Background: The androgen signaling pathway mediated through the AR is critical in prostate tumorigenesis. However, the precise role of AR in prostate tumorigenesis still remains largely unknown. Specifically, it is unclear whether overexpression of AR is sufficient to induce prostate tumor formation in vivo.
Results: Conditional expression of the human AR transgene in R26hARloxP:Osr1-Cre+ mice induces mouse prostatic intraepithelial neoplasia (mPIN) and prostatic adenocarcinoma formation.
Conclusion: We demonstrated that conditional expression of transgenic AR induces prostate tumor formation in mice.
Significance: This new AR transgenic mouse line mimics the human prostate cancer and can be used for study of prostate tumorigenesis and drug development.
Keywords: Androgen, Androgen Receptor, Prostate Cancer, Transcription, Transgenic Mice, Osr-cre
Abstract
The androgen signaling pathway, mediated through the androgen receptor (AR), is critical in prostate tumorigenesis. However, the precise role of AR in prostate cancer development and progression still remains largely unknown. Specifically, it is unclear whether overexpression of AR is sufficient to induce prostate tumor formation in vivo. Here, we inserted the human AR transgene with a LoxP-stop-loxP (LSL) cassette into the mouse ROSA26 locus, permitting “conditionally” activated AR transgene expression through Cre recombinase-mediated removal of the LSL cassette. By crossing this AR floxed strain with Osr1-Cre (odd skipped related) mice, in which the Osr1 promoter activates at embryonic day 11.5 in urogenital sinus epithelium, we generated a conditional transgenic line, R26hARloxP:Osr1-Cre+. Expression of transgenic AR was detected in both prostatic luminal and basal epithelial cells and is resistant to castration. Approximately one-half of the transgenic mice displayed mouse prostatic intraepithelial neoplasia (mPIN) lesions. Intriguingly, four mice (10%) developed prostatic adenocarcinomas, with two demonstrating invasive diseases. Positive immunostaining of transgenic AR protein was observed in the majority of atypical and tumor cells in the mPIN and prostatic adenocarcinomas, providing a link between transgenic AR expression and oncogenic transformation. An increase in Ki67-positive cells appeared in all mPIN and prostatic adenocarcinoma lesions of the mice. Thus, we demonstrated for the first time that conditional activation of transgenic AR expression by Osr1 promoter induces prostate tumor formation in mice. This new AR transgenic mouse line mimics the human disease and can be used for study of prostate tumorigenesis and drug development.
Introduction
Prostate cancer is the most common malignancy among males in the Western world and affects about 2,276,000 men in the United States. Androgen signaling promotes prostate cancer development and progression (1, 2). Androgens exert their biological effects mainly through the androgen receptor (AR),2 a member of the steroid hormone receptor superfamily (3). The AR is expressed in virtually all primary prostate cancers and in most castration-resistant prostate cancers (CRPCs) (4, 5). AR proteins containing shorter polyglutamine tracts are more transcriptionally active and correlate with an increased risk of developing primary and advanced prostate cancers (6–8). Higher testosterone levels and lower levels of sex steroid-binding globulin, which sequesters androgens, also increases the risk of prostate cancer (9, 10). AR gene amplification appears in almost one-third of prostate cancers after androgen ablation therapy (11, 12). Global gene expression profiling shows AR as the only gene to be consistently up-regulated in CRPCs (13). Mutations within the AR gene and dysregulation of AR co-regulators have also been identified in a significant portion of CRPCs (14, 15). These multiple lines of evidence elucidate AR action as a critical determinant of prostate cancer initiation, invasion, and metastasis.
In the past decade, significant effort has been devoted to generating relevant animal models to characterize the biological significance of the AR-signaling pathway in prostate tumorigenesis. In rat models, administration of testosterone has been shown to increase the expression of AR in prostate epithelium, and castration causes down-regulation of AR (16). The effects of selectively increasing AR expression in prostate epithelium have been assessed using transgenic mice. Mice expressing the mouse AR gene driven by the probasin promoter developed focal areas of prostatic intraepithelial neoplasia (PIN) at old age (17). However, no other proliferative lesions, including overt neoplasia, were observed in this and other similar AR transgenic mouse models. Additionally, only androgen-regulated effects can be evaluated in these mouse models because expression of the AR transgene was regulated in a ligand-dependent manner. Therefore, there is an urgent need for developing biologically relevant animal models that can mimic the human disease and be used for characterizing the molecular mechanisms underlying CRPCs and for drug development.
In this study, we developed a conditional AR transgenic mouse strain in which the human AR transgene was specifically targeted into the ROSA26 locus (18, 19). Expression of the AR transgene in this mouse model can be achieved in a constitutive manner through the activation of a Cre recombinase. It has been shown that the mouse Osr1 (odd skipped related) promoter becomes active at embryonic day 11.5 in urogenital sinus epithelium and maintains its activity in prostatic epithelial cells of prostate glands throughout development (20). We generated a conditional AR transgenic mouse line (R26hARloxP:Osr1-Cre+) by intercrossing the AR floxed mice with Osr1-Cre line. The specific expression of the AR transgene was detected in both luminal and basal epithelial cells of mouse prostatic glands. A total 18 of 40 transgenic mice showed PIN lesions between 6 and 20 months of age. Most intriguingly, four mice developed prostatic adenocarcinomas, two of which demonstrated an invasive phenotype. Histological analyses showed specific expression of the human AR transgene in both PIN and adenocarcinomas, providing a link between transgenic AR expression and tumorigenic transformation in the prostate of these transgenic mice. Our data demonstrate that this AR transgenic mouse model is a new and unique strain that can be used to characterize AR action in prostate tumorigenesis and drug development.
EXPERIMENTAL PROCEDURES
Generation of the Target Vector
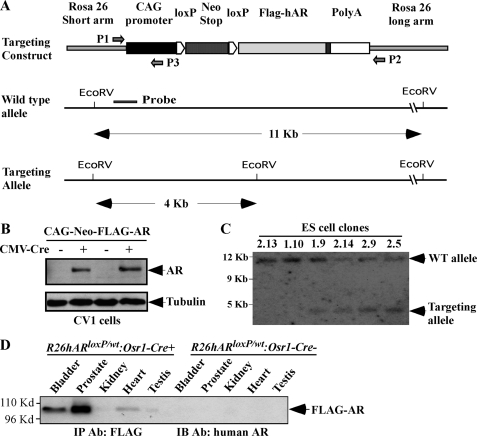
The targeting construct used to generate this AR transgenic line, pROSA26-1, is a gift from Dr. Philippe M. Soriano. A loxP-PGK-neomycin-STOP-loxP cassette was inserted between the CMV early enhancer/chicken β-actin (CAG) promoter and the human AR coding sequence with nine polyglutamine repeats followed by a polyadenylation signal (see Fig. 1A). The targeting construct was linearized by PacI digestion. DNA (25 μg) was electroporated into R1 ES cells as described previously (21, 22). Correctly targeted ES clones were screened by their acquired puromycin resistance (positive selection) and identified by genomic PCR with P1 (5′-TCCTCAGAGAGCCTCGGCTAGGTAG-3′) and P2 (5′-TCTGTCTAGGGGTTGGATAAGCCAG-3′) primers. Southern blot analyses were performed for further confirmation as described previously (18, 23). In brief, genomic DNA of the ES clones was digested with EcoRV and subjected to Southern blotting with the DNA probe as indicated in Fig. 1A.
FIGURE 1.
Generating the AR conditional transgenic mice. A, a scheme of the conditional human AR transgene targeting construct is shown. A PGK-neomycin cassette with flanked loxP sites (LSL cassette) was inserted between the CAG promoter and a FLAG-tagged human AR coding sequence containing a nine-polyglutamine repeat tract. The DNA fragment isolated from the short arm region used as the probe in the Southern blot is marked as a solid line. The primers used for genotyping are marked with arrows. B, CV-1 cells were transfected with the targeting vector plasmid in the presence or absence of CMV-Cre expression vector to assess the activation of the AR transgene expression through the loxP/Cre recombination. Western blot was performed on cell lysates using the antibody against the human AR or β-tubulin. The expression of FLAG-tagged AR protein was detected in cells co-transfected with CMV-Cre vectors, demonstrating that a loxP/Cre recombination can result in the deletion of the LSL cassette and activation of AR transgene expression. C, Southern blot analysis was performed to examine ES cells transfected with the targeting vectors. Genomic DNA was digested by EcoRV and hybridized to a 32P-labeled probe (represented in Fig. 1a) located on the short arm. The wild type allele and targeting allele were differentiated by size and labeled. In four clones, two bands of expected size, 11.5 and 3.8 kb, were detected, representing the endogenous and targeted ROSA26 locus, respectively. D, whole protein lysates were isolated from different mouse tissues of 16-week-old R26hARloxP/wt:Osr1-Cre+ and R26hARloxP/wt:Osr1-Cre− mice. Equal amounts of protein lysates were subjected to immunoprecipitation (IP) with FLAG antibody and then analyzed by Western blotting with a specific antibody against the human AR to detect the specific expression of the human AR transgene. IB, immunoblot.
Mouse Breeding, Genotyping, and Manipulation (Castration)
The production of chimeras from the ES cells was done as described previously (24). Progenies were genotyped and male chimeras were identified. Germ line transmission was achieved by backcrossing male chimeras to wild type C57BL/6J females. Daughters carrying the targeted allele were bred to C57BL/6J males. To generate the conditional AR transgenic mice, we intercrossed R26hARloxP/wt mice with the Osr1-Cre strain, which was kindly provided by Dr. Gail Martin at UCSF and backcrossed more than seven times with C57BL/6J mice (20), and PB-Cre4 mice, carrying the Cre transgene under the control of a modified probasin promoter (ARR2PB) (25). For genotyping, mouse tail tips were incubated in lysis buffer (catalog number 102-T; Viagen Biotech, Los Angeles, CA) overnight at 55 °C, and briefly spun down. Genomic DNA was dissolved in TE buffer. Three primers that can distinguish the wild type from the mutant allele were used for genomic PCR amplification. The forward primer, 5′-TCCTCAGAGAGCCTCGGCTAGGTAG-3′, was used for both the wild type and targeted alleles, and the reverse primer for the wild type allele was 5′-TCTGTCTAGGGGTTGGATAAGCCAG-3′, and that for targeted allele was 5′-CCGTAAGTTATGTAACGCGGAACTC-3′. To assess recombination, the forward primer 5′-TTCGGCTTCTGGCGTGTGAC-3′ and the reverse primer 5′-GCTGTGATGATGCGGTAGTC-3′ were used in genomic PCR. PCR fragments were amplified at 95 °C for 5 min; then 95 °C for 45 s, 63 °C for 50 s, and 72 °C for 45 s for 40 cycles; and then 72 °C for 5 min.
For castration, either the AR conditional transgenic or control mice were anesthetized by intraperitoneal injection of ketamine and xylazine. Both testicles and epididmides were removed through a scrotal approach. The distal end of the spermatic cord was ligated with silk thread as described previously (26). All of the animal experiments performed in this study were approved by the Administrative Panel on Laboratory Animal Care at Stanford University.
Immunohistochemistry, Immunofluorescence, and Histological Analyses
Mouse tissues were fixed in 10% neutral-buffered formalin and processed into paraffin for immunohistochemistry. Samples were cut into 5-μm sections, deparaffinized in xylene, and rehydrated using a decreasing ethanol gradient followed by PBS. Tissues were then blocked with 3% hydrogen peroxide in methanol and protein blocked for 15 min each to inhibit endogenous peroxidase activity and nonspecific antibody binding, respectively. Samples were exposed to a 1:500 dilution of anti-human AR antibody (Santa Cruz Biotechnology; sc-7305), 1:500 dilution of anti-mouse/human AR (Santa Cruz; sc-816, N-20), 1:300 dilution of anti-p63 antibody (Santa Cruz; sc-8431), 1:3000 of anti Ki67 antibody (Novacastsra; NCL-ki67), 1:300 of E-cadherin antibody (Transduction Laboratories; c20820), 1:800 of CK-5 antibody (Covance; PRB-160P), 1:800 of CK8 antibody (Covance; MMS-162P), and 1:200 of synaptophysin antibody (Invitrogen; Z66) in 1% of goat serum at 4 °C overnight. The slides were then incubated with biotinylated anti-rabbit or anti-mouse secondary antibody (Vector Laboratories; BA-1000 or BA-9200) for 1 h and horseradish peroxidase streptavidin (Vector Laboratories; SA-5004) for 30 min at room temperature and then visualized by DAB kit (Vector Laboratories; SK-4100). Slides were subsequently counterstained with 5% (w/v) Harris hematoxylin. For histological analysis, 5-μm serial sections were processed from xylene to water through a decreasing ethanol gradient, stained with hematoxylin and eosin, and processed back to xylene through an increasing ethanol gradient. For immunofluorescence assays, 5-μm sections were boiled in 0.01 m citrate buffer (pH 6.0) for 20 min after redehydration from xylene to water, and blocked by 5% goat serum. Tissue sections were then incubated with 1:300 dilution of anti-human AR antibody (Santa Cruz Biotechnology; sc-7305), 1:500 dilution of anti-mouse/human AR (Santa Cruz; sc-816), or 1:300 dilution of anti-p63 antibody (Santa Cruz; sc-8343) in 1% of goat serum at 4 °C overnight. Goat anti-mouse Alexa Fluor 594 (Molecular Probes; A21203), or goat anti-rabbit Alexa Fluor 488 (Molecular Probes; A11034) was incubated at 1:1000 dilution for 1 h at room temperature. Sections were mounted by VECTASHIELD mounting medium with DAPI (Vector Laboratories; H-1200). Images for all hematoxylin and eosin and immunohistochemistry experiments in this study were acquired on a Leica dissecting microscope (model MZ95) using Zeiss Axiovision software. Immunofluorescence images were taken using an Olympus BX-52 microscope.
Immunoprecipitation and Western Blotting
Mouse tissues were homogenized in ice-cold radioimmune precipitation assay buffer (150 mm sodium chloride, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mm Tris, pH 8.0). Protein concentrations were measured using a protein assay kit (Bio-Rad; catalog number 500-0006). For immunoprecipitation assays, cell lysates containing 150 μg of total protein were diluted in buffer containing 20 mm HEPES (pH 8.0), 0.5% Nonidet P-40, 100 mm NaCl, 1 mm EDTA, 5 mm MgCl2, 1 mm CaCl2, 10 mm ZnCl2, 1 mm DTT, 1 mm PMSF, 5 mg/ml leupeptin, and 5% glycerol and then incubated with rabbit normal IgG or anti-FLAG antibody conjugated with pre-equilibrated protein A-Sepharose beads at 4 °C with gentle rotation for 7 h. The beads were collected by centrifugation and gently washed three times with the same buffer as described above. Equal amounts of immunoprecipitates were eluted using 2× sample buffer (125 mm Tris-HCl, pH 6.8, 4% SDS, 20% (v/v) glycerol, 0.004% bromphenol blue) and analyzed by Western blot. A 1:500 dilution of a monoclonal antibody against the human AR (Santa Cruz Biotechnology; catalog number sc-7305) was used. Protein detection was performed using ECL kit according to the manufacturer's protocol (Amersham Biosciences).
Cell Cultures and Transient Transfections
The monkey kidney cell line, CV-1, was maintained in DMEM supplemented with 5% FCS (HyClone, Denver, CO). Transient transfections were carried out using a Lipofectamine transfection kit (Invitrogen). Transfection and whole cell collection were performed as described previously (27, 28). Whole cell lysates were prepared from transfected cells and subjected to Western blot analyses.
Statistical Analyses
We presented the data as the means ± S.D. We made comparisons between groups, using a two-sided Student's t test. p < 0.05 and p < 0.01 were considered significant.
RESULTS
Generation of the AR Conditional Transgenic Mice
Previous AR transgenic mouse models were developed through random insertion transgenesis in ES cells or pronuclear microinjection (17). To achieve high targeting efficiency and comparable expression of the AR transgene between animals, we developed a “floxed” AR allele in which the human AR transgene containing a short polyglutamine repeat tract was targeted into the ROSA26 locus (18, 19). A loxP flanked transcriptional silencing element was inserted between the CAG promoter, a hybrid CMV enhancer coupled to a modified chicken β-actin promoter, and the AR coding sequence in the targeting vector (Fig. 1A). Because the CAG promoter is ubiquitously active in most mouse tissues in vivo (29), AR transgene expression in this mouse model can be achieved in a constitutive but tissue-specific manner through Cre-recombinase-mediated removal of the LSL cassette. Thus, this mouse model will enable us to characterize the specific role of AR in prostate tissues in a ligand-independent manner to avoid the complication observed in previous mouse models regulated by androgen-induced promoters. AR transgene expression was assessed in CV-1 cells through recombinase-mediated removal of the transcriptional silencer, the LSL cassette. Expression of FLAG-tagged AR was observed in the cells co-transfected with the targeted vector and CMV-Cre plasmids (Fig. 1B). Genomic DNA samples were isolated from ES cells and digested with EcoRV and subjected to Southern blot analyses (18, 23). Four positive clones displayed a 11.5-kb hybridization band corresponding to the wild type locus and a 3.8-kb band that represents the targeted ROSA26 locus (Fig. 1C). Two independent positive ES cell clones were injected into C57BL/6J blastocysts that were then implanted into pseudopregnant recipients to create chimeric animals.
Conditional Expression of the AR Transgene in Mouse Prostatic Epithelium
The Osr1-Cre mouse line is a newly established tool strain, in which the Osr1 promoter activates at embryonic day 11.5 in urogenital sinus epithelium and retains its activity in epithelium of the prostate throughout development (20). Although the activity of the Osr1 promoter is not fully restricted to the prostate gland, its early activation in prostatic epithelial cells makes Osr1-Cre a unique tool in assessing AR action in prostate development and tumorigenesis. We crossed the floxed AR strain with Osr1-Cre mice to generate both R26hARloxP/loxP:Osr1-Cre+ and R26hARloxP/wt:Osr1-Cre+ mice. Using genomic PCR approaches, we examined the activity of Osr1-Cre in the prostate gland of R26hARloxP/wt:Osr1-Cre+ mice at different ages. We observed a 300-bp PCR fragment corresponding to the deletion of the LSL cassette through loxP/Cre recombination in four prostatic lobes and the bladder of R26hARloxP/wt:Osr1-Cre+ mice at 4, 8, and 24 weeks of age and at a low level in the testis and kidney of 8 and 12-week-old mice (supplemental Fig. S1). In contrast, only a 1.6-kb nonrecombined fragment was observed in mouse tissues isolated from age matched R26hARloxP/wt:Osr1-Cre− controls. To confirm the expression of transgenic human AR protein in the mice through LoxP/Cre recombination, we then analyzed different mouse tissues using immunoprecipitation and immunoblotting. As shown in Fig. 1D, FLAG tagged human AR proteins were detected in the prostate gland, bladder, and heart of 4-week-old R26hARloxP/wt:Osr1-Cre+ mice. Among these tissues, the prostate gland showed the highest expression of AR protein when samples containing equal amounts of total proteins from different tissues were used. There is no expression in the samples isolated from age matched R26hARloxP/wt:Osr1-Cre− control mice. These results demonstrate that the Osr1-Cre transgene can selectively activate expression of the human AR transgene in the prostate gland and other tissues, which is consistent with previous reports (20).
Next, we performed immunohistochemistry to visualize transgenic AR expression in R26hARloxP/wt:Osr1-Cre+ mice. Using an antibody specifically against the human AR protein (441; Santa Cruz; sc-7305), we surveyed transgenic AR expression in the transgenic mice. We observed clear nuclear staining of human AR protein in luminal cells of all four prostate lobes, including anterior, dorsal, lateral, and ventral prostate in 8-week-old male R26hARloxP/wt:Osr1-Cre+ and R26hARloxP/loxP:Osr1-Cre+mice, but not in age-matched R26hARloxP/wt:Osr1-Cre− control mice. Representative images are shown in Fig. 2. There is no significant difference in intensity between heterozygous (R26hARloxP/wt:Osr1-Cre+) and homozygous (R26hARloxP/loxP:Osr1-Cre+) mice. Notably, staining of AR was limited to a portion of epithelial cells in some prostate glands. Using immunofluorescence, we further investigated expression of the AR transgene in the prostate tissues of differently aged mice. The immunofluorescence signal of the human AR protein appeared consistently in all prostate lobes between 4- and 48-week-old R26hARloxP/wt:Osr1-Cre+ mice (data not shown).
FIGURE 2.
Expression of the AR transgene in R26hARloxP:Osr1-Cre+ mice. Immunohistochemistry staining was used to assess expression of the human AR transgene in the AR transgenic mice. Different paraffin-embedded prostate lobes dissected from 8-week-old male R26hARloxP/wt:Osr1-Cre+, R26hARloxP/loxP:Osr1-Cre+, and R26hARloxP/loxP:Osr1-Cre− mice were stained with an antibody specifically against the human AR protein (Santa Cruz; sc-7305; 1:300 dilution). The sections were also counterstained with hematoxylin. Representative images are shown. Note that in the absence of human AR transgene expression in R26hARloxP/loxP:Osr1-Cre− mice, the human specific AR antibody fails to detect endogenous mouse AR. AP, anterior prostate; DP, dorsal prostate; LP, lateral prostate; VP, ventral prostate.
Detection of the AR Transgene Expression in Prostatic Basal Cells in AR Conditional Transgenic Mice
Observation of the local staining pattern of the AR transgene in R26hARloxP/wt:Osr1-Cre+ mice is novel and interesting. To confirm transgenic AR protein expression, we repeated immunohistochemistry with either an antibody (441; Santa Cruz; sc-7305) specifically against human AR protein or an antibody (N-20; Santa Cruz; sc-816) against both human and mouse AR proteins. Using these two antibodies allowed us to distinguish exogenous human AR from endogenous mouse AR. Uniform AR staining with the N20 antibody appears in the nucleus of prostatic luminal cells in R26hARloxP/wt:Osr1-Cre+ mice and R26hARloxP/wt:Osr1-Cre− controls (Fig. 3, A2 and B2). However, positive staining with the human AR specific antibody (441) was only observed in prostate tissues of R26hARloxP/wt:Osr1-Cre+ mice, indicating that expression of the AR transgene is a result of the LoxP/Cre recombination through activation of Cre transgene (Fig. 3, A1 versus B1). We then used immunofluorescence to co-localize both human AR and endogenous mouse AR in the above mouse tissues. A uniform nuclear immunofluorescence signal was observed with the antibody (N20) against human and mouse AR proteins in the prostate of R26hARloxP/wt:Osr1-Cre+ mice (Fig. 3C2, green), but only a portion of prostatic epithelial cells showed a positive immunofluorescence with the human AR antibody (Fig. 3C1, red). A significant amount of overlay was observed especially in luminal epithelial cells with these two antibodies (Fig. 3C3, arrows). Interestingly, positive staining of the human AR protein also appeared in prostatic basal cells in the AR transgenic mice.
FIGURE 3.
Analysis of the AR transgene expression in the prostate of theR26hARloxP/wt:Osr1-Cre+ mice. A and B, adjacent prostate sections from 16-week old male R26hARloxP/wt:Osr1-Cre+(A1 and A2) or R26hARloxP/wt:Osr1-Cre− (B1 and B2) mice were prepared and stained with either the antibody specifically against the human AR (A1 and B1) or human and mouse AR (A2 and B2) to distinguish exogenous human AR from endogenous mouse AR. Representative images are shown. C, a prostate section isolated from a 16-week-old R26hARloxP/wt:Osr1-Cre+ mouse was co-stained with the antibody against the human AR protein (C1) and the antibody against both human and mouse AR proteins (C2). The merged image (C3) shows overlap of both exogenous and endogenous AR proteins (arrows). D, the antibody against the human AR protein (red, D1 and D4) and the antibody against the p63 protein (green, D2 and D5) were used to co-stain prostate tissues isolated from R26hARloxP/wt:Osr1-Cre+ mice. The merged images show overlay of immunostaining with two antibodies (arrows, D3 and D6). E, similar co-stain analyses were performed using prostate tissues isolated from 16-week-old male R26hARloxP/wt:PB-Cre+ mice, in which there is no overlay of AR and p63 co-staining.
As described above, we observed positive AR immunostaining in both prostatic luminal and basal epithelial cells of R26hARloxP/wt:Osr1-Cre+ mice (Fig. 3C). It has been suggested that prostatic basal cells may contain prostate “stem” or “progenitor” cells (30, 31). We then used an antibody against p63, a prostatic basal cell marker, to confirm the expression of the AR transgene in prostatic basal epithelial cells (32). In these experiments, we included prostate tissues isolated from 16-week-old male R26hARloxP/wt:PB-Cre4+ mice in which expression of the AR transgene was limited in prostatic luminal cells through the ARR2PB promoter activation. As expected, positive immunostaining of p63 was observed exclusively in prostatic basal cells in both R26hARloxP/wt:Osr1-Cre+ and R26hARloxP/wt:PB-Cre4+ mice (Fig. 3, panels D2 and D5 and panels E2 and E5, respectively). Importantly, as observed previously, positive nuclear staining with the human AR antibody (441) appeared in a portion of prostatic basal cells in the prostate of R26hARloxP/wt:Osr1-Cre+ but not in R26hARloxP/wt:PB-Cre4+ mice (Fig. 3, panels D1 and D4 versus panels E1 and E4). Merging of these images showed a significant amount of overlay between transgenic human AR and endogenous mouse p63 proteins in the basal epithelial cells of the above mouse prostate tissues (Fig. 3, D3 and D6, blue arrows). The observation that expression of transgenic AR protein in prostatic basal cells of R26hARloxP/wt:Osr1-Cre+ mice is novel and interesting, suggesting that the mouse model is a new tool to assess AR action in prostatic epithelial basal cells.
Hyperplasia and Intraepithelial Neoplasia in Prostate Glands of the AR Conditional Transgenic Mice
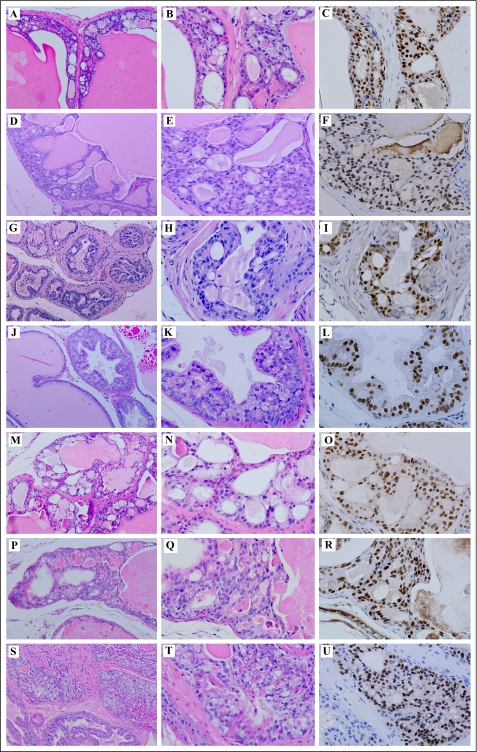
Both R26hARloxP/loxP:Osr1-Cre+ and R26hARloxP/wt:Osr1-Cre+ mice were born at the expected Mendelian ratios, suggesting that there is no significant prenatal lethality associated with genotypes of these mice. All of the transgenic mice appeared normal and did not show significant difference in appearance with age-matched R26hARloxP/loxP:Osr1-Cre− and R26hARloxP/wt:Osr1-Cre− controls as well as wild type littermates. In an effort to search for phenotypes of these AR transgenic mice, we thoroughly examined the mice at 2, 4, 8, 12, and 16 weeks and after 16 months of age. We observed atypical proliferative lesions consistent with mouse prostatic intraepithelial neoplasia (mPIN) in both R26hARloxP/loxP:Osr1-Cre+ and R26hARloxP/wt:Osr1-Cre+ mice as early as 8 weeks. Specifically, mPIN lesions appeared mainly as cribriform structures along with occasional stratification of cells, papilliferous structures, and tufts of cells. Atypical epithelial cells that appeared irregular, larger than adjacent normal cells and lacking normal polarity were observed in all prostatic lobes, including anterior prostate (Fig. 4, A, B, M, P, and S), dorsal prostate (Fig. 4G), and ventral prostate (Fig. 4J). The fibromuscular stroma was intact, and the glandular and duct profiles were undisturbed (Fig. 4, G and J). In Fig. 4 (M, P, and S), the foci of atypical cells partially fill the lumen of the ducts. Intraluminal glands forming within the original glands in the dysplastic lesions are pronounced in these cases, which are further characterized by epithelial cell crowding, and enlarged vesicular nuclei that often contained one or more prominent nucleoli (Fig. 4, N, Q, and T). Using the antibody against the human AR protein (440), we detected positive immunostaining of transgenic AR in almost all atypical cells within mPIN lesions (Fig. 4, C, F, I, L, O, R, and U). These results provide a direct link between expression of transgenic AR protein and development of the dysplastic lesions. We observed mPIN lesions in nine of 22 R26hARloxP/wt:Osr1-Cre+ mice (40.9%) and in nine of 18 R26hARloxP/loxP:Osr1-Cre+ (50%) mice (Table 1). Among those with mPINs, more than half of the mice (11 of 18) developed lesions at less than 12 months old. No pathological abnormalities in the prostate glands were observed in control littermates and wild type mice. Occurrence of mPIN lesions in R26hARloxP/wt:Osr1-Cre+ and R26hARloxP/loxP:Osr1-Cre+ mice is earlier and more frequent than in the previous AR transgenic mice (17), suggesting the potential significance of this mouse model in prostate tumorigenesis.
FIGURE 4.
Immunohistochemistry analyses of the prostate tissues isolated from R26hARloxP/wt:Osr1-Cre+ and R26hARloxP/loxP:Osr1-Cre+ mice. Prostate tissues isolated from seven R26hARloxP/wt:Osr1-Cre+ and R26hARloxP/loxP:Osr1-Cre+ male mice between 9 and 33 weeks old were analyzed histologically. Two adjacent sections from each mouse were stained with hematoxylin and eosin or with the antibody against the human AR (Santa Cruz; sc-7305) in which tissues were also counterstained with hematoxylin. Representative sections of anterior prostate (A, M, P, and S), dorsal prostate (G), and ventral prostate (J) lobes stained with hematoxylin and eosin show several typical dysplastia lesions and mPIN. Corresponding high power images (400×) of hematoxylin and eosin staining are shown in B, E, H, K, N, Q, and T, accordingly. Immunohistochemical analyses with the human AR specific antibody were used to detect the expression of the human AR transgene within dysplastic prostatic glands (C, F, I, L, O, R, and U).
TABLE 1.
Pathological abnormalities of R26hAR transgenic mice
| Genotype | Total number | Number of PIN | Number of adenocarcinoma |
|---|---|---|---|
| R26hARLoxP/WT:Osr1-Cre− | 14 | ||
| <12Mo months | 10 | 0 | 0 |
| >12 months | 4 | 0 | 0 |
| R26hARLoxP/LoxP:Osr1-Cre− | 23 | ||
| <12 months | 14 | 0 | 0 |
| >12 months | 9 | 0 | 0 |
| R26hARLoxP/WT:Osr1-Cre+ | 22 | 9 (40.9%) | 3 (13.6%) |
| <12 months | 13 | 6 | 1 |
| >12 months | 9 | 3 | 2 |
| R26hARLoxP/LoxP:Osr1-Cre+ | 18 | 9 (50.0%) | 1 (5.6%) |
| <12 months | 11 | 5 | 0 |
| >12 months | 7 | 4 | 1 |
Development of Prostate Adenocarcinoma in the AR Conditional Transgenic Mice
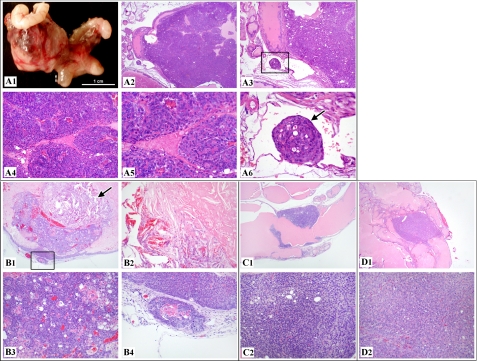
Because there is consensus that mPINs can progress toward prostate adenocarcinomas, we continued examining more AR transgenic mice at progressively older ages. Most intriguingly, we identified prostatic adenocarcinomas in three R26hARloxP/wt:Osr1-Cre+ mice and one R26hARloxP/loxP:Osr1-Cre+ mouse from 8 to 21 months of age. In two of the mice (Fig. 5, A and B), the neoplasms were grossly evident, being large, extensive tumor masses in the pelvis (Fig. 5, A1). The tumors were poorly circumscribed and unencapsulated (Fig. 5, A2 and B1) and comprised of haphazard acini and lobules of pleomorphic cells (Fig. 5, A4, A5, and B3) with no or limited amounts of fibrovascular stroma. Tumor necrosis was also noted in one of the tumors (Fig. 5B2). In both, malignancy was evident (in addition to the architectural, cellular, and nuclear features) by obvious vascular invasion by neoplastic cells (Fig. 5, A3 and A6) or local invasion of the tumor beyond the basement membrane into surrounding stromal tissues that show an early desmoplastic response (Fig. 5B4). In the other two mice (Fig. 5, C and D), smaller prostatic adenocarcinomas were noted by microscopic evaluation only. Specifically, the tumors were discrete, circumscribed unencapsulated masses comprised of haphazard solid epithelial sheets (with only rare glandular formation) of pleomorphic cells with scant fibrovascular stroma. Although neither obvious local nor vascular invasion was noted in these tumors, the architectural, cellular, and nuclear features of neoplasms suggest malignant neoplastic transformation of prostatic epithelial cells. Despite extreme attention to the presence of metastases in distant organs in the mice showing prostatic adenocarcinomas, distant dissemination of neoplastic prostatic epithelial cells was not noted in all four mice.
FIGURE 5.
Development of prostatic adenocarcinoma was observed in R26hARloxP/wt:Osr1-Cre+ and R26hARloxP/loxP:Osr1-Cre+. A, a 20-month-old R26hARloxP/wt:Osr1-Cre+ male mouse was grossly examined, which revealed a 1.8 × 1.3 × 1.3-cm mass at the base of the right seminal vesicle, replacing normal tissues of the right coagulating gland or anterior prostate area (A1). Histologically, the mass was an extensive, expansile, demarcated, unencapsulated neoplasm that intraluminally expanded a gland of the anterior prostate lobe (A2). The neoplasm consisted of haphazard solid lobules of cells with rare acinar formation (A4 and A5) with small amounts of fibrovascular stroma. The cells were pleomorphic with a large degree of anisocytosis and anisokaryosis, and occasional mitoses were noted. A tumor embolus was noted within an adjacent thin-walled vessel (A3 and A6). B, histologic analysis of a 19-month-old R26hARloxP/wt:Osr1-Cre+ male mouse revealed a focal, expansile, demarcated neoplasm filling the lumen of a gland of the anterior prostate (B1), with the remaining glandular mucosa demonstrating epithelial stratification consistent with high grade mPIN. The neoplasm consisted of haphazard acini of cells (B3) with small amounts of fibrovascular stroma. A sole, discrete invasive focus of the tumor is noted within the stroma next to the mass (B4), with the stroma presenting with an early desmoplastic response. A focal area of necrosis with abundant acicular (cholesterol) cleft formation is present within the main tumor mass itself (B2). C, histologic examination of a 34-week-old R26hARloxP/wt:Osr1-Cre+ male mouse revealed a likely circumferential, expansile, demarcated neoplasm focally expanding the mucosa of the anterior prostate gland (C1), with multifocal areas of the remaining glandular mucosa demonstrating epithelial cribriform changes consistent with high grade mPIN. The neoplasm consisted of haphazard solid sheet of cells with rare acinar formation (C2) with scant fibrovascular stroma. The cells were pleomorphic with a large degree of anisocytosis and anisokaryosis, and rare mitoses were noted. D, histologic assessment of a 80-week-old R26hARloxP/loxP:Osr1-Cre+ male mouse revealed a focal, expansile, demarcated neoplasm expanding the mucosa of the anterior prostate gland (D1), with multifocal areas of the remaining glandular mucosa demonstrating epithelial cribriform changes consistent with high grade mPIN. The neoplasm consisted of haphazard solid sheet of cells with rare acinar formation (D2) with scant fibrovascular stroma. The cells were pleomorphic, with a large degree of anisocytosis and anisokaryosis, and regular mitoses were noted.
Conditional Expression of the Human AR Transgene Induces Prostatic Cell Proliferation and Contributes to Development of Adenocarcinomas
A promotional role of AR in cell proliferation has been demonstrated previously (1, 33, 34). To understand the cellular effects resulting by conditional expression of the AR transgene in the mouse prostate, we assessed for proliferation using Ki67 immunohistochemistry in three mice from each genotype. A significant increase in Ki67 immunostaining in both mPIN and prostatic adenocarcinoma lesions was observed when compared with wild type samples (Fig. 6, A–I). Ki67 immunostaining was quantified by counting a total of 1,000 epithelial cells from five high power fields in each sample. Experiments were repeated three times, and three different slides prepared independently from three mice in each genotype were analyzed. Representative data are shown that the epithelial proliferative index increased from 10 in wild type cells to 50 in mPIN lesions and 140 in prostatic adenocarcinoma lesions (Fig. 6J). These results demonstrate a tumor-promoting role of the AR human transgene expression in the prostate of the transgenic mice.
FIGURE 6.
Expression of the human AR transgene induces cell proliferative advantage in prostate tissues. Normal prostatic tissues (A, D, and G) or tissues containing mPIN (B, E, and H) or adenocarcinomas (C, F, and I) were isolated from the AR conditional transgenic mice or controls and assessed for cell proliferation. All of the tissues were stained with a Ki67 antibody, and the proliferation indexes for the above tissues were measured in total 1,000 epithelial cells counted from five high power fields. The experiments were repeated three times, and three different slides prepared independently from three mice in each genotypy were analyzed. Representative data show the number of Ki67-positive cells in graphs (J). * or ** indicates samples showing a significant difference, p < 0.05 or p < 0.01, respectively.
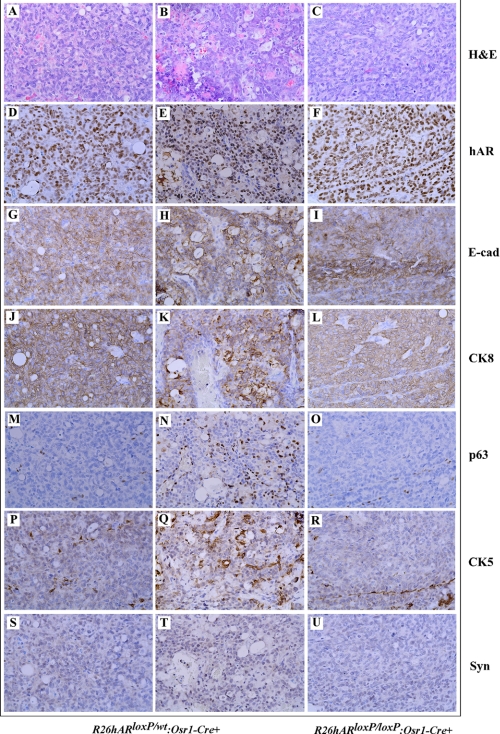
In prostatic epithelium, there are basal and luminal epithelial cells as well as neuroendocrine cells. Development of both adenocarcinoma and neuroendocrine carcinoma in mouse prostates has been demonstrated in previous animal models (35–37). To further define the origin of tumors in these AR transgenic mice, we performed comprehensive immunohistochemical analyses to examine a series of prostatic cellular markers. As shown in Fig. 7, prostatic tumor cells showed positive immunostaining with the human AR antibody (Fig. 7, D–F), suggesting a direct link between expression of transgenic human AR and oncogenic transformation in the mice. The tumor cells also showed positive staining for E-cadherin and CK8 (Fig. 7, G–L), the hallmarks of secretory epithelium, but no staining for the neuroendocrine cell marker synaptophysin (Fig. 7, S–V). Interestingly, staining of p63 and CK5 was also observed in some tumor cells in one of the R26hARloxP/wt:Osr1-Cre+ mice (Fig. 7, N and Q), suggesting the possibility that prostatic basal epithelial cells may also be tumor initiation cells in this animal model.
FIGURE 7.
Immunohistochemical analyses of prostate adenocarcinomas in R26hARloxP/wt:Osr1-Cre+ and R26hARloxP/loxP:Osr1-Cre+. Adjacent prostate tissue slides were prepared from prostatic adenocarcinoma regions of R26hARloxP/wt:Osr1-Cre+ and R26hARloxP/loxP:Osr1-Cre+ mice that reported on Fig. 5 and stained with different antibodies as labeled in the figure. A–C, hematoxylin and eosin staining; D–U, immunohistochemistry with different antibodies as labeled in the figure.
DISCUSSION
The androgen signaling pathway plays a key role in prostate cancer initiation and progression. The pioneering work of Charles Huggins and Clarence Hodges (38) demonstrated that depletion of androgens resulted in significant regression of prostate tumors, heralding the now ubiquitous and most effective strategy to treat prostate cancer, androgen deprivation therapy. The fundamental premise of this therapy has remained almost unchanged since then, despite different medications that have been developed and applied to patients for the purpose of reducing the level of androgens or competitively repressing AR function. Most patients develop hormone refractory tumors within 2–3 years following initiation of therapy, for which there is no effective treatment. One of the main reasons for the limited progress is the lack of biologically relevant animal models that mimic the human disease and can be used to investigate AR action in androgen-induced prostate tumor initiation and disease progression.
In past years, many genetically modified mouse models have been established for studying prostate tumorigenesis. They involve either overexpression of oncogenes or targeted deletion of tumor suppressors in the prostatic epithelium. Transgenic (gain-of-function) models that express SV40 T antigen (i.e. the TRAMP and LADY models) and c-Myc have been developed previously (39–41). Knock-out mice with specific deletion of various tumor suppressors in the prostate have also been developed (39, 42–44). One of the best characterized mouse models is the conditional pten knock-out (37). Loss of both alleles of pten results in invasive prostate cancer that metastasizes to lymph nodes and lung in some mice (37). Combining PTEN loss with other genetic abnormalities has led to several additional mouse models (45–47). Deletion of Nkx3.1 and PTEN results in androgen-independent prostate cancer (39, 47). However, despite the progress offered by these genetically modified mouse models in analyses of different molecules and signaling pathways in prostate tumorigenesis, there is a great need for new animal models for characterizing the androgen axis, a key pathway, in prostate development and tumorigenesis.
In this study, we report a new AR conditional transgenic mouse model, R26hARloxP:Osr1-Cre+. In this mouse model expression of the human AR transgene is regulated in a constitutive but prostate-specific manner by the hybrid CAG promoter-coupled CMV enhancer and chicken β-actin promoter through loxP/Cre recombination. We used newly established Osr1-Cre mice to activate AR transgene expression. The Osr1 promoter is active at embryonic day 11.5 in urogenital sinus epithelium and maintains its activity in prostatic epithelial cells throughout development (20). We detected transgenic AR expression in both luminal and basal epithelial cells of prostatic glands of 4-week-old R26hARloxP:Osr1-Cre+ mice. The robust activity of the Osr1 promoter is detected in all four prostatic lobes, although it is not fully restricted to the prostate as described previously (20). Through loxP/Cre mediated recombination, deletion of the LSL cassette resulted in the activation of the AR transgene expression in a constitutive manner. These unique and novel features distinguish our AR transgenic mice from other genetically modified mouse models, which should allow us to assess and validate the AR action in both prostatic luminal and basal epithelial cells and in a ligand-dependent or -independent manner.
The majority of human primary prostate cancers are androgen-dependent. Data from previous AR transgenic mouse models also showed that overexpression of the mouse AR transgene in prostate luminal epithelial cells promotes proliferation of the epithelium, with the subsequent development of precancerous lesions and mouse PIN in aged mice. However, the precise role of AR in promoting prostate cancer development still remains unclear. In this study, we showed that almost half of R26hARloxP/loxP:Osr1-Cre+ or R26hARloxP/wt:Osr1-Cre+ mice developed mouse PIN lesions. The onset of mPIN has been observed as early as 8 weeks of age. Most importantly, prostatic adenocarcinomas were developed in four of 40 AR transgenic mice between 8 and 20 months of age. It has been well documented that wild type mice have a very low incidence of spontaneous prostate tumors (please see the review in Ref. 48). Therefore, identifying prostate adenocarcinomas in these AR conditional transgenic mice directly demonstrates a promoting role of the AR in prostate cancer development. In this study, we also examined the role of the human AR transgene in R26hARloxP/wt:PB-Cre+ and R26hARloxP/loxed:PB-Cre+ mice, in which the AR transgene expression was selectively targeted in prostatic luminal epithelial cells through ARR2PB promoter (25). We only observed mPIN lesions but no prostatic adenocarcinomas in those mice. These results are consistent with the previous studies and implicate that selective activation of the AR transgene expression in different cells of the prostate may regulate cell proliferation distinctly during the course of mouse prostate cancer development.
The promoting role of AR in stimulating prostatic cell growth has been implicated in human prostate tumorigenesis. However, it is unclear whether increasing AR expression, a steroid hormone receptor, is sufficient to induce prostate cancer development in mice because previous AR transgenic mice only showed mPIN lesions (17). Thus, the finding in this study is very interesting and suggests that this mouse model is novel and should be characterized further. In this mouse model, positive immunostaining of transgenic human AR protein appears in the majority of atypical cells in mPIN lesions and tumor cells of prostatic adenocarcinomas, providing a direct link between AR transgene expression and oncogenic transformation in mouse prostates. In addition, an increase of Ki67-positive cells has been observed in all above prostatic adenocarcinoma and PIN samples. In this study, we also examined cell apoptosis in the above samples with mPIN and adenocarcinoma lesions and did not observe any significant changes. These data further support a promotional role of transgenic AR protein in inducing prostatic epithelial proliferation.
Using immunohistochemistry, we observed that most tumor cells in the adenocarcinoma and PIN lesions were E-cadherin- and CK8-positive but synaptophysin-negative. These data suggest that tumor cells are immunoreactive to luminal epithelial cellular markers. Interestingly, we also observed some immunolabeling with p63 and CK5 antibodies in some tumor cells from one R26hARloxP/wt:Osr1-Cre+ mouse. Expression of the human AR transgene has been observed in prostatic basal epithelial cells in R26hARloxP/loxP:Osr1-Cre+ and R26hARloxP/wt:Osr1-Cre+. These data suggest that oncogenic transformation can be initiated in both basal and luminal epithelial cells through the activation of the androgen signaling pathway. Previous studies showing that both prostatic luminal and basal epithelial cells are competent to function as tumor initiating cells support this hypothesis (30, 31). In general, most prostatic basal epithelial cells have no or low expression of the AR. Enforcement of transgenic AR expression in basal cells may disrupt the normal differentiation pathway and induce oncogenic transformation. Although it is unclear whether activation of the AR transgene through Osr1-Cre can directly promote and induce normal basal epithelial cells into “primary” tumor-initiating cells, occurrence of prostate adenocarcinomas in this AR transgenic mouse model provides a direct line of evidence that dysregulation of the AR signaling pathway can promote prostate tumor formation in mice. Interestingly, we only observed prostatic adenocarcinomas in about 10% of the transgenic mice that have been examined in this study. The low penetrance of adenocarcinoma in this model further implies that other additional “hits” may be required to enhance AR-mediated oncogenic transformation in prostate tumorigenesis. Thus, the current AR transgenic mouse model mimics features of the human disease and can be used to identify other factors and pathways that promote AR action in inducing prostate cancer initiation and progression.
The probasin promoter has been widely used to create prostate genetically modified mouse models in the past. It is activated postnatally in an androgen-inducible manner and is targeted selectively to luminal cells (25). One of the most important features for our AR conditional transgenic mouse model is that AR expression is regulated in a constitutive but prostate-specific manner by the hybrid CAG promoter through loxP/Cre recombination (Fig. 1A). In this study, we compared expression of the AR transgene between castrated versus uncastrated R26hARloxP/wt:Osr1-Cre+ and R26hARloxP/wt:Osr1-Cre− mice. Expression of transgenic human AR protein appeared unchanged between castrated and intact R26hARloxP/wt:Osr1-Cre+ mice (supplemental Fig. S2). Thus, this model allows us to characterize the role of AR in the absence of androgens. Further studies with larger cohorts of the AR transgenic mice will allow us to fully assess the effect of castration in growth and progression of prostatic adenocarcinomas in this new mouse model.
Supplementary Material
This work was supported by Public Health Service Grant CA-070297 from the National Cancer Institute.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- AR
- androgen receptor
- mPIN
- mouse prostatic intraepithelial neoplasia
- LSL
- LoxP-stop-loxP
- CRPC
- castration-resistant prostate cancer.
REFERENCES
- 1. Kyprianou N., Isaacs J. T. (1988) Endocrinology 122, 552–562 [DOI] [PubMed] [Google Scholar]
- 2. Abate-Shen C., Shen M. M. (2000) Genes Dev. 14, 2410–2434 [DOI] [PubMed] [Google Scholar]
- 3. Jenster G. (1999) Semin. Oncol. 26, 407–421 [PubMed] [Google Scholar]
- 4. Culig Z., Hobisch A., Bartsch G., Klocker H. (2000) Urol. Res. 28, 211–219 [DOI] [PubMed] [Google Scholar]
- 5. Koivisto P., Kolmer M., Visakorpi T., Kallioniemi O. P. (1998) Am. J. Pathol. 152, 1–9 [PMC free article] [PubMed] [Google Scholar]
- 6. Salinas C. A., Austin M. A., Ostrander E. O., Stanford J. L. (2005) Prostate 65, 58–65 [DOI] [PubMed] [Google Scholar]
- 7. Palazzolo I., Gliozzi A., Rusmini P., Sau D., Crippa V., Simonini F., Onesto E., Bolzoni E., Poletti A. (2008) J. Steroid Biochem. Mol. Biol. 108, 245–253 [DOI] [PubMed] [Google Scholar]
- 8. Stanford J. L., Just J. J., Gibbs M., Wicklund K. G., Neal C. L., Blumenstein B. A., Ostrander E. A. (1997) Cancer Res. 57, 1194–1198 [PubMed] [Google Scholar]
- 9. Gann P. H., Hennekens C. H., Ma J., Longcope C., Stampfer M. J. (1996) J. Natl. Cancer Inst. 88, 1118–1126 [DOI] [PubMed] [Google Scholar]
- 10. Shaneyfelt T., Husein R., Bubley G., Mantzoros C. S. (2000) J. Clin. Oncol. 18, 847–853 [DOI] [PubMed] [Google Scholar]
- 11. Koivisto P., Kononen J., Palmberg C., Tammela T., Hyytinen E., Isola J., Trapman J., Cleutjens K., Noordzij A., Visakorpi T., Kallioniemi O. P. (1997) Cancer Res. 57, 314–319 [PubMed] [Google Scholar]
- 12. Ruizeveld de Winter J. A., Janssen P. J., Sleddens H. M., Verleun-Mooijman M. C., Trapman J., Brinkmann A. O., Santerse A. B., Schröder F. H., van der Kwast T. H. (1994) Am. J. Pathol. 144, 735–746 [PMC free article] [PubMed] [Google Scholar]
- 13. Chen C. D., Welsbie D. S., Tran C., Baek S. H., Chen R., Vessella R., Rosenfeld M. G., Sawyers C. L. (2004) Nat. Med. 10, 33–39 [DOI] [PubMed] [Google Scholar]
- 14. Taplin M. E., Bubley G. J., Shuster T. D., Frantz M. E., Spooner A. E., Ogata G. K., Keer H. N., Balk S. P. (1995) N. Engl. J. Med. 332, 1393–1398 [DOI] [PubMed] [Google Scholar]
- 15. Gaddipati J. P., McLeod D. G., Heidenberg H. B., Sesterhenn I. A., Finger M. J., Moul J. W., Srivastava S. (1994) Cancer Res. 54, 2861–2864 [PubMed] [Google Scholar]
- 16. Bruchovsky N., Lesser B., Van Doorn E., Craven S. (1975) Vitam Horm 33, 61–102 [DOI] [PubMed] [Google Scholar]
- 17. Stanbrough M., Leav I., Kwan P. W., Bubley G. J., Balk S. P. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 10823–10828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soriano P. (1999) Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- 19. Srinivas S., Watanabe T., Lin C. S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001) BMC Dev. Biol. 1, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grieshammer U., Agarwal P., Martin G. R. (2008) Genesis 46, 69–73 [DOI] [PubMed] [Google Scholar]
- 21. Nagy A., Rossant J., Nagy R., Abramow-Newerly W., Roder J. C. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 8424–8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Soriano P., Montgomery C., Geske R., Bradley A. (1991) Cell 64, 693–702 [DOI] [PubMed] [Google Scholar]
- 23. Yanagawa Y., Kobayashi T., Ohnishi M., Kobayashi T., Tamura S., Tsuzuki T., Sanbo M., Yagi T., Tashiro F., Miyazaki J. (1999) Transgenic Res. 8, 215–221 [DOI] [PubMed] [Google Scholar]
- 24. Robertson E., Bradley A., Kuehn M., Evans M. (1986) Nature 323, 445–448 [DOI] [PubMed] [Google Scholar]
- 25. Wu X., Wu J., Huang J., Powell W. C., Zhang J., Matusik R. J., Sangiorgi F. O., Maxson R. E., Sucov H. M., Roy-Burman P. (2001) Mech. Dev. 101, 61–69 [DOI] [PubMed] [Google Scholar]
- 26. Sugimura Y., Cunha G. R., Donjacour A. A. (1986) Biol. Reprod 34, 973–983 [DOI] [PubMed] [Google Scholar]
- 27. Huang C. Y., Beliakoff J., Li X., Lee J., Li X., Sharma M., Lim B., Sun Z. (2005) Mol. Endocrinol. 19, 2915–2929 [DOI] [PubMed] [Google Scholar]
- 28. Li X., Thyssen G., Beliakoff J., Sun Z. (2006) J. Biol. Chem. 281, 23748–23756 [DOI] [PubMed] [Google Scholar]
- 29. Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y. (1997) FEBS Lett. 407, 313–319 [DOI] [PubMed] [Google Scholar]
- 30. Kasper S. (2008) Stem Cell Rev. 4, 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matusik R. J., Jin R. J., Sun Q., Wang Y., Yu X., Gupta A., Nandana S., Case T. C., Paul M., Mirosevich J., Oottamasathien S., Thomas J. (2008) Differentiation 76, 682–698 [DOI] [PubMed] [Google Scholar]
- 32. Weinstein M. H., Signoretti S., Loda M. (2002) Mod. Pathol. 15, 1302–1308 [DOI] [PubMed] [Google Scholar]
- 33. Culig Z., Klocker H., Bartsch G., Steiner H., Hobisch A. (2003) J. Urol. 170, 1363–1369 [DOI] [PubMed] [Google Scholar]
- 34. Gelmann E. P. (2002) J. Clin. Oncol. 20, 3001–3015 [DOI] [PubMed] [Google Scholar]
- 35. Greenberg N. M., DeMayo F., Finegold M. J., Medina D., Tilley W. D., Aspinall J. O., Cunha G. R., Donjacour A. A., Matusik R. J., Rosen J. M. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 3439–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garabedian E. M., Humphrey P. A., Gordon J. I. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 15382–15387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang S., Gao J., Lei Q., Rozengurt N., Pritchard C., Jiao J., Thomas G. V., Li G., Roy-Burman P., Nelson P. S., Liu X., Wu H. (2003) Cancer Cell 4, 209–221 [DOI] [PubMed] [Google Scholar]
- 38. Huggins C., Hodges C. V. (2002) J. Urol 168, 9–12 [DOI] [PubMed] [Google Scholar]
- 39. Abate-Shen C. (2006) Clin. Cancer Res. 12, 5274–5276 [DOI] [PubMed] [Google Scholar]
- 40. Ellwood-Yen K., Graeber T. G., Wongvipat J., Iruela-Arispe M. L., Zhang J., Matusik R., Thomas G. V., Sawyers C. L. (2003) Cancer Cell 4, 223–238 [DOI] [PubMed] [Google Scholar]
- 41. Gingrich J. R., Barrios R. J., Kattan M. W., Nahm H. S., Finegold M. J., Greenberg N. M. (1997) Cancer Res. 57, 4687–4691 [PubMed] [Google Scholar]
- 42. Kerkhofs S., Denayer S., Haelens A., Claessens F. (2009) J. Mol. Endocrinol. 42, 11–17 [DOI] [PubMed] [Google Scholar]
- 43. Pienta K. J., Abate-Shen C., Agus D. B., Attar R. M., Chung L. W., Greenberg N. M., Hahn W. C., Isaacs J. T., Navone N. M., Peehl D. M., Simons J. W., Solit D. B., Soule H. R., VanDyke T. A., Weber M. J., Wu L., Vessella R. L. (2008) Prostate 68, 629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Winter S. F., Cooper A. B., Greenberg N. M. (2003) Prostate Cancer Prostatic Dis. 6, 204–211 [DOI] [PubMed] [Google Scholar]
- 45. Chen Z., Trotman L. C., Shaffer D., Lin H. K., Dotan Z. A., Niki M., Koutcher J. A., Scher H. I., Ludwig T., Gerald W., Cordon-Cardo C., Pandolfi P. P. (2005) Nature 436, 725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gao H., Ouyang X., Banach-Petrosky W. A., Gerald W. L., Shen M. M., Abate-Shen C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 14477–14482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gao H., Ouyang X., Banach-Petrosky W. A., Shen M. M., Abate-Shen C. (2006) Cancer Res. 66, 7929–7933 [DOI] [PubMed] [Google Scholar]
- 48. Shappell S. B., Thomas G. V., Roberts R. L., Herbert R., Ittmann M. M., Rubin M. A., Humphrey P. A., Sundberg J. P., Rozengurt N., Barrios R., Ward J. M., Cardiff R. D. (2004) Cancer Res. 64, 2270–2305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.