ca2+ channels. β2AR may also activate alternative signaling pathways that may have detrimental effects in asthma. Glucocorticoids are the most effective anti-inflammatory treatments and switch off multiple activated inflammatory genes through recruitment of histone deacetylase-2, activating anti-inflammatory genes, and through increasing mRNA stability of inflammatory genes. There are beneficial molecular interactions between β2AR and glucocorticoid-activated pathways. Understanding these signaling pathways may lead to even more effective therapies in the future.
Keywords: Adrenergic Receptor, Cyclic AMP (cAMP), Histone Deacetylase, Inflammation, Lipoxygenase Pathway, NF-κB Transcription Factor, β2-Adrenergic Receptor, Cysteinyl leukotriene, Glucocorticoid, Theophylline
Introduction
Asthma has become one of the most prevalent diseases and is increasing throughout the world, particularly in developing countries. It is characterized by variable airflow obstruction that is secondary to an allergic pattern of inflammation in the airways, which involves infiltration with inflammatory cells (eosinophils and T-helper 2 (TH2)2 cells) and the activation of resident mast cells and dendritic cells by allergens (1, 2). Structural cells, such as airway epithelial cells and smooth muscle cells, are important sources of inflammatory mediators as well as activated inflammatory cells. Chronic inflammation may lead to structural changes in the airways, including increased airway smooth muscle cells, fibrosis, angiogenesis, and hyperplasia of mucus-secreting cells. Multiple inflammatory mediators are involved, and many cytokines and chemokines orchestrate this complex chronic inflammation (3).
Although asthma is a complex inflammatory disease, current therapies (if taken correctly) are very effective in the majority of patients (4). There is now a good understanding of how current asthma therapies work at a biochemical level, and this has formed the basis for a search for new treatments (5). Drugs used to treat asthma include bronchodilators, which act mainly by reversing airway smooth muscle contraction, and anti-inflammatory drugs, which suppress inflammation in the airways. The inflammation of asthma is confined to the airways, so inhalational therapy has been found to be the most effective treatment modality and largely avoids systemic side effects.
β2-Adrenergic Agonists
β2-Agonists are by far the most effective bronchodilators for asthma, as they act as functional antagonists and relax airway smooth muscle cells whatever the constricting stimulus. Long-acting β2-agonists (LABA), such as salmeterol and formoterol, have a 12-h duration of action, but once-daily drugs, including indacaterol, vilanterol, and olodaterol, have been developed recently (6). LABA should always be used in combination with a corticosteroid, as they are potentially dangerous if used alone because they do not effectively treat the underlying inflammation.
Bronchodilator Mechanisms
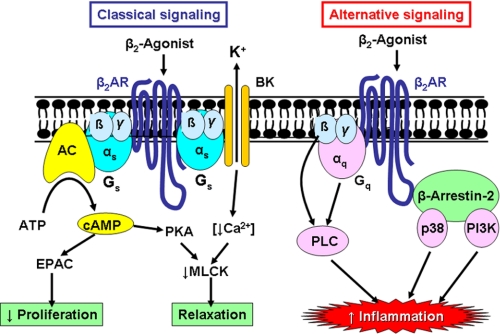
Occupation of β2-adrenergic receptors (β2AR) by agonists results in the activation of adenylyl cyclase via the stimulatory G-protein (Gs). This increases intracellular cAMP, leading to activation of PKA, which phosphorylates several target proteins within the cell, leading to activation of myosin light chain phosphatase and inhibition of myosin light chain kinase and thus relaxation of airway smooth muscle (Fig. 1). In addition, β2-agonists open large conductance calcium-activated potassium channels (BKCa), which repolarize the smooth muscle cell so that Ca2+ is sequestrated into intracellular stores. β2AR are also directly coupled to KCa via Gs, so relaxation of airway smooth muscle may occur independently of an increase in cAMP. Some actions of β2-agonists are mediated via other cAMP-regulated proteins, such as EPAC (exchange protein activated by cAMP) (7). For example, the inhibition of airway smooth muscle cell proliferation by β2-agonists appears to be dependent on EPAC rather than PKA (8).
FIGURE 1.
β2-Agonist signaling pathways. In the classical pathway, β2-agonists bind to β2AR, which are coupled via a stimulatory G-protein (Gs) to adenylyl cyclase (AC), resulting in formation of cAMP. cAMP activates PKA, which phosphorylates myosin light chain kinase (MLCK) in airway smooth muscle cells, resulting in relaxation. Increased cAMP may also activate EPAC to mediate effects such as inhibition of cell proliferation. β2AR are also coupled via Gs to a large conductance calcium-activated potassium channels (BK), leading to decreased intercellular Ca2+ and inhibition of myosin light chain kinase activation. Alternative signaling by β2AR may activate βγ-subunits of Gα and Gq and via Gαq, resulting in activation of PLC. β2AR also interact with β-arrestin-2, which interacts with p38 MAPK and PI3K. These alternative signaling pathways may increase the expression of inflammatory proteins and therefore may have a deleterious effect in asthma.
Airway smooth muscle shows resistance to β2AR desensitization, and this may be due to a large receptor reserve and a very low level of expression of the enzyme GRK2 (G-protein receptor kinase-2), which phosphorylates and inactivates occupied β2-receptors (9). IL-1β uncouples pulmonary β2-receptors in rats in vivo by increasing GRK2/5 activity and expression and thus reducing responsiveness to β2-agonists (10). However, uncertainty remains as to whether β2AR signaling is abnormal in the airway smooth muscle of asthmatic patients.
Other Airway Effects
β2-Agonists may have additional effects on airways, as β2-receptors are localized to several different cells types in the airways. β2-Agonists may therefore cause bronchodilatation in vivo not only via a direct action on airway smooth muscle but also indirectly by inhibiting the release of bronchoconstrictor mediators from inflammatory cells and of neurotransmitters from airway nerves. For example, β2-agonists inhibit mediator release from mast cells though closing an intermediate conductance Ca2+-activated K+ channel (KCa3.1) coupled to Gs (11). Stem cell factor, which is secreted by epithelial cells in asthmatic patients, is an important factor in keeping mast cells at the airway surface in asthma (2) and counteracts this effect of β2-agonists (12). Whether β2-agonists have anti-inflammatory effects in asthma is controversial. The inhibitory effects of β2-agonists on mast cell mediator release and microvascular leakage are clearly anti-inflammatory, suggesting that β2-agonists may modify acute inflammation. However, β2-agonists do not have a significant inhibitory effect on the chronic inflammation of asthmatic airways, which is suppressed by glucocorticoids. This has now been confirmed by several biopsy and bronchoalveolar lavage studies in patients with asthma who are taking regular β2-agonists (including LABA), which demonstrate no significant reduction in the number or activation of inflammatory cells in the airways, in contrast to resolution of the inflammation, which occurs with inhaled glucocorticoids (13). This is likely to be related to the fact that β2-agonist effects on macrophages, eosinophils, and lymphocytes are rapidly desensitized due to a low density of β2-receptors on these cells and high expression of GRK2 (9). Indeed, exposure to LABA increases the expression of GRK2 and GRK5 in human peripheral lung (14).
β2-Receptor Polymorphisms
There are several single-nucleotide polymorphisms and haplotypes of the human β2AR gene (ADRB2) that may affect β2AR function. The common variants are G16R and Q27E, which have in vitro effects on receptor desensitization, but clinical studies have shown inconsistent effects on the bronchodilator responses to short- and long-acting β2-agonists (15). Some studies have shown that patients with the common homozygous Arg16/Arg variant have more frequent adverse effects and a poorer response to short-acting β2-agonists than heterozygotes or Gly16/Gly homozygotes (16), but, overall, these differences are small, and there appears to be no clinical value in measuring ADRB2 genotype. No differences have been found with responses to LABA between these genotypes (17).
Alternative Signaling of β2-Receptors
It is now recognized that, although β2AR are coupled through Gs to relax airway smooth muscle, they may activate alternative signaling pathways that may have deleterious effects, such as increasing inflammation (Fig. 1). In β2AR-overexpressing mice, Gq coupled to phospholipase Cβ1 (PLCβ1) is activated, resulting in an enhanced bronchoconstrictor response to mediators, such as ACh and histamine, that signal through Gq (18). βγ-Subunits of Gs may also signal through PLC activation (PLCη2) (19). β-Arrestin-1 and -2 are adaptor proteins involved in uncoupling phosphorylated β2-receptors from Gs, leading to internalization by clathrin-coated pits. β-Arrestins determine whether β2-receptors are degraded within the cell by endocytosis or are recycled to the cell membrane (20). Interaction of β2-receptors with β-arrestin-2 leads to ubiquitination of each protein and subsequent proteasomal destruction (21). As well as terminating receptor function, β-arrestins act as a scaffold to allow receptors to enhance other signaling pathways, such as MAPK and PI3K, independently of G-proteins and therefore allow β2AR to regulate different responses in the cell (22, 23). This may contribute to the adverse effects of LABA that have been reported (24). Inverse agonists, such as nadolol and carvedilol, block β2AR and inhibit the signaling of constitutively active β2AR and paradoxically have been found to have beneficial effects in murine models of asthma. Furthermore, β2AR knock-out mice are protected from development of asthma (25). β2AR antagonists without inverse agonist activity, such as alprenolol, fail to reverse the asthma phenotype, however. A pilot study in asthma patients showed that, although nadolol reduced lung function in the short-term, there was a reduction in airway hyper-responsiveness after 9 weeks of therapy (26). Deletion of the β-arrestin-2 gene prevents the recruitment of inflammatory cells and airway hyper-responsiveness in mice sensitized and exposed to allergen (27), and both structural and inflammatory cells are involved (28). It is possible that β2-receptor activation in epithelial cells activates β-arrestin-1/2, leading to activation of proinflammatory kinase pathways, such as p38 MAPK, with the activation of proinflammatory genes (29). Biased β2-agonists that favor Gs signaling pathways rather than β-arrestin recruitment may prove to be more effective as bronchodilators in the future (20).
Anticholinergics
Anticholinergics are antagonists of muscarinic receptors, and their only action at therapeutic doses is to block the effects of endogenous acetylcholine (ACh). In animals and humans, there is a small degree of resting bronchomotor tone due to tonic vagal nerve impulses that release ACh in the vicinity of airway smooth muscle because it can be blocked by anticholinergic drugs. ACh may also be released from other airway cells, including epithelial and inflammatory cells (30). Airway epithelial cells contain all of the machinery needed for ACh synthesis and release (31, 32). Tiotropium bromide is a once-daily inhaled anticholinergic that dissociates slowly from muscarinic M1- and M3-receptors and more rapidly from M2-receptors. Although β2-agonists are the most effective bronchodilators in asthma, anticholinergics may have some additive bronchodilator effect, particularly in patients with severe disease (33). M3-receptors via Gq and the activation of PLC result in hydrolysis of phosphatidylinositol 4,5-bisphosphate and generation of inositol 1,4,5 trisphosphate, which releases Ca2+ from intracellular stores, thus resulting in contraction of airway smooth muscle cells and mucus secretion. M3-receptors may also be involved in the structural remodeling that occurs in some patients with asthma, as the increase in airway smooth muscle that occurs after chronic allergen exposure in mice is prevented by tiotropium (34). M2-receptors are also highly expressed in airway smooth muscle cells and inhibit adenylyl cyclase via Gi, thus counteracting the bronchodilator effect of β2-agonists. M2-receptors also counteract β2-agonists by inhibiting KCa in tracheal smooth muscle cells via Gβγ subunits (35). Presynaptic M2-receptors that limit ACh release are defective in animal models of asthma, and this may be secondary to eotaxin release from parasympathetic nerves with neural recruitment of eosinophils that release basic proteins to cause M2-receptor dysfunction (36). There is indirect evidence that presynaptic M2-receptor function is also impaired in asthmatic patients (37).
Glucocorticoids
Glucocorticoids are by far the most effective therapy for controlling asthma, and inhaled corticosteroids (ICS) have become the mainstay of treatment for all patients with persistent symptoms. There has been major progress in understanding the cellular and molecular mechanisms involved in their anti-inflammatory effects in asthma (38).
Anti-inflammatory Mechanisms
Glucocorticoids diffuse across the cell membrane and bind to glucocorticoid receptors (GR) in the cytoplasm (39, 40). Upon ligand binding, GR are activated and released from chaperone proteins (heat shock protein 90 and others) and rapidly translocate to the nucleus, where they exert their molecular effects. The mechanism of nuclear translocation involves the nuclear import proteins importin-α (karyopherin-β) and importin-13 (41, 42). There is only one form of GR that binds glucocorticoids, termed GRα. GRβ is an alternatively spliced form of GR that interacts with DNA but not with glucocorticoids, so it may theoretically act as a dominant-negative inhibitor of glucocorticoid action by interfering with the binding of GR to DNA (43).
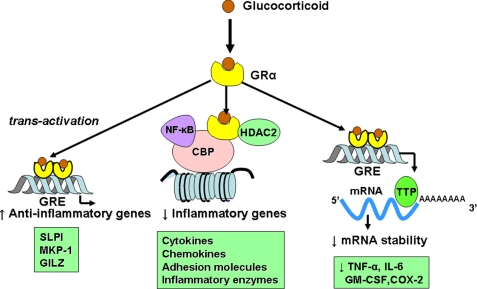
GR homodimerize and bind to glucocorticoid response elements (GRE) in the promoter region of glucocorticoid-responsive genes, and this interaction switches on (or occasionally switches off) gene transcription (Fig. 2). Activation of glucocorticoid-responsive genes occurs via an interaction between the DNA-bound GR and transcriptional coactivator molecules, such as CBP (cAMP-responsive element-binding protein-binding protein), which have intrinsic histone acetyltransferase activity and cause acetylation of core histones (particularly histone 4). This tags histones to recruit chromatin-remodeling engines, such as SWI/SNF, and subsequent association with RNA polymerase II, resulting in gene activation (44, 45). Genes that are switched on by glucocorticoids include genes encoding β2AR and the anti-inflammatory proteins secretory leukoprotease inhibitor (SLPI) and MKP-1 (MAPK phosphatase-1), which inhibits MAPK pathways. These effects may contribute to the anti-inflammatory actions of glucocorticoids (46, 47). GR interaction with negative GRE or with GRE that cross the transcriptional start site may suppress gene transcription, and this may be important in mediating many of the side effects of glucocorticoids, such as inhibition of osteocalcin, which is involved in bone synthesis (48).
FIGURE 2.
Anti-inflammatory effects of glucocorticoids. Glucocorticoids cross the cell membrane and bind to GRα in the cytoplasm, which translocates to the nucleus. GR homodimers bind to GRE in glucocorticoid-responsive genes, which may trans-activate genes encoding anti-inflammatory proteins, such as SLPI, MKP-1, and glucocorticoid-induced leucine zipper (GILZ). GR also interacts with coactivator molecules, such as CBP, which have been activated by proinflammatory transcription factors, such as NF-κB. GR recruits HDAC2, which deacetylates core histones to suppress inflammatory gene transcription. GR also has post-translational effects by increasing the expression of tristetraprolin (TTP), which binds to the AU-rich untranslated ends of mRNAs of some inflammatory cytokines, resulting in destabilization and thus reduced expression of these cytokines.
The major action of glucocorticoids is to switch off multiple activated inflammatory genes that code for cytokines, chemokines, adhesion molecules, inflammatory enzymes, and receptors (49). These genes are switched on in the airways by proinflammatory transcription factors, such as NF-κB and AP-1 (activator protein-1), both of which are usually activated at sites of inflammation in asthma and chronic obstructive pulmonary disease (COPD), resulting in the switching on of multiple inflammatory genes. These genes are activated through interactions with transcriptional coactivator molecules in a manner similar to that described above for GR-mediated gene transcription (38).
Activated GR interact with corepressor molecules to attenuate NF-κB-associated coactivator activity, thus reducing histone acetylation, chromatin remodeling, and RNA polymerase II actions (38, 44). More importantly, reduction of histone acetylation occurs through the specific recruitment of HDAC2 (histone deacetylase-2) to the activated inflammatory gene complex by activated GR, thereby resulting in effective suppression of activated inflammatory genes within the nucleus (Fig. 2). GR becomes acetylated upon ligand binding, allowing it to bind to GRE, and HDAC2 can target acetylated GR, thereby allowing it to associate with the NF-κB complex (50). The site of acetylation of GR is the lysine-rich region −495 to −492 with the sequence KKTK. Site-directed mutagenesis of Lys494 and Lys495 prevents GR acetylation and reduces activation of the SLPI gene by glucocorticoids, whereas repression of NF-κB is unaffected.
Additional mechanisms are also important in the anti-inflammatory actions of glucocorticoids. Glucocorticoids have potent inhibitory effects on MAPK signaling pathways through the induction of MKP-1, and this may inhibit the expression of multiple inflammatory genes (Fig. 2) (46, 47). An important effect of glucocorticoids in the treatment of asthma is through suppression of TH2 cells and TH2 cytokines (IL-4, IL-5, and IL-13), and this may be mediated via inhibition of the transcription factor GATA3, which regulates the transcription of TH2 cytokine genes (51). This is controlled by translocation of GATA3 from the cytoplasm to the nucleus via importin-α after phosphorylation by p38 MAPK. Glucocorticoids potently inhibit GATA3 nuclear translocation, as GR competes for nuclear import via importin-α and also induces MKP-1 to reverse the phosphorylation of GATA3 by p38 MAPK (52). A further immunosuppressive effect of glucocorticoids is through enhanced activity and expression of indoleamine 2,3-dioxygenase, a tryptophan-degrading enzyme that plays a key role in the regulation of T lymphocyte function in allergic diseases though increased secretion of the anti-inflammatory cytokine IL-10 (53). Interestingly, this effect of glucocorticoids on indoleamine 2,3-dioxygenase is further enhanced by statins (54).
Post-transcriptional Effects
Some proinflammatory genes, such as TNF-α, have unstable mRNA that is rapidly degraded by certain RNases but stabilized when cells are stimulated by inflammatory mediators. Glucocorticoids reverse this effect, resulting in rapid degradation of mRNA and reduced inflammatory protein secretion (55). This may be mediated through the increased gene expression of proteins that destabilize mRNAs of inflammatory proteins, such as the zinc finger protein tristetraprolin, which binds to the AU-rich 3′-untranslated region of mRNAs of certain cytokine mRNAs (Fig. 2) (56).
Interaction with β2-Adrenergic Receptors
Inhaled β2-agonists and glucocorticoids are frequently used together (usually as a fixed-combination inhaler of a glucocorticoid with a LABA), and it is now recognized that there are important biochemical interactions between these two classes of drug (57, 58). Glucocorticoids increase transcription of the β2-receptor gene, resulting in increased expression of cell surface receptors. This has been demonstrated in human lung in vitro (59) and nasal mucosa in vivo (60) after topical application of a glucocorticoid. In this way, glucocorticoids protect against the down-regulation of β2-receptors after long-term administration (61). This may be important for the non-bronchodilator effects of β2-agonists, such as mast cell stabilization. Glucocorticoids may also enhance the coupling of β2-receptors to Gs, thus enhancing β2-agonist effects and reversing the uncoupling of β2-receptors that may occur in response to inflammatory mediators, such as IL-1β, through a stimulatory effect on GRK2 (14).
There is increasing evidence that β2-agonists may affect GR function and thus enhance the anti-inflammatory effects of glucocorticoids. LABA increase the translocation of GR from the cytoplasm to the nucleus after activation by glucocorticoids (62). This effect has been demonstrated in sputum macrophages of asthmatic patients after treatment with an inhaled glucocorticoid and inhaled LABA (63). This suggests that LABA and glucocorticoids enhance each other's beneficial effects in asthma therapy, and this may contribute to the greater efficacy of combination inhalers compared with increased doses of ICS in clinical trials (64).
Glucocorticoid Resistance Pathways
Patients with severe asthma and asthmatics who smoke have a poor response to glucocorticoids, which necessitates the need for high doses, and a few patients are completely resistant (65). Several biochemical mechanisms have now been identified to account for glucocorticoid resistance. In smoking asthmatics and patients with severe asthma, there is a reduction in HDAC2 activity and expression, which prevents glucocorticoids from switching off activated inflammatory genes (66, 67). This reduction in HDAC2 may be secondary to oxidative and nitrative stress and the generation of peroxynitrite, which nitrates critical tyrosine residues on HDAC2, leading to its ubiquitination and proteasomal degradation (Fig. 3) (68). Oxidative stress activates PI3Kδ, which results in subsequent HDAC2 phosphorylation and inactivation (69). Hypoxia reduces transcription of the HDAC2 gene via activation of hypoxia-inducible factor-1α, which binds to the promoter region, leading to transcriptional repression (70). Other mechanisms may also contribute to glucocorticoid insensitivity, including reduced translocation of GR as a result of phosphorylation by p38 MAPK (71) and JNK, which phosphorylates GR at Ser226 (72). Some asthmatic patients with severe glucocorticoid resistance show abnormal histone acetylation patterns (73). Another proposed mechanism is that increased GRβ may prevent GRα binding to DNA, but the amounts of GRβ appear to be too low (74). Although glucocorticoids do not bind to GRβ, it is transcriptionally active, and the GR antagonist mifepristone (RU-486) binds to GRβ, making it translocate to the nucleus, but the endogenous ligand of GRβ is currently unidentified (75).
FIGURE 3.
Signaling pathways involved in glucocorticoid resistance in severe asthma and smoking asthmatics. Oxidative and nitrative stress generates peroxynitrite, which nitrates specific tyrosine residues (NO-Tyr) on HDAC2, resulting in its ubiquitination (Ub) and proteasomal degradation. Oxidative stress also activates PI3Kδ, which also leads to subsequent phosphorylation (P) and ubiquitination of HDAC2, resulting in glucocorticoid resistance.
Theophylline
Methylxanthines, such as theophylline, which are related to caffeine, have been used in the treatment of asthma since 1930, and theophylline is still widely used in developing countries because it is inexpensive. However, the frequency of side effects and the relatively low efficacy of theophylline have recently led to reduced usage in many countries because inhaled β2-agonists are more effective as bronchodilators and ICS have greater anti-inflammatory effects. In patients with severe asthma, theophylline still remains a very useful add-on therapy, and there is increasing evidence that it has anti-inflammatory effects and may enhance the anti-inflammatory effects of glucocorticoids (76).
The mechanism of action of theophylline is still uncertain. The bronchodilator effect seen at high plasma concentrations (10–20 mg/liter) is due to inhibition of phosphodiesterases (PDE) in airway smooth muscle, particularly PDE3, which results in increased cAMP concentrations. Inhibition of PDE4 accounts for the common side effects of nausea, diarrhea, and headaches. At therapeutic concentrations, theophylline antagonizes adenosine receptors, particularly A2B-receptors, on mast cells to inhibit adenosine-mediated mediator release (77). Antagonism of A1-receptors may account for the serious side effects of cardiac arrhythmias and seizures. Theophylline prevents the translocation of NF-κB into the nucleus, thus potentially reducing the expression of inflammatory genes in asthma, and this appears to be due to a protective effect against the degradation of the inhibitory protein IκBα (78). However, these effects are seen only at high concentrations and may be mediated by inhibition of PDE. Theophylline promotes apoptosis in eosinophils and neutrophils in vitro. This is associated with a reduction in the anti-apoptotic protein Bcl-2 (79). This effect is not mediated via PDE inhibition but, in neutrophils, may be mediated by antagonism of adenosine A2A-receptors (80).
Theophylline is an activator of histone deacetylases (HDAC) at low therapeutic concentrations (1–5 mg/liter), thus enhancing the anti-inflammatory effects of glucocorticoids in vitro and in animal models in vivo (81, 82). This mechanism is independent of PDE inhibition or adenosine antagonism and appears to be mediated in vitro and in vivo by direct inhibition of oxidant-activated PI3Kδ, which is activated by oxidative stress (69, 83). The anti-inflammatory effects of theophylline are inhibited by the HDAC inhibitor trichostatin A. Low doses of theophylline increase HDAC activity in bronchial biopsies of asthmatic patients and correlate with reduction in eosinophils in the airway wall (81).
Leukotriene Modifiers
Cysteinyl leukotrienes (CysLT) are produced in asthma and have potent effects on airway function, inducing bronchoconstriction, plasma exudation, and mucus secretion, and possibly on eosinophilic inflammation (84). Blocking leukotriene pathways may be beneficial in asthma, and this has led to the development of 5′-lipoxygenase (5-LOX) enzyme inhibitors (of which zileuton is the only drug marketed) and several antagonists of the CysLT1 receptor, including montelukast, zafirlukast, and pranlukast. Although there are clinical improvements in symptoms and lung function and reduced exacerbations, these drugs are significantly less effective than low doses of ICS in asthma but are used because they are effective orally and appear to be safe. The pathways involved in CysLT synthesis are strictly compartmentalized within the cell. Ca2+ stimulates the binding of type IVA cytosolic phospholipase A2 (cPLA2α) to the cell membrane, with release of arachidonic acid, and the binding of 5-LOX to the nuclear membrane, where it co-localizes with FLAP (five-LOX-activating protein) and converts arachidonic acid to LTA4 (Fig. 4). In certain cells that express LTC4 synthase (mast cells and eosinophils), 5-LOX also co-localizes with these enzymes to generate CysLT from LTA4 (85). FLAP, LTC4 synthase, and PLA2 inhibitors are now in development as potential asthma therapies. Secreted PLA2 enzymes cooperate with cPLA2; secreted PLA2-X is increased in the airways of asthmatic patients and potently stimulates CysLT release from eosinophils, which is inhibited by p38 MAPK and JNK inhibitors (86).
FIGURE 4.
Leukotriene modifiers. Cell activation increases intracellular Ca2+ ions, leading to association of cPLA2α with the nuclear membrane, generating arachidonic acid. This activates 5-LOX, which is also located in the nuclear membrane bound to FLAP. This generates LTA4, which is converted by membrane-bound LTC4 synthase (LTC4S) in cells, such as mast cells and eosinophils, into LTC4, LTD4, and LTE4, which then activate CysLT1 receptors on target cells, such as airway smooth muscle cells. This pathway may be blocked by various types of drugs shown in green boxes.
Concluding Remarks
There is now a relatively good understanding of how drugs used to treat asthma work in terms of their biochemical mechanisms, providing opportunities to improve existing treatments and to discover novel therapies in the future (5). For example, once-daily β2-agonists, such as indacaterol and vilanterol, that dissociate more slowly from β2AR have been developed (6). The recognition that β2-agonists may have potentially adverse effects through activating inflammatory pathways via interaction with β-arrestins might lead to the development of biased β2-agonists that are less likely to have this activity and, at the same time, are less likely to result in tolerance (20). Several once-daily anticholinergics have been developed for treating COPD, but they may also be useful in treating patients with severe asthma (33). There are additive effects between β2-agonists and anticholinergics, which may be explained by cross-talk between signaling pathways, such that inhibition of M2-receptors may enhance the stimulatory effect of β2-agonists on adenylyl cyclase, and inhibition of phosphatidylinositol hydrolysis via M3-receptors may also increase signaling effects of β2-agonists. This has led to the development of fixed-combination inhalers that contain a once-daily β2-agonist and anticholinergic (87).
Understanding the biochemical pathways involved in suppression of inflammation by glucocorticoids and the molecular mechanisms of glucocorticoid resistance has led to new therapeutic approaches. Nonsteroidal selective glucocorticoid receptor agonists (so-called dissociated steroids) that target the trans-repression pathway linked to inhibition of NF-κB-induced inflammatory genes with relative sparing of trans-activation pathways that involve DNA binding should theoretically reduce the side effects of glucocorticoids that appear to be mediated mainly via trans-activation (88). In reality, it has been difficult to demonstrate marked dissociation of beneficial and adverse effects with the compounds currently in development. Approaches to treating glucocorticoid resistance in asthma include alternative anti-inflammatory treatments such as MAPK inhibitors and drugs that activate HDAC2, such as theophylline and other PI3Kδ inhibitors. PDE4 inhibitors (now approved in Europe for severe COPD) may be useful in severe asthma (89). Although leukotriene receptor antagonists have been disappointing in the treatment of asthma, drugs acting higher up the leukotriene synthesis pathway, such as 5-LOX and subtype-selective PLA2 inhibitors, might be more effective through inhibiting the synthesis of more inflammatory mediators (90). It has so far proved difficult to development novel anti-inflammatory treatments for asthma that are as safe or effective as ICS, but perhaps a better understanding of the inflammatory signaling pathways in asthmatic airway cells might lead to more effective novel classes of therapy in the future.
This is the fourth article in the Thematic Minireview Series on Molecular Bases of Disease: Asthma. This minireview will be reprinted in the 2011 Minireview Compendium, which will be available in January, 2012.
- TH2
- T-helper 2
- LABA
- long-acting β2-agonist(s)
- β2AR
- β2-adrenergic receptor(s)
- PLC
- phospholipase C
- ACh
- acetylcholine
- ICS
- inhaled corticosteroid(s)
- GR
- glucocorticoid receptor(s)
- GRE
- glucocorticoid response element(s)
- SLPI
- secretory leukoprotease inhibitor
- COPD
- chronic obstructive pulmonary disease
- PDE
- phosphodiesterase(s)
- HDAC
- histone deacetylase(s)
- CysLT
- cysteinyl leukotriene(s)
- 5-LOX
- 5′-lipoxygenase
- cPLA2
- cytosolic phospholipase A2.
REFERENCES
- 1. Barnes P. J. (2008) Nat. Immunol. Rev. 8, 183–192 [DOI] [PubMed] [Google Scholar]
- 2. Hamid Q., Tulic M. (2009) Annu. Rev. Physiol. 71, 489–507 [DOI] [PubMed] [Google Scholar]
- 3. Barnes P. J. (2008) J. Clin. Invest. 118, 3546–3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bateman E. D., Hurd S. S., Barnes P. J., Bousquet J., Drazen J. M., FitzGerald M., Gibson P., Ohta K., O'Byrne P., Pedersen S. E., Pizzichini E., Sullivan S. D., Wenzel S. E., Zar H. J. (2008) Eur. Respir. J. 31, 143–178 [DOI] [PubMed] [Google Scholar]
- 5. Barnes P. J. (2010) Trends Pharmacol. Sci. 31, 335–343 [DOI] [PubMed] [Google Scholar]
- 6. Cazzola M., Matera M. G. (2009) Eur. Respir. J. 34, 757–769 [DOI] [PubMed] [Google Scholar]
- 7. Holz G. G., Kang G., Harbeck M., Roe M. W., Chepurny O. G. (2006) J. Physiol. 577, 5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kassel K. M., Wyatt T. A., Panettieri R. A., Jr., Toews M. L. (2008) Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L131–L138 [DOI] [PubMed] [Google Scholar]
- 9. McGraw D. W., Liggett S. B. (1997) J. Biol. Chem. 272, 7338–7344 [DOI] [PubMed] [Google Scholar]
- 10. Mak J. C., Hisada T., Salmon M., Barnes P. J., Chung K. F. (2002) Br. J. Pharmacol. 135, 987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duffy S. M., Cruse G., Lawley W. J., Bradding P. (2005) FASEB J. 19, 1006–1008 [DOI] [PubMed] [Google Scholar]
- 12. Cruse G., Yang W., Duffy S. M., Chachi L., Leyland M., Amrani Y., Bradding P. (2010) J. Allergy Clin. Immunol. 125, 257–263 [DOI] [PubMed] [Google Scholar]
- 13. Howarth P. H., Beckett P., Dahl R. (2000) Respir. Med. 94, S22–S25 [DOI] [PubMed] [Google Scholar]
- 14. Mak J. C., Chuang T. T., Harris C. A., Barnes P. J. (2002) Eur. J. Pharmacol. 436, 165–172 [DOI] [PubMed] [Google Scholar]
- 15. Hawkins G. A., Weiss S. T., Bleecker E. R. (2008) Pharmacogenomics 9, 349–358 [DOI] [PubMed] [Google Scholar]
- 16. Israel E., Chinchilli V. M., Ford J. G., Boushey H. A., Cherniack R., Craig T. J., Deykin A., Fagan J. K., Fahy J. V., Fish J., Kraft M., Kunselman S. J., Lazarus S. C., Lemanske R. F., Jr., Liggett S. B., Martin R. J., Mitra N., Peters S. P., Silverman E., Sorkness C. A., Szefler S. J., Wechsler M. E., Weiss S. T., Drazen J. M. (2004) Lancet 364, 1505–1512 [DOI] [PubMed] [Google Scholar]
- 17. Bleecker E. R., Postma D. S., Lawrance R. M., Meyers D. A., Ambrose H. J., Goldman M. (2007) Lancet 370, 2118–2125 [DOI] [PubMed] [Google Scholar]
- 18. McGraw D. W., Almoosa K. F., Paul R. J., Kobilka B. K., Liggett S. B. (2003) J. Clin. Invest. 112, 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou Y., Sondek J., Harden T. K. (2008) Biochemistry 47, 4410–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Violin J. D., Lefkowitz R. J. (2007) Trends Pharmacol. Sci. 28, 416–422 [DOI] [PubMed] [Google Scholar]
- 21. Shenoy S. K., McDonald P. H., Kohout T. A., Lefkowitz R. J. (2001) Science 294, 1307–1313 [DOI] [PubMed] [Google Scholar]
- 22. Schmid C. L., Bohn L. M. (2009) Pharmacol. Ther. 121, 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeWire S. M., Ahn S., Lefkowitz R. J., Shenoy S. K. (2007) Annu. Rev. Physiol. 69, 483–510 [DOI] [PubMed] [Google Scholar]
- 24. Nelson H. S., Dorinsky P. M. (2006) Ann. Intern. Med. 145, 706–710 [DOI] [PubMed] [Google Scholar]
- 25. Nguyen L. P., Lin R., Parra S., Omoluabi O., Hanania N. A., Tuvim M. J., Knoll B. J., Dickey B. F., Bond R. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 2435–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanania N. A., Singh S., El-Wali R., Flashner M., Franklin A. E., Garner W. J., Dickey B. F., Parra S., Ruoss S., Shardonofsky F., O'Connor B. J., Page C., Bond R. A. (2008) Pulm. Pharmacol. Ther. 21, 134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walker J. K., Fong A. M., Lawson B. L., Savov J. D., Patel D. D., Schwartz D. A., Lefkowitz R. J. (2003) J. Clin. Invest. 112, 566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hollingsworth J. W., Theriot B. S., Li Z., Lawson B. L., Sunday M., Schwartz D. A., Walker J. K. (2010) Am. J. Respir. Cell Mol. Biol. 43, 269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gong K., Li Z., Xu M., Du J., Lv Z., Zhang Y. (2008) J. Biol. Chem. 283, 29028–29036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wessler I., Kirkpatrick C. J. (2008) Br. J. Pharmacol. 154, 1558–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kummer W., Lips K. S., Pfeil U. (2008) Histochem. Cell Biol. 130, 219–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bateman E. D., Rennard S., Barnes P. J., Dicpinigaitis P. V., Gosens R., Gross N. J., Nadel J. A., Pfeifer M., Racké K., Rabe K. F., Rubin B. K., Welte T., Wessler I. (2009) Pulm. Pharmacol. Ther. 22, 533–542 [DOI] [PubMed] [Google Scholar]
- 33. Peters S. P., Kunselman S. J., Icitovic N., Moore W. C., Pascual R., Ameredes B. T., Boushey H. A., Calhoun W. J., Castro M., Cherniack R. M., Craig T., Denlinger L., Engle L. L., DiMango E. A., Fahy J. V., Israel E., Jarjour N., Kazani S. D., Kraft M., Lazarus S. C., Lemanske R. F., Jr., Lugogo N., Martin R. J., Meyers D. A., Ramsdell J., Sorkness C. A., Sutherland E. R., Szefler S. J., Wasserman S. I., Walter M. J., Wechsler M. E., Chinchilli V. M., Bleecker E. R. (2010) N. Engl. J. Med. 363, 1715–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bos I. S., Gosens R., Zuidhof A. B., Schaafsma D., Halayko A. J., Meurs H., Zaagsma J. (2007) Eur. Respir. J. 30, 653–661 [DOI] [PubMed] [Google Scholar]
- 35. Zhou X. B., Wulfsen I., Lutz S., Utku E., Sausbier U., Ruth P., Wieland T., Korth M. (2008) J. Biol. Chem. 283, 21036–21044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fryer A. D., Stein L. H., Nie Z., Curtis D. E., Evans C. M., Hodgson S. T., Jose P. J., Belmonte K. E., Fitch E., Jacoby D. B. (2006) J. Clin. Invest. 116, 228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Minette P. A., Lammers J. W., Dixon C. M., McCusker M. T., Barnes P. J. (1989) J. Appl. Physiol. 67, 2461–2465 [DOI] [PubMed] [Google Scholar]
- 38. Barnes P. J. (2006) Br. J. Pharmacol. 148, 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rhen T., Cidlowski J. A. (2005) N. Engl. J. Med. 353, 1711–1723 [DOI] [PubMed] [Google Scholar]
- 40. Nicolaides N. C., Galata Z., Kino T., Chrousos G. P., Charmandari E. (2010) Steroids 75, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goldfarb D. S., Corbett A. H., Mason D. A., Harreman M. T., Adam S. A. (2004) Trends Cell Biol. 14, 505–514 [DOI] [PubMed] [Google Scholar]
- 42. Tao T., Lan J., Lukacs G. L., Haché R. J., Kaplan F. (2006) Am. J. Respir. Cell Mol. Biol. 35, 668–680 [DOI] [PubMed] [Google Scholar]
- 43. Lewis-Tuffin L. J., Cidlowski J. A. (2006) Ann. N.Y. Acad. Sci. 1069, 1–9 [DOI] [PubMed] [Google Scholar]
- 44. Ito K., Barnes P. J., Adcock I. M. (2000) Mol. Cell. Biol. 20, 6891–6903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. John S., Sabo P. J., Johnson T. A., Sung M. H., Biddie S. C., Lightman S. L., Voss T. C., Davis S. R., Meltzer P. S., Stamatoyannopoulos J. A., Hager G. L. (2008) Mol. Cell 29, 611–624 [DOI] [PubMed] [Google Scholar]
- 46. Barnes P. J. (2006) Eur. Respir. J. 27, 413–426 [DOI] [PubMed] [Google Scholar]
- 47. Clark A. R. (2003) J. Endocrinol. 178, 5–12 [DOI] [PubMed] [Google Scholar]
- 48. Dostert A., Heinzel T. (2004) Curr. Pharm. Des. 10, 2807–2816 [DOI] [PubMed] [Google Scholar]
- 49. Barnes P. J., Adcock I. M. (2003) Ann. Intern. Med. 139, 359–370 [DOI] [PubMed] [Google Scholar]
- 50. Ito K., Yamamura S., Essilfie-Quaye S., Cosio B., Ito M., Barnes P. J., Adcock I. M. (2006) J. Exp. Med. 203, 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maneechotesuwan K., Xin Y., Ito K., Jazrawi E., Lee K. Y., Usmani O. S., Barnes P. J., Adcock I. M. (2007) J. Immunol. 178, 2491–2498 [DOI] [PubMed] [Google Scholar]
- 52. Maneechotesuwan K., Yao X., Ito K., Jazrawi E., Usmani O. S., Adcock I. M., Barnes P. J. (2009) PLoS Med. 6, e1000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maneechotesuwan K., Supawita S., Kasetsinsombat K., Wongkajornsilp A., Barnes P. J. (2008) J. Allergy Clin. Immunol. 121, 43–50 [DOI] [PubMed] [Google Scholar]
- 54. Maneechotesuwan K., Ekjiratrakul W., Kasetsinsombat K., Wongkajornsilp A., Barnes P. J. (2010) J. Allergy Clin. Immunol. 126, 754–762 [DOI] [PubMed] [Google Scholar]
- 55. Bergmann M. W., Staples K. J., Smith S. J., Barnes P. J., Newton R. (2004) Am. J. Respir. Cell Mol. Biol. 30, 555–563 [DOI] [PubMed] [Google Scholar]
- 56. Smoak K., Cidlowski J. A. (2006) Mol. Cell. Biol. 26, 9126–9135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barnes P. J. (2002) Eur. Respir. J. 19, 182–191 [DOI] [PubMed] [Google Scholar]
- 58. Newton R., Leigh R., Giembycz M. A. (2010) Pharmacol. Ther. 125, 286–327 [DOI] [PubMed] [Google Scholar]
- 59. Mak J. C., Nishikawa M., Barnes P. J. (1995) Am. J. Physiol. 268, L41–L46 [DOI] [PubMed] [Google Scholar]
- 60. Baraniuk J. N., Ali M., Brody D., Maniscalco J., Gaumond E., Fitzgerald T., Wong G., Yuta A., Mak J. C., Barnes P. J., Bascom R., Troost T. (1997) Am. J. Respir. Crit. Care Med. 155, 704–710 [DOI] [PubMed] [Google Scholar]
- 61. Mak J. C., Nishikawa M., Shirasaki H., Miyayasu K., Barnes P. J. (1995) J. Clin. Invest. 96, 99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roth M., Johnson P. R., Rüdiger J. J., King G. G., Ge Q., Burgess J. K., Anderson G., Tamm M., Black J. L. (2002) Lancet 360, 1293–1299 [DOI] [PubMed] [Google Scholar]
- 63. Usmani O. S., Ito K., Maneechotesuwan K., Ito M., Johnson M., Barnes P. J., Adcock I. M. (2005) Am. J. Respir. Crit. Care Med. 172, 704–712 [DOI] [PubMed] [Google Scholar]
- 64. Gibson P. G., Powell H., Ducharme F. M. (2007) J. Allergy Clin. Immunol. 119, 344–350 [DOI] [PubMed] [Google Scholar]
- 65. Barnes P. J., Adcock I. M. (2009) Lancet 373, 1905–1917 [DOI] [PubMed] [Google Scholar]
- 66. Hew M., Bhavsar P., Torrego A., Meah S., Khorasani N., Barnes P. J., Adcock I., Chung K. F. (2006) Am. J. Respir. Crit. Care Med. 174, 134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barnes P. J. (2009) Annu. Rev. Physiol. 71, 451–464 [DOI] [PubMed] [Google Scholar]
- 68. Osoata G. O., Yamamura S., Ito M., Vuppusetty C., Adcock I. M., Barnes P. J., Ito K. (2009) Biochem. Biophy. Res. Commun. 384, 366–371 [DOI] [PubMed] [Google Scholar]
- 69. To Y., Ito K., Kizawa Y., Failla M., Ito M., Kusama T., Elliott W. M., Hogg J. C., Adcock I. M., Barnes P. J. (2010) Am. J. Resp. Crit. Care Med. 182, 897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Charron C. E., Chou P. C., Coutts D. J., Kumar V., To M., Akashi K., Pinhu L., Griffiths M., Adcock I. M., Barnes P. J., Ito K. (2009) J. Biol. Chem. 284, 36047–36054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Irusen E., Matthews J. G., Takahashi A., Barnes P. J., Chung K. F., Adcock I. M. (2002) J. Allergy Clin. Immunol. 109, 649–657 [DOI] [PubMed] [Google Scholar]
- 72. Ismaili N., Garabedian M. J. (2004) Ann. N.Y. Acad. Sci. 1024, 86–101 [DOI] [PubMed] [Google Scholar]
- 73. Matthews J. G., Ito K., Barnes P. J., Adcock I. M. (2004) J. Allergy Clin. Immunol. 113, 1100–1108 [DOI] [PubMed] [Google Scholar]
- 74. Pujols L., Mullol J., Picado C. (2007) Curr. Allergy Asthma Rep. 7, 93–99 [DOI] [PubMed] [Google Scholar]
- 75. Lewis-Tuffin L. J., Jewell C. M., Bienstock R. J., Collins J. B., Cidlowski J. A. (2007) Mol. Cell. Biol. 27, 2266–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Barnes P. J. (2003) Am. J. Respir. Crit. Care Med. 167, 813–818 [DOI] [PubMed] [Google Scholar]
- 77. Wilson C. N. (2008) Br. J. Pharmacol. 155, 475–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ichiyama T., Hasegawa S., Matsubara T., Hayashi T., Furukawa S. (2001) Naunyn-Schmiedebergs Arch. Pharmacol. 364, 558–561 [DOI] [PubMed] [Google Scholar]
- 79. Chung I. Y., Nam-Kung E. K., Lee N. M., Chang H. S., Kim D. J., Kim Y. H., Park C. S. (2000) Cell. Immunol. 203, 95–102 [DOI] [PubMed] [Google Scholar]
- 80. Yasui K., Agematsu K., Shinozaki K., Hokibara S., Nagumo H., Nakazawa T., Komiyama A. (2000) J. Leukocyte Biol. 67, 529–535 [DOI] [PubMed] [Google Scholar]
- 81. Ito K., Lim S., Caramori G., Cosio B., Chung K. F., Adcock I. M., Barnes P. J. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 8921–8926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cosio B. G., Tsaprouni L., Ito K., Jazrawi E., Adcock I. M., Barnes P. J. (2004) J. Exp. Med. 200, 689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Marwick J. A., Caramori G., Stevenson C. S., Casolari P., Jazrawi E., Barnes P. J., Ito K., Adcock I. M., Kirkham P. A., Papi A. (2009) Am. J. Respir. Crit. Care Med. 179, 542–548 [DOI] [PubMed] [Google Scholar]
- 84. Peters-Golden M., Henderson W. R., Jr. (2007) N. Engl. J. Med. 357, 1841–1854 [DOI] [PubMed] [Google Scholar]
- 85. Newcomer M. E., Gilbert N. C. (2010) J. Biol. Chem. 285, 25109–25114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lai Y., Oslund R. C., Bollinger J. G., Henderson W. R., Jr., Santana L. F., Altemeier W. A., Gelb M. H., Hallstrand T. S. (2010) J. Biol. Chem. 285, 41491–41500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. van Noord J. A., Buhl R., Laforce C., Martin C., Jones F., Dolker M., Overend T. (2010) Thorax 65, 1086–1091 [DOI] [PubMed] [Google Scholar]
- 88. Schäcke H., Berger M., Rehwinkel H., Asadullah K. (2007) Mol. Cell. Endocrinol. 275, 109–117 [DOI] [PubMed] [Google Scholar]
- 89. Hatzelmann A., Morcillo E. J., Lungarella G., Adnot S., Sanjar S., Beume R., Schudt C., Tenor H. (2010) Pulm. Pharmacol. Ther. 23, 235–256 [DOI] [PubMed] [Google Scholar]
- 90. Garcia-Garcia H. M., Serruys P. W. (2009) Curr. Opin. Lipidol. 20, 327–332 [DOI] [PubMed] [Google Scholar]