Abstract
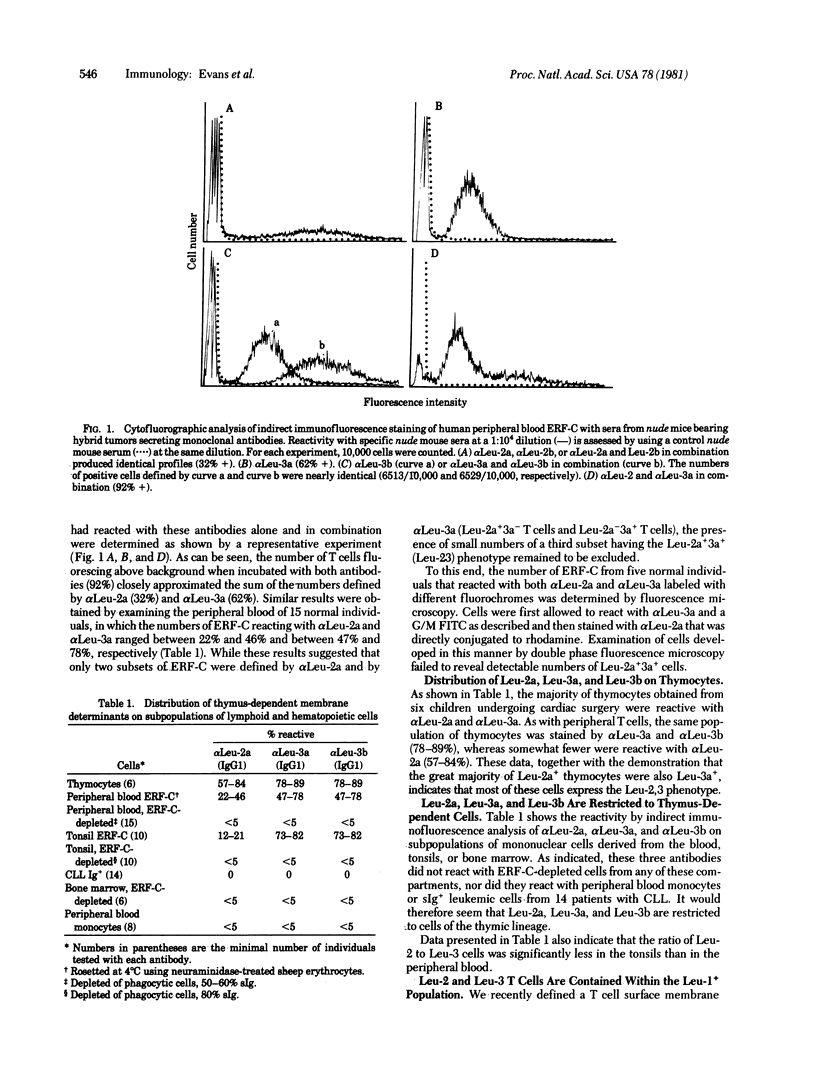
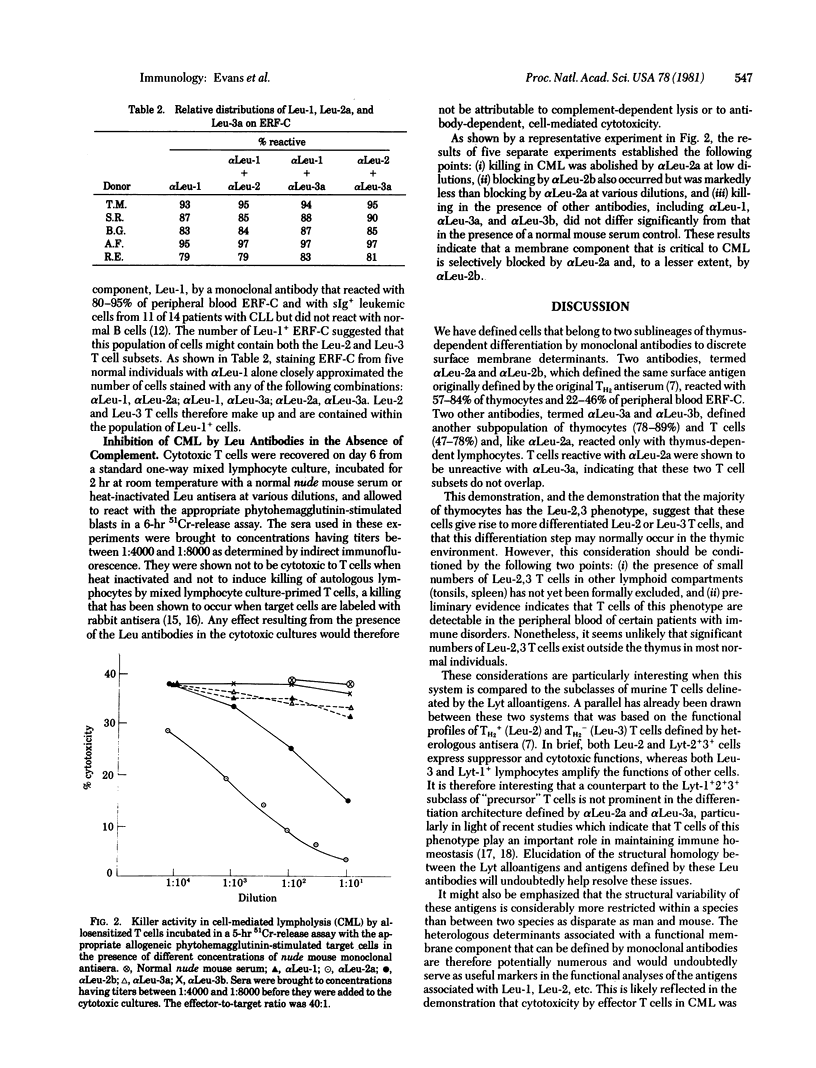
In prior studies a heteroantiserum to a surface membrane component termed TH2 was used to define two subsets of human T cells (TH2+ and TH2-), which were found to express distinct sets of activities in vitro. In the present studies we prepared monoclonal antibodies to surface determinants that are restricted to T cells belonging to each of these two subsets. Two antibodies, termed αLeu-2a and αLeu-2b, which seem to define the same surface antigen identified by the original TH2 antiserum, reacted with 57-84% of thymocytes and 22-46% of the erythrocyte-rosette-forming cells (ERF-C) in peripheral blood. Two other monoclonal antibodies, termed αLeu-3a and αLeu-3b, reacted with the same subpopulation of thymocytes (78-89%) and peripheral blood ERF-C (47-78%) but, unlike αLeu-2a and αLeu-2b, did not exhibit cross-blocking; i.e., labeling cells with αLeu-3a did not inhibit the subsequent binding of αLeu-3b. T cells reactive with αLeu-2a were shown to be unreactive with αLeu-3a, indicating that two separate subpopulations of T cells, Leu-2 (formerly TH2+) and Leu-3 (TH2-) T cells, were thereby defined. These two T cell subsets make up the subpopulation of ERF-C (80-95%) previously defined by a monoclonal antibody to a T cell membrane antigen (Leu-1) that has a thymus-dependent distribution on normal lymphocytes but is expressed by some surface-immunoglobulin-positive (sIg+) leukemic lymphocytes. None of the Leu antibodies reported here reacted with sIg+, Leu-1+ leukemic cells, nor did they react with normal hematopoietic cells or lymphoid cells that had surface markers characteristic of B cells. Studies of the blocking effects of Leu antibodies on killing in cell-mediated lympholysis by effector T cells were carried out in the absence of complement. These experiments established the following points: (i) αLeu-2a abolished the killing by cytotoxic T cells of allogeneic phytohemagglutinin-stimulated blasts, (ii) inhibition of killing by αLeu-2b was markedly less than inhibition by αLeu-2a, and (iii) other antibodies, including αLeu-1, αLeu-3a, and αLeu-3b, had little or no effect on killing in cell-mediated lympholysis. The relevance of these findings to prior studies done in the mouse and in man are discussed.
Keywords: T cell antigen, receptor, functional T cell subsets, cytotoxicity
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyse E. A., Itakura K., Stockert E., Iritani C. A., Miura M. Ly-C: a third locus specifying alloantigens expressed only on thymocytes and lymphocytes. Transplantation. 1971 Mar;11(3):351–353. [PubMed] [Google Scholar]
- Boyse E. A., Miyazawa M., Aoki T., Old L. J. Ly-A and Ly-B: two systems of lymphocyte isoantigens in the mouse. Proc R Soc Lond B Biol Sci. 1968 Jun 11;170(1019):175–193. doi: 10.1098/rspb.1968.0032. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975 Jun 1;141(6):1390–1399. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., McVay-Boudreau L., Hugenberger J., Naidorf K., Shen F. W., Gershon R. K. Immunoregulatory circuits among T-cell sets. II. Physiologic role of feedback inhibition in vivo: absence in NZB mice. J Exp Med. 1978 Apr 1;147(4):1116–1125. doi: 10.1084/jem.147.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eardley D. D., Hugenberger J., McVay-Boudreau L., Shen F. W., Gershon R. K., Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978 Apr 1;147(4):1106–1115. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Breard J. M., Lazarus H., Schlossman S. F., Chess L. Detection, isolation, and functional characterization of two human T-cell subclasses bearing unique differentiation antigens. J Exp Med. 1977 Jan 1;145(1):221–233. doi: 10.1084/jem.145.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Chess L., Levine H., Schlossman S. F. Antibody-dependent cellular cytotoxicity by allosensitized human T cells. J Exp Med. 1978 Feb 1;147(2):605–610. doi: 10.1084/jem.147.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Lazarus H., Penta A. C., Schlossman S. F. Two functionally distinct subpopulations of human T cells that collaborate in the generation of cytotoxic cells responsible for cell-mediated lympholysis. J Immunol. 1978 Apr;120(4):1423–1428. [PubMed] [Google Scholar]
- Gibson D. M., Taylor B. A., Cherry M. Evidence for close linkage of a mouse light chain marker with the Ly-2,3 locus. J Immunol. 1978 Oct;121(4):1585–1590. [PubMed] [Google Scholar]
- Gottlieb P. D. Genetic correlation of a mouse light chain variable region marker with a thymocyte surface antigen. J Exp Med. 1974 Nov 1;140(5):1432–1437. doi: 10.1084/jem.140.5.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Nakayama E., Shiku H., Stockert E., Oettgen H. F., Old L. J. Cytotoxic T cells: Lyt phenotype and blocking of killing activity by Lyt antisera. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1977–1981. doi: 10.1073/pnas.76.4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Pichler W. J., Nelson D. L. Fc receptors on human T-lymphocytes. III. Characterization of subpopulations involved in cell-mediated lympholysis and antibody-dependent cellular cytotoxicity. J Immunol. 1979 Feb;122(2):599–604. [PubMed] [Google Scholar]
- Shiku H., Kisielow P., Bean M. A., Takahashi T., Boyse E. A., Oettgen H. F., Old L. J. Expression of T-cell differentiation antigens on effector cells in cell-mediated cytotoxicity in vitro. Evidence for functional heterogeneity related to the surface phenotype of T cells. J Exp Med. 1975 Jan 1;141(1):227–241. doi: 10.1084/jem.141.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Good R. A., Ammirati P., Dymbort G., Evans R. L. Identification of a p69,71 complex expressed on human T cells sharing determinants with B-type chronic lymphatic leukemic cells. J Exp Med. 1980 Jun 1;151(6):1539–1544. doi: 10.1084/jem.151.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]



