Background: We investigated the role of mad-9/syntenin, a cancer metastasis inducer, in ILK adaptor function during adhesion to type I collagen.
Results: mda-9/syntenin positively regulates the scaffold function of ILK.
Conclusion: mda-9/syntenin regulates the activation of ILK signaling molecules by facilitating ILK adaptor function during adhesion to type I collagen.
Significance: We describe a new mechanism of mda-9/syntenin for regulation of cancer metastasis.
Keywords: Adaptor Proteins, Akt PKB, Cell Migration, Integrin, Oncogene, Signal Transduction, Tumor Metastases, Integrin-linked Kinase, mda-9/Syntenin, Plasma Membrane Translocation
Abstract
The integrin-linked kinase (ILK)-PINCH1-α-parvin (IPP) complex functions as a signaling platform for integrins that modulates various cellular processes. ILK functions as a central adaptor for the assembly of IPP complex. We report here that mda-9/syntenin, a positive regulator of cancer metastasis, regulates the activation of Akt (also known as protein kinase B) by facilitating ILK adaptor function during adhesion to type I collagen (COL-I) in human breast cancer cells. COL-I stimulation induced the phosphorylation and plasma membrane translocation of Akt. Inhibition of mda-9/syntenin or expression of mutant ILK (E359K) significantly blocked the translocation of both ILK and Akt to the plasma membrane. mda-9/syntenin associated with ILK, and this association was increased at the plasma membrane by COL-I stimulation. Knockdown of mda-9/syntenin impaired COL-I-induced association of ILK with Akt and plasma membrane targeting of ILK-Akt complex. These results demonstrated that mda-9/syntenin regulates the activation of Akt by controlling the plasma membrane targeting of Akt via a mechanism that facilitates the association of Akt with ILK at the plasma membrane during adhesion to COL-I. On a striking note, inhibition of mda-9/syntenin impaired COL-I-induced plasma membrane translocation of the IPP complex and assembly of integrin β1-IPP signaling complexes. Thus, our study defines the role of mda-9/syntenin in ILK adaptor function and describes a new mechanism of mda-9/syntenin for regulation of cell migration.
Introduction
PDZ2 (postsynaptic density protein, disc large, and zonula occludens) domain-containing proteins are ubiquitous scaffolding proteins that are involved in the organization of multiprotein complexes at the membrane (1, 2). mda-9/syntenin is a 32-kDa protein that comprises a 113-amino acid N-terminal domain with no obvious structural motifs followed by two adjacent tandem PDZ domains and a short 24-amino acid C-terminal domain (3). The PDZ domains of mda-9/syntenin are essential for assembly and organization of diverse cell signaling processes occurring at the plasma membrane (4, 5) and bind to multiple peptide motifs with low to medium affinity (6, 7).
Several laboratories, including our own, have shown that mda-9/syntenin, which is overexpressed in several cancer cells and tissues, may regulate tumor cell invasion and metastasis (8–12). mda-9/syntenin is colocalized with focal adhesion kinase (FAK) and facilitates fibronectin (FN)-induced phosphorylation of FAK, with subsequent activation of p38 and JNK MAPKs as well as nuclear factor-κB (9, 10). mda-9/syntenin may interact directly with c-Src, and this interaction correlates with increased formation of active FAK-c-Src signaling complexes (12, 13), which are important for regulation of migration machinery (14). We recently showed that transient induction of mda-9/syntenin during adhesion to FN is dependent on PKCα activation and enhances the FN-induced assembly of integrin β1 signaling complexes with FAK and c-Src, activating FAK and its downstream pathways (15). The role of mda-9/syntenin in regulation of signaling of extracellular matrices other than FN has not been well elucidated. mda-9/syntenin also increases the migratory and invasive potential of HEK 293T cells toward COL-I though a pathway involving phosphoinositol 3-kinase (PI3K), Akt, and ERK1/2 (11); however, the mechanisms by which mda-9/syntenin regulates activation of these kinases in response to COL-I is still unknown.
Integrins are α and β heterodimeric cell-surface receptors that mediate cell-extracellular matrix proteins (16). Ligand binding to the extracellular integrin domain induces conformational changes and integrin clustering for activation of signaling cascades and recruitment of multiprotein complexes to focal adhesions (17). Subsequently, kinase activity-lacking integrins transmit messages through a variety of intracellular protein kinases and adaptor molecules, including FAK, ILK, and paxillin (18–20). ILK plays a central role in signaling transductions that are initiated by cell-matrix interactions and that regulate biochemical processes, such as growth, proliferation, survival, differentiation, migration, invasion, and angiogenesis (21). ILK was initially described as an integrin β1 subunit-binding protein with involvement in kinase signaling pathways (18). Structurally, ILK comprises an N-terminal ankyrin repeat domain and a C-terminal kinase-like domain (18, 22, 23). By means of its ankyrin repeat domain, ILK binds to PINCH1 (24, 25). The kinase-like domain interacts directly with other components of integrin-based adhesion plaques, including paxillin and α-parvin (22, 26). ILK, PINCH1, and α-parvin form a ternary complex termed IPP, which localizes to focal adhesion and functions as a signaling platform for at least β1 and β3 integrin by interfacing with the actin cytoskeleton and many diverse signaling pathways (20). Formation of the ILK-PINCH1-α-parvin triad is a prerequisite for recruitment of ILK, PINCH1, and α-parvin to integrin adhesions (27), implying a functional interdependency of these proteins. ILK has also been shown to act as a serine/threonine kinase and to directly activate several signaling pathways, including that of Akt, glycogen synthase kinase (GSK)-3α/β and members of the MAPK signaling pathway (28–30). However, recent studies have shown that ILK is a pseudokinase and that it acts as a distinct adaptor protein linking integrin and α-parvin (22, 23, 31).
In this study we provide evidence to show that mda-9/syntenin positively regulates the ILK adaptor function for the assembly of integrin β1-IPP signaling complexes, which activate integrin signaling pathways, including Akt, ERK1/2, and Rac1, during adhesion to COL-I in human breast cancer cells.
EXPERIMENTAL PROCEDURES
Cells and Cell Culture
Human breast cancer cell lines MCF-7 and MDA-MB-231 and HEK 293T17 cell line were purchased from the American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640. Human melanoma line C8161 was a gift from Dr. Danny R. Welch (The University of Alabama at Birmingham) and maintained in DMEM. All media were supplemented with penicillin-streptomycin (Invitrogen) and 10% heat-inactivated FBS (Hyclone, Logan, UT). All cells were maintained in a humidified 5% CO2 atmosphere at 37 °C.
Antibodies and Other Reagents
Antibodies for ILK, phospho-PKCα (Thr-638/641), phospho-Akt (Ser-473), phospho-GSK-3α/β (Ser-9/21), phospho-mTOR (Ser-2448), phospho-p70S6K (Thr-389), phospho-4EBP (Ser-65), phospho-ERK1/2 (Thr-202/Tyr-204), phospho-FAK (Tyr-397), FAK, and Myc were from Cell Signaling Technology (Danvers, MT). Antibodies for α-tubulin, PINCH1, and FLAG were from Sigma. Protein A/G plus agarose bead and antibodies for syntenin, integrin β1, α-parvin, β-parvin, Akt, hemagglutinin (HA), GST, ErbB2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for PKCα, FN, and COL-I were from BD Biosciences. Alexa 488-conjugated goat anti-mouse and Alexa 546-conjugated goat anti-rabbit IgGs were from Invitrogen. Fluorescein isothiocyanate (FITC)-conjugated phalloidin was from Enzo Life Sciences (Alexis Biochemicals, San Diego, CA).
Plasmids and RNA Interference
Expression vectors for FLAG-tagged mda-9/syntenin, PKCα, the dominant negative (DN)-PKCα, and DN-Rac1 were described previously (15). Expression vectors for the FLAG-Akt, DN-Akt, and DN-PI3K were obtained from Dr. N. Fujita (Japanese Foundation for Cancer Research, Tokyo, Japan). A mutant ILK (E359K) was created using QuikChange site-directed mutagenesis kit according to the manufacturer's protocol (Agilent Technologies, Santa Clara, CA). For construction of HA-ILK, Myc-α-parvin, and FLAG-PINCH1, the open reading frames of ILK, α-parvin, and PINCH1 were amplified by PCR, digested with appropriate restriction enzymes, and cloned in-frame into the polylinker of the pCMV-HA (Clontech, Mountain View, CA), pCMV-Myc (Clontech), and pCMV-Tag2B (Agilent Technologies), respectively. Three kinds of syntenin mutants were generated from syntenin cDNA as a template using the following primer sets: Syn-M1 (Arg-105—Pro-271), 5′-CGCGGATCCAGAGCAGAAATTAAGCAA-3′ (sense) and 5′-CGCCTCGAGTTAAGGCATGATTGTAATAGTAAC-3′ (antisense); Syn-M2 (deletion from Arg-113—Asp-192), 5′-CGCTCTAGAAGGCCCTTTGAACGGACG-3′ (sense) and 5′-CGCTCTAGAACGAATCCCTTGCTTAAT-3′ (antisense); Syn-M3 (Met-1—Arg-193), 5′-CGCGGATCCTCTCTCTATCCATCTCTC-3′ (sense) and 5′-CGCCTCGAGTTACCTGTCACGAATGGTCAT-3′ (antisense). The PCR products were ligated into pCMV-Tag2B (Agilent Technologies) with in-frame. All constructs and mutations were confirmed by DNA sequencing. The pSUPER shRNA-producing plasmids (pSUPER/syntenin and pSUPER-scramble) were previously described (15). siRNAs for mda-9/syntenin, ILK and scramble control were from Santa Cruz Biotechnology. Transfections were performed using Lipofectamine Plus reagent according to the manufacturer's instructions (Invitrogen).
Preparation of GST Fusion Protein
For construction of GST-syntenin1–298 and GST-Akt379–480, the corresponding open reading frames were amplified by PCR, digested with appropriate restriction enzymes, and cloned in-frame into the polylinker of the pGEX4T-1. Each construct was transformed into Escherichia coli BL21 cells, and ampicillin was applied to select bacteria carrying the expression constructs. Isopropyl-d-thiogalactopyranoside was added at 0.1 mm and purified by the affinity column of glutathione-Sepharose 4B resin (GE Healthcare).
Immunoprecipitation and Western Blotting
Immunoprecipitation and Western blotting were described previously (8, 15). Briefly, cells were lysed in lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 5 mm sodium orthovanadate, 1% Nonidet P-40 and protease inhibitors mixture (BD Biosciences)) and centrifuged at 15,000 rpm for 30 min at 4 °C. For immunoprecipitation, equivalent amounts of cell lysates were incubated with the appropriate antibodies and followed by incubation with protein A/G-agarose beads. Immunoprecipitates were extensively washed, and the eluted precipitates were resolved by SDS-PAGE, transferred, and probed with the proper antibodies. The signal was detected using an ECL system (Intron, Seongnam, Korea).
In Vitro Kinase Assays
Kinase assays were performed as described previously with some modifications (32). MDA-MB-231 cells were serum-starved for 12 h and allowed to adhere to COL-I-coated dishes (10 μg/ml) for the indicated periods of time in the absence of serum. The cells were lysed and immunoprecipitated with anti-ILK or anti-Akt, then the activity of ILK or Akt was measured. Briefly, immunoprecipitates were extensively washed with cell lysis buffer and wash buffer (50 mm HEPES, pH 7.0, 2 mm MgCl2, 2 mm MnCl2, 5 mm sodium orthovanadate, and protease inhibitors mixture) and subjected to kinase assay in kinase buffer (added 200 μm ATP in wash buffer); 2 μg of GST-GSK-3α/β (Cell Signaling Technology) or GST-Akt379–480 protein was added as the kinase substrate, and cells were incubated at 37 °C for 30 min. Phosphorylation of GSK3 or AKT was measured by Western blot analysis using phospho-GSK-3α/β (Ser-9/21) or phospho-AKT (Ser-473) antibody (Cell signaling).
In Vitro Binding Assays
In vitro binding assays were performed as described previously (33). The GST-fused syntenin or GST (2 μg each) was immobilized on the glutathione-Sepharose beads (40 μl volume of 80% beads slurry) and equilibrated in the binding buffer consisting of phosphate-buffered saline (PBS), 10% glycerol, 0.1% (v/v) Nonidet P-40. The recombinant Myc-ILK (Origene Technologies, Rockville, MD) was added in the affinity beads then incubated at 4 °C for 2 h. The beads were washed 4 times, and the bound proteins were eluted in 30 μl of the 20 mm reduced glutathione in the buffer and analyzed by SDS-PAGE followed by Western blotting.
Cell Fractionation
Cells were washed with PBS, incubated in hypotonic lysis buffer (50 mm Tris-HCl, pH 7.0, 1 mm EDTA, 0.1% β-mercaptoethanol, 5 mm sodium orthovanadate, protease inhibitors mixture), and lysed by 15 strokes of a prechilled 1-ml Dounce homogenizer with a tight-fitting pestle. Unbroken cells and nuclei were pelleted at 1000 × g at 4 °C for 10 min. The cytoplasmic fraction was obtained by centrifuging supernatants at 21,000 × g at 4 °C for 45 min, and the pellets containing cellular membranes were washed 3 times in hypotonic lysis buffer and resuspended in lysis buffer.
Cell Migration and Invasion Assays
Cell migration and invasion assays were performed as described previously (8, 34). Briefly, the lower surface of the filters was coated with 6.5 μg COL-I. Cells were seeded in triplicate on the upper part of each chamber. The cells that had migrated to the lower surface of the filter were fixed with methanol. Then the cells were stained and counted in at least five randomly selected microscopic fields (×100) per filter.
Rac1 Activity ELISA
A commercially available ELISA Rac1 activation assay kit was used according to the manufacturer's instructions (Cytoskeleton, Denver, CO). Briefly, captured Rac1 was cross-linked to the plate and detected by sequential incubations with an anti-Rac1 primary antibody and an HRP-conjugated secondary antibody. The relative amount of bound Rac1 was then measured by developing the plate with an HRP-detection reagent and recording the luminescence signal.
Immunofluorescence and Confocal Microscopy
Immunofluorescence staining was performed as described (15). Cells grown on glass coverslips were fixed with 4% paraformaldehyde, permeabilized in 0.2% Triton X-100, and incubated with primary and appropriate secondary antibodies. F-actin was stained with FITC-conjugated phalloidin. Confocal images were acquired by using a Zeiss LSM510 META NLO inverted laser scanning confocal microscope (Carl Zeiss, Jena, Germany; Korea Basic Science Institute Chuncheon Center) equipped with an external argon, HeNe laser, and HeNe laser II. Using a C-Apochromat 63X NA1.2 water immersion objective (Zeiss), images were captured at the colony midsection.
Statistical Analysis
The data are expressed as the mean ± S.D. unless otherwise specified. Statistical significance was assessed by 2-tailed unpaired student's t test, and p < 0.05 was considered statistically significant.
RESULTS
mda-9/Syntenin Regulates COL-I-induced Activation of Akt and Cell Migration
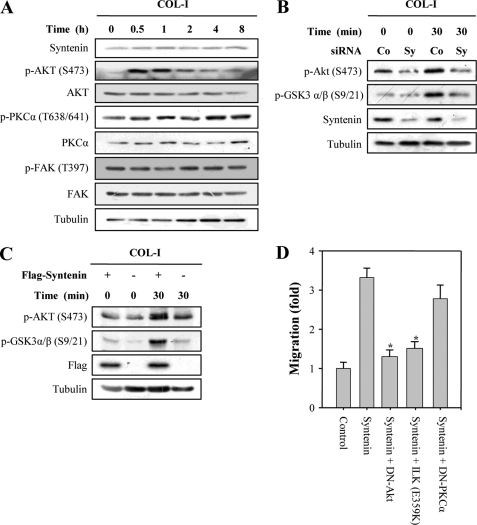
To investigate the molecular mechanism of mda-9/syntenin for inducing cell migration and invasion, we analyzed the effect of COL-I stimulation on mda-9/syntenin expression and PKCα, Akt, and FAK activation in MDA-MB-231 (Fig. 1A) and C8161 cells (supplemental Fig. 1), which have high endogenous mda-9/syntenin expression levels (8, 9). Unlike FN stimulation (15), COL-I stimulation did not significantly increase the expression levels of mda-9/syntenin, phospho-PKCα, and phospho-FAK in either cell line. However, COL-I stimulation significantly increased the expression level of phospho-Akt within 30 min, after which this induction showed a gradual return to the basal level (Fig. 1A and supplemental Fig. 1), suggesting that Akt activation could be predominantly induced at an early time during adhesion to COL-I in these cancer cells.
FIGURE 1.
mda-9/syntenin regulates COL-I-induced activation of Akt and cell migration. A, MDA-MB-231 cells were serum-starved for 12 h and allowed to adhere to COL-I-coated dishes (10 μg/ml) for the indicated periods of time in the absence of serum. Whole cell lysates were blotted with the indicated antibodies. B, MDA-MB-231 cells transfected with a control siRNA (Co) or mda-9/syntenin-targeted siRNA (Sy) were plated onto COL-I-coated dishes for the indicated periods of time. Whole cell lysates were blotted with the indicated antibodies. C, MCF-7 cells transfected with a control vector or FLAG-tagged mda-9/syntenin vector were plated onto COL-I-coated dishes for the indicated periods of time. Whole cell lysates were blotted with the indicated antibodies. D, MCF-7 cells stably transfected with a FLAG-tagged mda-9/syntenin vector were transfected the indicated constructs. Cells that migrate through the pores in the filter were fixed, stained, and counted in five random fields visualized by microscopy (×100). Data represent the average of three independent experiments performed in triplicate; *, statistically significant (p < 0.01).
We next examined the question of whether mda-9/syntenin regulates Akt activation during adhesion to COL-I. Transfection of mda-9/syntenin siRNA into MDA-MB-231 significantly reduced the phosphorylation levels of Akt and GSK-3α/β, a well known downstream target molecule of Akt that is induced by COL-I stimulation (Fig. 1B). In contrast, overexpression of mda-9/syntenin into MCF-7 cells increased COL-I-induced Akt and GSK-3α/β phosphorylation (Fig. 1C). Using a DN of Akt, we sought to determine whether or not Akt affected mda-9/syntenin-induced cell migration toward COL-I. mda-9/syntenin overexpression into MCF-7 cells induced cell migration toward COL-I; however, this effect was blocked by the DN of Akt but not PKCα. Of particular interest, mutant ILK (E359K), which is known to disrupt the interaction between ILK and both α-parvin and paxillin (24, 35), significantly suppressed mda-9/syntenin-induced cell migration toward COL-I (Fig. 1D). These results suggest that mda-9/syntenin may be involved in regulation of COL-I-induced activation of Akt and cell migration through the mediation of ILK.
ILK Is Required for Akt Activation during Adhesion to COL-I
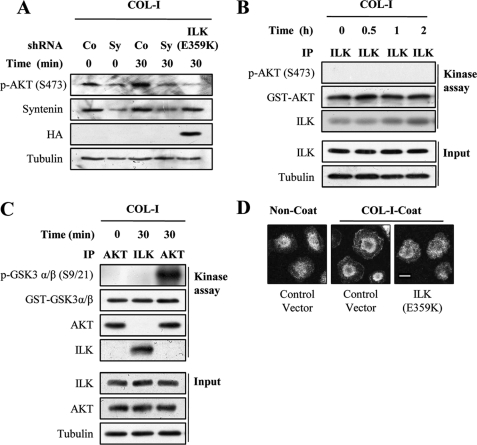
Overexpression of mutant ILK (E359K) suppressed COL-I-induced Akt phosphorylation (Fig. 2A), as did transfection of mda-9/syntenin shRNA. Because ILK has been shown to directly phosphorylate Akt (28–30), we next determined whether or not the kinase activity of ILK is required for COL-I-induced Akt phosphorylation. We thus performed in vitro kinase assays using Akt and GSK-3α/β as ILK substrates. ILK coimmunoprecipitated from MDA-MB-231 cells stimulated with COL-I showed no effect on Akt phosphorylation (Fig. 2B) and GSK-3α/β phosphorylation (Fig. 2C), confirming that ILK is a pseudokinase (22, 23, 31). In contrast, Akt coimmunoprecipitated from MDA-MB-231 cells stimulated with COL-I significantly phosphorylated GSK-3α/β. We next determined whether ILK affected the translocation of Akt to plasma membrane. Transfection of mutant ILK (E359K) significantly impaired the plasma membrane translocation of Akt (Fig. 2D). These results suggest that ILK adaptor function could be required for Akt activation during adhesion to COL-I.
FIGURE 2.
ILK is required for Akt activation during adhesion to COL-I. A, MDA-MB-231 cells transfected a control shRNA (Co), mda-9/syntenin-targeted shRNA (Sy), or HA-tagged ILK (E359K) vector were allowed to adhere to COL-I-coated dishes for the indicated periods of time. Whole cell lysates were blotted with the indicated antibodies. B and C, serum-starved MDA-MB-231 cells were allowed to adhere to COL-I-coated dishes (10 μg/ml) for the indicated periods of time. The cells were lysed, and ILK or Akt immunoprecipitates (IP) were conducted in in vitro kinase assays using GST-Akt (B) or GST-GSK-3α/β (C) as a substrate. Whole cell lysates (Input) were blotted with the indicated antibodies. D, MDA-MB-231 cells transfected with a control vector or HA-tagged ILK (E359K) vector were plated onto COL-I-coated for 30 min and fixed and stained with appropriate primary and secondary antibodies to visualized endogenous Akt (green, Alexa 488). Bar, 10 μm.
mda-9/Syntenin Regulates Plasma Membrane Targeting of Akt
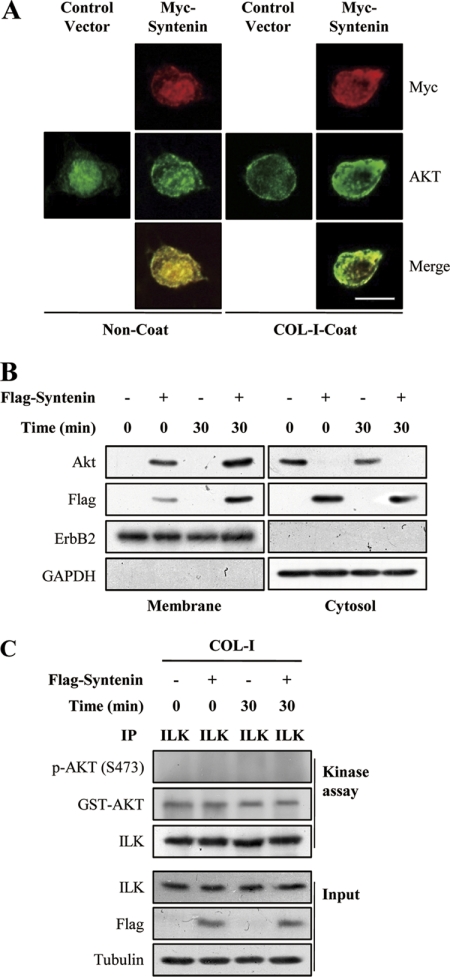
We next examined whether mda-9/syntenin affects the plasma membrane translocation of Akt during adhesion to COL-I. Overexpression of mda-9/syntenin increased the plasma membrane targeting of Akt in MCF-7 cells as assessed by immunofluorescence staining (Fig. 3A). COL-I stimulation further increased the colocalization of Akt with mda-9/syntenin at the plasma membrane. Moreover, translocation of mda-9/syntenin to the plasma membrane was increased by COL-I stimulation. We further confirmed this result with membrane fractionation, where overexpression of mda-9/syntenin again increased the membrane targeting of Akt and COL-I stimulation further increased translocation of Akt and mda-9/syntenin to the plasma membrane (Fig. 3B). Moreover, transfection of mda-9/syntenin siRNA into MDA-MB-231 significantly reduced plasma membrane translocation of Akt after COL-I stimulation (see Fig. 5C). However, overexpression of mda-9/syntenin did not show any effect on ILK kinase activity during adhesion to COL-I (Fig. 3C). Taken together, these results suggest that mda-9/syntenin may be involved in the activation of Akt by regulating translocation of Akt to plasma membrane during adhesion to COL-I.
FIGURE 3.
mda-9/syntenin regulates translocation of Akt to plasma membrane during adhesion to COL-I. A, MCF-7 cells transfected with a control vector or Myc-tagged mda-9/syntenin vector were plated onto COL-I-coated or uncoated coverslips for 30 min and fixed and stained with appropriate primary and secondary antibodies to visualized the Myc epitope (red, Alexa 546) and endogenous Akt (green, Alexa 488). Bar, 10 μm. B, MCF-7 cells transfected with a control vector or FLAG-tagged mda-9/syntenin vector were plated onto COL-I-coated dishes for the indicated period of time and then fractionated into cytosol and plasma membrane fraction. Both fractions were blotted with the indicated antibodies. ErbB2 was used as the membrane protein marker, and GAPDH was used as the cytosol protein marker. C, MCF-7 cells transfected with a control vector or FLAG-tagged mda-9/syntenin vector were plated onto COL-I-coated dishes for the indicated periods of time. The cells were lysed, and ILK immunoprecipitates (IP) were conducted in vitro kinase assays using GST-Akt as substrates. Whole cell lysates (Input) were blotted with the indicated antibodies.
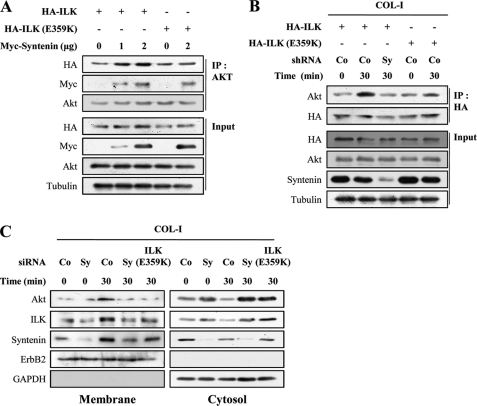
FIGURE 5.
mda-9/syntenin regulates the association between ILK and Akt at the plasma membrane in response to COL-I. A, MDA-MB-231 cells were transfected with HA-tagged ILK or HA-tagged ILK (E359K) vector together with the indicated amount of Myc-tagged mda-9/syntenin vector. Whole cell lysates were immunoprecipitated with Akt antibody. The immunoprecipitates (IP) and whole cell lysates (Input) were blotted with the indicated antibodies. B, MDA-MB-231 cells stably transfected a control shRNA (Co) or mda-9/syntenin-targeted shRNA (Sy) were transfected with HA-tagged ILK vector or HA-tagged ILK (E359K) vector, and then the cells were adhered to COL-I-coated dishes for the indicated periods of time. Whole cell lysates were immunoprecipitated with HA antibody. The immunoprecipitates and whole cell lysates (Input) were blotted with the indicated antibodies. C, MDA-MB-231 cells transfected with a control siRNA (Co), mda-9/syntenin-targeted siRNA (Sy), or HA-tagged ILK (E359K) vector were plated onto COL-I-coated dishes for the indicated periods of time and then fractionated into cytosol and plasma membrane fraction. Both fractions were blotted with the indicated antibodies. ErbB2 was used as the membrane protein marker, and GAPDH was used as the cytosol protein marker.
mda-9/Syntenin Facilitates Plasma Membrane Targeting of ILK
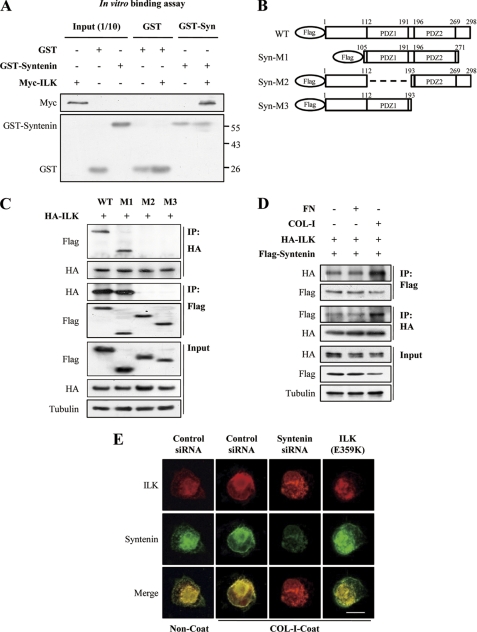
mda-9/syntenin physically associated with ILK both in vitro (Fig. 4A) and in cells (Fig. 4D, supplemental Fig. 2A). To determine which domain of mda-9/syntenin is responsible for interacting with ILK, we constructed a series of deletion mutants of mda-9/syntenin (Fig. 4B) and tested their interaction with ILK by coimmunoprecipitation assay (Fig. 4C). The mda-9/syntenin mutant containing both PDZ1 and PDZ2 domain associated with ILK. On the other hand, the mda-9/syntenin mutant lacking PDZ1 or PDZ2 domain did not associate with ILK, suggesting that the tandem two PDZ domains of mda-9/syntenin could be responsible for the interaction with ILK.
FIGURE 4.
mda-9/syntenin associates with ILK. A, recombinant GST-mda-9/syntenin interacts with recombinant Myc-ILK in vitro. In vitro GST pulldown analyses were performed by mixing 2 μg of recombinant GST or GST-mda-9/syntenin with 2 μg of recombinant Myc-ILK and incubating at 4 °C. GST pulldown assays were then analyzed by SDS-PAGE and immunoblotting by specific antibodies for GST and Myc. Ten percent of input recombinant GST or GST-mda-9/syntenin or Myc-ILK proteins were loaded and detected as a control. B, shown is schematic depiction of mda-9/syntenin mutants. C, HEK 293T17 cells were transfected with HA-tagged ILK and the indicated FLAG-tagged mda-9/syntenin mutant, and whole cell lysates were immunoprecipitated with HA and FLAG antibody. The immunoprecipitates (IP), and whole cell lysates (Input) were blotted with the indicated antibodies. D, MDA-MB-231 cells cotransfected with HA-tagged ILK and FLAG-tagged mda-9/syntenin vectors were adhered to COL-I-coated, FN-coated, or uncoated dishes for 30 min. Whole cell lysates were immunoprecipitated with HA and FLAG antibody. The immunoprecipitates and whole cell lysates (Input) were blotted with the indicated antibodies. E, MDA-MB-231 cells transfected with a control siRNA, mda-9/syntenin-targeted siRNA, or HA-tagged ILK (E359K) vector were plated onto COL-I-coated or uncoated coverslips for 30 min, fixed, and stained with appropriate primary and secondary antibodies to visualized the endogenous ILK (red, Alexa 546) and endogenous mda-9/syntenin (green, Alexa 488). Bar, 10 μm.
We next examined the effect of COL-I stimulation on the association of mda-9/syntenin with ILK. COL-I stimulation, but not FN, increased this association in MDA-MB-231 cells (Fig. 4D). Double immunofluorescence revealed that colocalization of mda-9/syntenin with ILK in the plasma membrane was significantly increased by COL-I stimulation (Fig. 4E); however, knockdown of mad-9/syntenin blocked translocation of ILK to the plasma membrane, as did overexpression of mutant ILK (E359K), suggesting that mda-9/syntenin may mediate the plasma membrane translocation of ILK in response to COL-I.
mda-9/Syntenin Facilitates Plasma Membrane Targeting of Akt by Mediating ILK
We next sought to determine whether or not mda-9/syntenin modulates the association between ILK and Akt. As previously reported (36), ILK associated with Akt (supplemental Fig. 2B). This association was increased by overexpression of mda-9/syntenin (Fig. 5A). The mutant ILK (E359K) also associated with Akt; however, an association of Akt with mutant ILK (E359K) was not modulated by overexpression of mda-9/syntenin (Fig. 5A). Moreover, COL-I stimulation also significantly increased the association between ILK and Akt; however, this association was impaired by mda-9/syntenin siRNA (Fig. 5B). In contrast, an association of Akt with mutant ILK (E359K) was not affected after COL-I stimulation. These results suggest that mda-9/syntenin may regulate the association of ILK with Akt in response to COL-I.
We next determined whether mda-9/syntenin regulates the plasma membrane translocation of ILK-Akt complex during adhesion to COL-I. Membrane fractionation revealed that COL-I stimulation increased the plasma membrane translocation of both Akt and ILK (Fig. 5C). Knockdown of mda-9/syntenin or overexpression of mutant ILK (E359K) blocked the plasma membrane targeting of both Akt and ILK after COL-I stimulation (Fig. 5C). Taken together, these results suggest that mda-9/syntenin may facilitate the plasma membrane translocation of ILK-Akt complex by regulating the association of ILK with Akt during adhesion to COL-I.
mda-9/Syntenin Regulates Formation of IPP Complex at the Plasma Membrane during Adhesion to COL-I
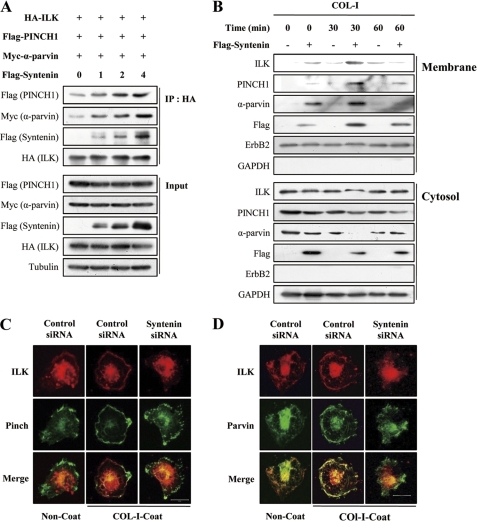
We investigated the question of whether mda-9/syntenin regulates formation and membrane translocation of the IPP complex, which functions as a signaling platform for at least β1 and β3 integrin (20, 29). mda-9/syntenin overexpression increased the associations of ILK with both PINCH1 and α-parvin (Fig. 6A). Overexpression of mda-9/syntenin into MCF-7 cells increased translocation of ILK, PINCH1, and α-parvin to the plasma membrane, and COL-I stimulation further increased plasma membrane translocation of ILK, PINCH1, and α-parvin (Fig. 6B). Double immunofluorescence also revealed that COL-I stimulation significantly increased the colocalization of ILK with PINCH1 and α-parvin at the plasma membrane in MDA-MB-231 cells. In contrast, mda-9/syntenin siRNA suppressed this colocalization (Fig. 6, C and D). These results suggest that mda-9/syntenin may facilitate translocation of IPP complex to plasma membrane through its association with ILK during adhesion to COL-I.
FIGURE 6.
Knockdown of mda-9/syntenin suppresses the plasma membrane translocation of ILK, PINCH1, and α-parvin complex during adhesion to COL-I. A, HEK 293T17 cells were transfected with the indicated constructs for 48 h. Whole cell lysates were immunoprecipitated with HA antibody, and then immunoprecipitates (IP) and whole cell lysates (Input) were blotted with the indicated antibodies. B, MCF-7 cells transfected with a control vector or FLAG-tagged mda-9/syntenin vector were plated onto COL-I-coated dishes for the indicated periods of time and then fractionated into cytosol and plasma membrane fraction. Both fractions were blotted with the indicated antibodies. ErbB2 was used as the membrane protein marker, and GAPDH was used as the cytosol protein marker. C, MDA-MB-231 cells were transfected with a control siRNA, or mda-9/syntenin-targeted siRNA were plated onto COL-I-coated or uncoated coverslips for 30 min, fixed, and stained with appropriate primary and secondary antibodies to visualized the endogenous ILK (red, Alexa 546) and PINCH1 (green, Alexa 488). D, MDA-MB-231 cells were prepared as described in c and stained with the appropriate primary and secondary antibodies to visualized the endogenous ILK (green, Alexa 488) and α-parvin (red, Alexa 546). Bar, 20 μm.
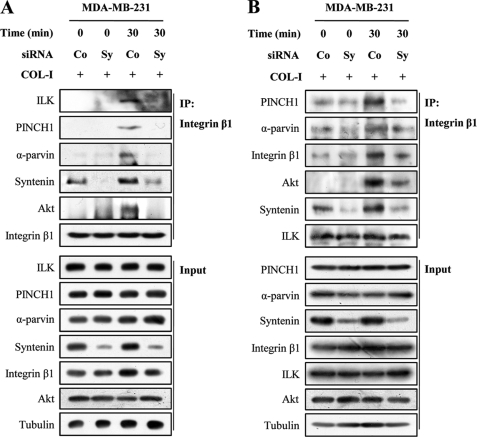
We next examined the question of whether mda-9/syntenin regulates COL-I-induced integrin β1-IPP complex associations. Integrin β1 immunoprecipitated from unstimulated MDA-MB-231 cells did not associate with ILK, PINCH1, α-parvin, Akt, and mda-9/syntenin (Fig. 7A). COL-I stimulation induced an integrin β1 association with ILK, PINCH1, α-parvin, Akt, and mda-9/syntenin; however, mda-9/syntenin siRNA blocked this effect. Similarly, for C8161 cells, transfection of mda-9/syntenin siRNA suppressed the COL-I-induced integrin β1 association with ILK, PINCH1, α-parvin, Akt, and mda-9/syntenin (supplemental Fig. 3). COL-I stimulation also increased the ILK association with integrin β1, PINCH1, α-parvin, Akt, and mda-9/syntenin; however, mda-9/syntenin siRNA blocked this effect (Fig. 7B). These results suggest that mda-9/syntenin could regulate formation of integrin β1 signaling complexes, including integrin β1, ILK, PINCH1, α-parvin, and Akt, at the plasma membrane during adhesion to COL-I.
FIGURE 7.
Knockdown of mda-9/syntenin suppresses COL-I-induced integrin β1-ILK-PINCH1-α-parvin associations in MDA-MB-231 cells. A and B, cells transfected with a control siRNA (Co) or mda-9/syntenin-targeted siRNA (Sy) were allowed to adhere to a COL-I-coated dishes for the indicated periods of time. Whole cell lysates were immunoprecipitated with integrin β1 (A) or ILK antibody (B). The immunoprecipitates (IP) and whole cell lysates (Input) were blotted with the indicated antibodies.
Inhibition of mda-9/Syntenin Impairs COL-I-induced Integrin β1 Signaling Pathways
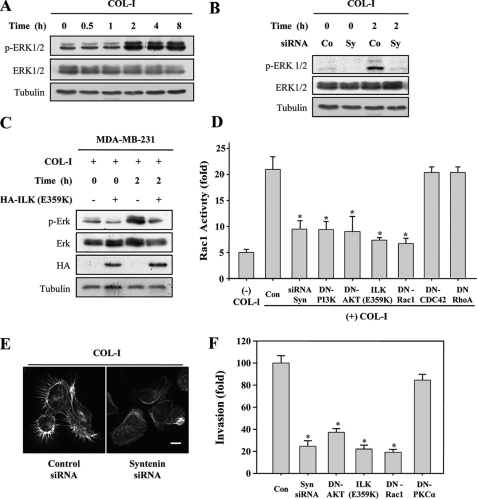
Because it has been known that the ternary complex of ILK, PINCH1, and α-parvin complex functions as a signaling platform for integrins (20, 30), we further investigated the role of mda-9/syntenin in COL-I-induced integrin signaling pathways. COL-I stimulation significantly increased the levels of phosphorylation of ERK1/2 in MDA-MB-231 (Fig. 8A) and C8161 cells (supplemental Fig. 1). COL-I-induced phosphorylation of ERK1/2 was blocked by mda-9/syntenin siRNA (Fig. 8B) and mutant ILK (E359K) (Fig. 8C). COL-I induced Rac1 activation; however, mda-9/syntenin siRNA or mutant ILK (E359K) blocked this activity (Fig. 8D). mda-9/syntenin siRNA showed consistent blockage of COL-I-induced reorganization of the actin cytoskeleton and formation of filopodia (Fig. 8E). mda-9/syntenin siRNA or mutant ILK (E359K) also blocked the invasiveness of MDA-MB-231 cells (Fig. 8F). These results further confirmed that mda-9/syntenin could be a critical regulator of formation of the integrin β1-IPP signaling complex at the plasma membrane during adhesion to COL-I.
FIGURE 8.
Knockdown of mda-9/syntenin suppresses COL-I-induced activation of ERK1/2 and Rac1 as well as cell invasion and reorganization of actin cytoskeleton. A, MDA-MB-231 cells were allowed to adhere to COL-I-coated dishes for the indicated periods of time. Whole cell lysates were blotted with the indicated antibodies. B, MDA-MB-231 cells transfected with a control siRNA (Co) or mda-9/syntenin-targeted siRNA (Sy) were plated onto COL-I-coated dishes for 2 h. Whole cell lysates were blotted with the indicated antibodies. C, MDA-MB-231 cells transfected with a control vector or HA-tagged ILK (E359K) vector were plated onto COL-I-coated dishes for 2 h. Whole cell lysates were blotted with the indicated antibodies. D, MDA-MB-231 cells transfected with the indicated constructs were plated onto COL-I-coated dishes for 30 min, and then Rac1 activity was determined. Data represent the average of two independent experiments performed in triplicate; *, statistically significant (p < 0.01) versus the control. E, MDA-MB-231 cells transfected with a control siRNA or mda-9/syntenin-targeted siRNA were plated onto COL-I-coated coverslips for 30 min and stained with FITC-labeled phalloidin to detect of F-actin filaments. Bar, 10 μm. F, MDA-MB-231 cells were transfected with the indicated constructs. Cells that invade through the pores in the filter were fixed, stained, and counted in five random fields visualized by microscopy (×100). Data represent average of three independent experiments performed in triplicate; *, statistically significant (p < 0.01) versus the control. Con, control.
DISCUSSION
ILK is a central component for the assembly of the ILK-PINCH1-α-parvin complex that functions as a signaling platform for integrins. In the present study we have demonstrated that mda-9/syntenin positively regulates the scaffold function of ILK by facilitating the formation of ILK-PINCH1-α-parvin complex at the plasma membrane during adhesion to COL-I, leading to activation of ILK-mediated signaling molecules, such as Akt, ERK1/2, and Rac1, in human breast and melanoma cells.
Akt activation is a complex process involving at least two major steps, namely membrane translocation and subsequent phosphorylation of Akt at Ser-473 and Thr-308 (37, 38). Activation of Akt could be regulated at either the membrane translocation or phosphorylation steps. The mechanism by which ILK functions in activation of Akt has been controversial. ILK has been widely claimed to act as a signaling serine/threonine kinase, which triggers diverse integrin signaling pathways (20, 29). ILK has been found to be capable of direct phosphorylation of diverse substrates, including myosin light-chain kinase (39) and Akt (20, 29, 36). Purified ILK from mammalian cell extracts has been shown to coimmunoprecipitate and phosphorylate Akt (28, 36). However, several inactivating point mutations in the kinase domain of ILK also impair interaction of ILK with essential binding partners, such as α-parvin (34, 40). For example, the E359K mutation has no effect on the kinase activity of ILK in vitro; however, this mutation disrupts the interaction between ILK and α-parvin and reduces phosphorylation of Akt (26, 35), suggesting that the kinase activity of ILK is dispensable for Akt activation. Moreover, depletion of ILK, PINCH1, or α-parvin prevented phosphorylation of Akt (32, 41, 42), and α-parvin is required for the translocation of Akt to the plasma membrane (41). Recently, it has been reported that the kinase domain of ILK is not an active site conformation but, rather, recognizes α-parvin for promoting effective assembly of ILK into focal adhesion (22, 23, 31). In further support of this, we showed that ILK coimmunoprecipitated from COL-I-stimulated cells was not capable of direct phosphorylation of Akt and GSK3α/β. Moreover, overexpression of mutant ILK (E359K) also impaired COL-I-induced plasma membrane translocation of Akt. Therefore, ILK activates Akt indirectly by facilitating the translocation of Akt to the plasma membrane, but not the subsequent phosphorylation steps, during adhesion to COL-I.
We showed that mda-9/syntenin regulated COL-I-induced activation of Akt. This finding raises intriguing questions as to how mda-9/syntenin activates Akt. The association of mda-9/syntenin with Akt was dependent on ILK in response to COL-I (supplemental Fig. 4). Knockdown of ILK impaired COL-I-induced association of mda-9/syntenin with Akt, suggesting that ILK could function as a scaffold for the interaction between mad-9/syntenin and Akt. mda-9/syntenin was directly associated with ILK and facilitated the association between ILK and Akt during adhesion to COL-I. Overexpression of mda-9/syntenin increased the translocation of ILK and Akt to plasma membrane. In contrast, transfection of mda-9/syntenin siRNA inhibited plasma membrane targeting of ILK and Akt. Thus, mda-9/syntenin may regulate Akt activation by facilitating the translocation of Akt to plasma membrane via enhancement of ILK scaffolding function during adhesion to COL-I. We find it worthy of mention that inhibition or overexpression of mda-9/syntenin modulated activation of Akt and its downstream signaling molecules at the basal level (supplemental Fig. 5). Future studies will be required to further test the question of whether mda-9/syntenin is involved in the activation processes of Akt induced by other stimuli, such as growth factors.
The ternary complex of the IPP complex has emerged as an essential constituent of integrin-containing adhesion sites, and it can function both as a structural module that connects integrins to the actin cytoskeleton and as a signaling platform that modulates various cellular processes (20, 21, 29). The IPP complex is a central constituent of at least β1 and β3 integrin containing adhesion sites, from where it regulates multiple signaling pathways, including Akt, ERK1/2, and Rac1 (20, 21). We demonstrated here that inhibition of mda-9/syntenin suppressed the plasma membrane translocation of the IPP complex as well as the associations of integrin β1-IPP complexes during adhesion to COL-I. Moreover, inhibition of mda-9/syntenin completely blocked reorganization of the actin cytoskeleton, invasion, and migration as well as activation of ILK-mediated signaling molecules, such as Akt, Rac1, and ERK1/2, in response to COL-I. Therefore, mda-9/syntenin could be an important regulator of ILK adaptor function by facilitating translocation of IPP complex to the plasma membrane during adhesion to COL-I. The detailed mechanisms by which mda-9/syntenin assembles COL-I-induced IPP complex remain to be elucidated.
ILK is well recognized as an oncogenic protein and is highly expressed in numerous human cancers (29, 32). The degree of ILK expression correlates with tumor stage and grade in many of these malignancies, and strong ILK expression is often a poor prognostic indicator (32, 43, 44). ILK functions as a key regulator of cellular events critical to cancer progression, including migration, invasion, and angiogenesis (29). The necessity of ILK for cancer progression, particularly in carcinomas, has centered on the capacity of ILK to activate Akt (29, 32). In this study we showed that mda-9/syntenin may function as an important regulator for ILK adaptor function to create signaling platform that activates the downstream signaling molecules such as Akt and ERK1/2. Therefore, targeted therapies that inactivate mda-9/syntenin may provide a rational molecular approach to improve the prognosis of cancer patients.
In summary, we demonstrated here that mda-9/syntenin regulates the assembly of IPP complex at the plasma membrane and activation of ILK-mediated signaling molecules and that it may function as an adaptor for assembly of integrin β1 signal complexes during adhesion to COL-I. Consequently, increased expression of mda-9/syntenin in certain cancers would result in the increase of its ability to assemble with sufficient efficiency to integrin β1-associated signaling platform, leading to activation of Akt, Rac1, and ERK1/2, and would, therefore, have a profound effect on integrins signaling pathways. Our findings also support a hypothesis that mda-9/syntenin functions as an adaptor protein for assembly of multimeric signaling complexes clustered at high concentrations on the plasma membrane.
This work was supported by Basic Science Research Program Grants 2009-0073826 and 2010-0007366 through the National Research Foundation of Korea and the Regional Research Core Research Program/Medical and Biomaterials Research Center funded by the Ministry of Education, Science, and Technology.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
- PDZ
- postsynaptic density protein, disc large, and zonula occludens
- ILK
- integrin-linked kinase
- COL-I
- type I collagen
- DN
- dominant negative
- FAK
- focal adhesion kinase
- FN
- fibronectin
- GSK
- glycogen synthase kinase
- IPP
- ILK, PINCH, α-parvin.
REFERENCES
- 1. Pawson T., Scott J. D. (1997) Science 278, 2075–2080 [DOI] [PubMed] [Google Scholar]
- 2. Hung A. Y., Sheng M. (2002) J. Biol. Chem. 277, 5699–5702 [DOI] [PubMed] [Google Scholar]
- 3. Koroll M., Rathjen F. G., Volkmer H. (2001) J. Biol. Chem. 276, 10646–10654 [DOI] [PubMed] [Google Scholar]
- 4. Sarkar D., Boukerche H., Su Z. Z., Fisher P. B. (2008) Cancer Res. 68, 3087–3093 [DOI] [PubMed] [Google Scholar]
- 5. Beekman J. M., Coffer P. J. (2008) J. Cell Sci. 121, 1349–1355 [DOI] [PubMed] [Google Scholar]
- 6. Kang B. S., Cooper D. R., Jelen F., Devedjiev Y., Derewenda U., Dauter Z., Otlewski J., Derewenda Z. S. (2003) Structure 11, 459–468 [DOI] [PubMed] [Google Scholar]
- 7. Grembecka J., Cierpicki T., Devedjiev Y., Derewenda U., Kang B. S., Bushweller J. H., Derewenda Z. S. (2006) Biochemistry 45, 3674–3683 [DOI] [PubMed] [Google Scholar]
- 8. Koo T. H., Lee J. J., Kim E. M., Kim K. W., Kim H. D., Lee J. H. (2002) Oncogene 21, 4080–4088 [DOI] [PubMed] [Google Scholar]
- 9. Boukerche H., Su Z. Z., Emdad L., Baril P., Balme B., Thomas L., Randolph A., Valerie K., Sarkar D., Fisher P. B. (2005) Cancer Res. 65, 10901–10911 [DOI] [PubMed] [Google Scholar]
- 10. Boukerche H., Su Z. Z., Emdad L., Sarkar D., Fisher P. B. (2007) Cancer Res. 67, 1812–1822 [DOI] [PubMed] [Google Scholar]
- 11. Meerschaert K., Bruyneel E., De Wever O., Vanloo B., Boucherie C., Bracke M., Vandekerckhove J., Gettemans J. (2007) Exp. Cell Res. 313, 1790–1804 [DOI] [PubMed] [Google Scholar]
- 12. Boukerche H., Su Z. Z., Prévot C., Sarkar D., Fisher P. B. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15914–15919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dash R., Su Z. Z., Lee S. G., Azab B., Boukerche H., Sarkar D., Fisher P. B. (2010) Oncogene 29, 4412–4423 [DOI] [PubMed] [Google Scholar]
- 14. Ishizawar R., Parsons S. J. (2004) Cancer Cell 6, 209–214 [DOI] [PubMed] [Google Scholar]
- 15. Hwangbo C., Kim J., Lee J. J., Lee J. H. (2010) Cancer Res. 70, 1645–1655 [DOI] [PubMed] [Google Scholar]
- 16. Watt F. M. (2002) EMBO J. 21, 3919–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hynes R. O. (2002) Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 18. Hannigan G. E., Leung-Hagesteijn C., Fitz-Gibbon L., Coppolino M. G., Radeva G., Filmus J., Bell J. C., Dedhar S. (1996) Nature 379, 91–96 [DOI] [PubMed] [Google Scholar]
- 19. Schaller M. D., Parsons J. T. (1994) Curr. Opin. Cell Biol. 6, 705–710 [DOI] [PubMed] [Google Scholar]
- 20. Legate K. R., Montañez E., Kudlacek O., Fässler R. (2006) Nat. Rev. Mol. Cell Biol. 7, 20–31 [DOI] [PubMed] [Google Scholar]
- 21. McDonald P. C., Fielding A. B., Dedhar S. (2008) J. Cell Sci. 121, 3121–3132 [DOI] [PubMed] [Google Scholar]
- 22. Fukuda K., Gupta S., Chen K., Wu C., Qin J. (2009) Mol. Cell 36, 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wickström S. A., Lange A., Montanez E., Fässler R. (2010) EMBO J. 29, 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiswell B. P., Zhang R., Murphy J. W., Boggon T. J., Calderwood D. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 20677–20682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li F., Zhang Y., Wu C. (1999) J. Cell Sci. 112, 4589–4599 [DOI] [PubMed] [Google Scholar]
- 26. Nikolopoulos S. N., Turner C. E. (2002) J. Biol. Chem. 277, 1568–1575 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y., Chen K., Tu Y., Velyvis A., Yang Y., Qin J., Wu C. (2002) J. Cell Sci. 115, 4777–4786 [DOI] [PubMed] [Google Scholar]
- 28. Delcommenne M., Tan C., Gray V., Rue L., Woodgett J., Dedhar S. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hannigan G., Troussard A. A., Dedhar S. (2005) Nat. Rev. Cancer 5, 51–63 [DOI] [PubMed] [Google Scholar]
- 30. Maydan M., McDonald P. C., Sanghera J., Yan J., Rallis C., Pinchin S., Hannigan G. E., Foster L. J., Ish-Horowicz D., Walsh M. P., Dedhar S. (2010) PLoS One 5, e12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fukuda K., Knight J. D., Piszczek G., Kothary R., Qin J. (2011) J. Biol. Chem. 286, 21886–218895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tan C., Cruet-Hennequart S., Troussard A., Fazli L., Costello P., Sutton K., Wheeler J., Gleave M., Sanghera J., Dedhar S. (2004) Cancer Cell 5, 79–90 [DOI] [PubMed] [Google Scholar]
- 33. Ling L., Goeddel D. V. (2000) J. Biol. Chem. 275, 23852–23860 [DOI] [PubMed] [Google Scholar]
- 34. Kim K. K., Lee J. J., Yang Y., You K. H., Lee J. H. (2008) Carcinogenesis 29, 704–712 [DOI] [PubMed] [Google Scholar]
- 35. Yamaji S., Suzuki A., Sugiyama Y., Koide Y., Yoshida M., Kanamori H., Mohri H., Ohno S., Ishigatsubo Y. (2001) J. Cell Biol. 153, 1251–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Persad S., Attwell S., Gray V., Mawji N., Deng J. T., Leung D., Yan J., Sanghera J., Walsh M. P., Dedhar S. (2001) J. Biol. Chem. 276, 27462–27469 [DOI] [PubMed] [Google Scholar]
- 37. Filippa N., Sable C. L., Hemmings B. A., Van Obberghen E. (2000) Mol. Cell Biol. 20, 5712–5721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Restuccia D. F., Hemmings B. A. (2009) Science 325, 1083–1084 [DOI] [PubMed] [Google Scholar]
- 39. Deng J. T., Van Lierop J. E., Sutherland C., Walsh M. P. (2001) J. Biol. Chem. 276, 16365–16373 [DOI] [PubMed] [Google Scholar]
- 40. Attwell S., Mills J., Troussard A., Wu C., Dedhar S. (2003) Mol. Biol. Cell 14, 4813–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fukuda T., Guo L., Shi X., Wu C. (2003) J. Cell Biol. 160, 1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nho R. S., Xia H., Kahm J., Kleidon J., Diebold D., Henke C. A. (2005) J. Biol. Chem. 280, 26630–26639 [DOI] [PubMed] [Google Scholar]
- 43. Dai D. L., Makretsov N., Campos E. I., Huang C., Zhou Y., Huntsman D., Martinka M., Li G. (2003) Clin. Cancer Res. 9, 4409–4414 [PubMed] [Google Scholar]
- 44. Papachristou D. J., Gkretsi V., Rao U. N, Papachristou G. I., Papaefthymiou O. A., Basdra E. K., Wu C., Papavassiliou A. G. (2008) Eur. J. Cancer 44, 2518–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]