Abstract
7-Ketocholesterol is a bioactive sterol, a potent competitive inhibitor of cytochrome P450 7A1, and toxic in liver cells. Multiple origins of this compound have been identified, with cholesterol being the presumed precursor. Although routes for formation of the 7-keto compound from cholesterol have been established, we found that 7-dehydrocholesterol (the immediate precursor of cholesterol) is oxidized by P450 7A1 to 7-ketocholesterol (kcat/Km = 3 × 104 m−1 s−1). P450 7A1 converted lathosterol (Δ5-dihydro-7-dehydrocholesterol) to a mixture of the 7-keto and 7α,8α-epoxide products (∼1:2 ratio), with the epoxide not rearranging to the ketone. The oxidation of 7-dehydrocholesterol occured with predominant formation of 7-ketocholesterol and with the 7α,8α-epoxide as only a minor product; the synthesized epoxide was stable in the presence of P450 7A1. The mechanism of 7-dehydrocholesterol oxidation to 7-ketocholesterol is proposed to involve a FeIII-O-C-C+ intermediate and a 7,8-hydride shift or an alternative closing to yield the epoxide (Liebler, D. C., and Guengerich, F. P. (1983) Biochemistry 22, 5482–5489). Accordingly, reaction of P450 7A1 with 7-[2H1]dehydrocholesterol yielded complete migration of deuterium in the product 7-ketocholesterol. The finding that 7-dehydrocholesterol is a precursor of 7-ketocholesterol has relevance to an inborn error of metabolism known as Smith-Lemli-Opitz syndrome (SLOS) caused by defective cholesterol biosynthesis. Mutations within the gene encoding 7-dehydrocholesterol reductase, the last enzyme in the pathway, lead to the accumulation of 7-dehydrocholesterol in tissues and fluids of SLOS patients. Our findings suggest that 7-ketocholesterol levels may also be elevated in SLOS tissue and fluids as a result of P450 7A1 oxidation of 7-dehydrocholesterol.
Keywords: Cholesterol Metabolism, Cytochrome P450, Enzyme Mechanisms, Inborn Errors of Metabolism, Oxidation-Reduction, Dehydrocholesterol, Oxysterols
Introduction
Cholesterol is an important component of membranes and a precursor for critical signaling molecules. The balance of synthesis and degradation of cholesterol and its conversions to androgens, estrogens, glucocorticoids, and other important steroids are regulated in part by a number of cytochrome P450 enzymes (1, 2). We have been interested in several issues regarding cholesterol metabolism. One is the fate of 7-dehydrocholesterol, the immediate precursor of cholesterol. 7-Dehydrocholesterol is observed at very low levels or is not normally detectable in tissues and fluids of humans except in skin (3–5), where it is converted to previtamin D3 under UV irradiation (6). Thus, 7-dehydrocholesterol is important aside from its reduction to cholesterol. Some humans are deficient in 7-dehydrocholesterol reductase (DHCR7) and exhibit Smith-Lemli-Opitz syndrome (SLOS),2 with elevated levels of 7-dehydrocholesterol and attenuated levels of cholesterol (3, 7–10). 7-Dehydrocholesterol is exceptionally susceptible to free radical oxidation (11), and more than one dozen biologically active oxysterol products have been characterized from 7-dehydrocholesterol oxidation in solution (12–14). In particular, we have been interested in the enzymatic conversion of 7-dehydrocholesterol to oxysterols.
With regard to oxysterols, there is considerable interest in this general class of compounds because of their presence in humans and their biological properties (15–19). One oxysterol of interest is 7-ketocholesterol, which is a strong inhibitor of cytochrome P450 7A1, the cholesterol 7α-hydroxylase (IC50 ∼ 1 μm) (20). 7-Ketocholesterol can also exert biological activities, e.g. regulation of cholesterol homeostasis, cytotoxicity, and apoptosis and induction of inflammation, growth inhibition, and vascular endothelial growth factor (16, 19, 21–24). The level of 7-ketocholesterol in human plasma is about one-half that of 7α-hydroxycholesterol, the product of the first committed step of bile acid synthesis (16, 25–27). 7-Ketocholesterol is one of the major oxysterols in human atherosclerotic plaques and accumulates in photodamaged rat retina (15, 28). Levels of 7-ketocholesterol are also elevated in patients with some inherited diseases, e.g. cerebrotendinous xanthomatosis (25) and Niemann-Pick C1 disease (29), and in several animal models of diseases (30, 31). Cholesterol has been the presumed precursor of 7-ketocholesterol, but the mechanism of formation of 7-ketocholesterol in the body is unclear. Much of the literature attributes its source to lipid peroxidation and other radical processes acting on cholesterol (16, 28, 32–35). A 7-hydroxycholesterol dehydrogenase was purified from hamster liver microsomes, although the enzyme is apparently not present in rats or (most) humans (36). 7-Ketocholesterol was not among the many identified oxysterols in the free radical oxidation of 7-dehydrocholesterol (12). However, in a recent study, 7-ketocholesterol was one of the major oxysterols observed in a rat model for SLOS, in which 7-dehydrocholesterol is the dominant sterol (37).
We demonstrate the conversion of 7-dehydrocholesterol to 7-ketocholesterol in human liver microsomes. Of several human P450 enzymes examined, only P450 7A1, the classic cholesterol 7α-hydroxylase, catalyzed the reaction. Detailed studies showed what clearly appears to be a direct oxidation (of the 7,8-olefin) to 7-ketocholesterol, with only trace formation of a 7,8-epoxide but not its required intermediacy (see Fig. 1). P450-catalyzed direct oxidation of an olefin to a carbonyl has been reported before (38, 39), but this appears to be the first case of a dominant conversion with only minor epoxide formation and is relevant to a physiological reaction.
FIGURE 1.
Proposed oxidation mechanisms. A, lathosterol; B, 7-dehydrocholesterol. Step a, direct oxidation to 7-keto product; step b, epoxidation.
EXPERIMENTAL PROCEDURES
Chemicals
Cholesterol, 7-dehydrocholesterol, 2-hydroxypropyl-β-cyclodextrin (HPβCD), l-α-1,2-dioleoyl-sn-glycero-3-phosphocholine, l-α-1,2-dilauroyl-sn-glycero-3-phosphoserine, m-chloroperbenzoic acid, and Tween 20 were purchased from Sigma-Aldrich, and l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine was purchased from Enzo Life Sciences, Inc. (Plymouth Meeting, PA). m-Chloroperbenzoic acid (in CH2Cl2) was washed with 100 mm potassium phosphate buffer (pH 7.4) and dried over Na2SO4 before use. Lathosterol (Fig. 1), 7-ketocholesterol, 7-ketocholestanol, and 7α-hydroxycholesterol were purchased from Steraloids Inc. (Newport, RI). [25,26,26,26,27,27,27-d7]-7-Dehydrocholesterol was synthesized as described previously (13). [25,26,26,26,27,27,27-d7]-7-Ketocholesterol was purchased from Toronto Research Chemicals (North York, Ontario, Canada). HPLC-grade solvents were purchased from Fisher.
Enzyme Preparations
Recombinant P450 7A1 with a C-terminal His6 tag was expressed in Escherichia coli using a plasmid originally provided by I. Pikuleva (Case Western Reserve University, Cleveland, OH) and purified as described elsewhere (40). Recombinant P450 3A4 with a C-terminal His5 tag (41) was expressed in E. coli and purified as described elsewhere (42). Rat NADPH:P450 reductase (43) and human cytochrome b5 (44) were expressed in E. coli and purified as described elsewhere.
MS
FinniganTM TSQ Quantum and Orbitrap (high resolution mass spectrometry (HRMS)) mass spectrometers (ThermoFisher Scientific, Sunnydale, CA) connected to a Waters ACQUITY UPLC® system were used for analyses of sterol products. Electron impact HRMS was done at the University of Notre Dame facility (South Bend, IN).
Measurement of 7-Dehydrocholesterol Oxidation with P450 7A1 and P450 3A4 Reconstituted Systems
Enzyme reaction mixtures for P450 7A1 reactions typically contained 0.2 μm P450 7A1, 3.0 μm NADPH:P450 reductase, 60 μm l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine, 7-dehydrocholesterol in 3.1 mm HPβCD (0.45%, w/v), and 0.41 mm Tween 20 (0.05%, v/v) in 50 mm potassium phosphate buffer (pH 7.4) (40). For P450 3A4 reactions, a “5× P450 protein premix” was prepared with slight modification of a previously described method (45). Briefly, a 5× protein premix including 2.5 μm P450 3A4, 5 μm NADPH:P450 reductase, 2.5 μm cytochrome b5, 150 μg/ml phospholipid mixture (1:1:1 (w/w/w) l-α-1,2-dioleoyl-sn-glycero-3-phosphocholine/l-α-1,2-dilauroyl-sn-glycero-3-phosphoserine/l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine), 0.50 mg/ml potassium CHAPS (0.77 mm), and 3.0 mm GSH in 50 mm potassium HEPES buffer (pH 7.4) and a 5× buffer mix including 12 mm GSH and 150 mm MgCl2 in 200 mm potassium HEPES buffer (pH 7.4) were prepared. A typical 500-μl enzyme reaction mixture was prepared by mixing 100 μl of 5× P450 3A4 protein premix, an equal volume of 5× buffer mix, 5 μl of 7-dehydrocholesterol solution in 310 mm HPβCD (45%, w/v), and 220 μl of H2O. Incubations (at 37 °C) were initiated by the addition of an NADPH-generating system (46). Reactions were quenched with 2.0 ml of CH2Cl2 and mixed with a Vortex device. Following centrifugation (2000 × g, 5 min), 1.4 ml of the organic layer was transferred and taken to dryness under an N2 stream. The resulting samples were dissolved in 100 μl of the solution including 70% (v/v) CH3CN, 30% (v/v) CH3OH, 0.5 mm butylated hydroxytoluene (Sigma), and 0.5 mm triphenylphosphine (Sigma) for analysis.
Samples were analyzed by LC-MS using a UPLC system connected to a TSQ Quantum mass spectrometer with an ACQUITY UPLC BEH C18 octadecylsilane column (2.1 × 100 mm, 1.7 μm). LC conditions were as follow: solvent A contained 95% (v/v) H2O and 5% (v/v) CH3CN, and solvent B contained 95% (v/v) CH3CN and 5% (v/v) CH3OH. The column was maintained at an initial condition of 80% (v/v) solvent B for 1.5 min with a flow rate of 350 μl/min, followed by a linear gradient increasing to 100% (v/v) solvent B over 2.0 min. This condition was maintained for 7.0 min and then returned to the initial condition over 0.1 min and maintained until the end of a 13-min run. The column temperature was maintained at 40 °C. The injection volume (on the column) was 15 μl. MS analyses were performed with atmospheric pressure chemical ionization (APCI) in the positive ion mode. Full-scan detection mode was used, with the mass range set between m/z 200 and 500. The following parameters were used for the detection of the analytes and the internal standard: N2 sheath gas, 30 p.s.i.; N2 auxiliary gas, 5 p.s.i.; spray voltage, 5.0 kV; APCI vaporizer temperature, 400 °C; capillary temperature, 300 °C; capillary offset, 35 V; tube lens voltage, 112 V; argon collision gas, 1.5 millitorr; scan time, 100 ms; Q3 scan width, 1 m/z; and Q1/Q3 peak widths at half-maximum, 0.7 m/z. The data were collected using Finnigan Xcalibur® version 1.0 software.
For the measurement of 7-ketocholesterol formation rates, a UPLC system connected to an ACQUITY UV detector (using an ACQUITY UPLC BEH C18 octadecylsilane column, 2.1 × 100 mm, 1.7 μm) was used, and 15 nmol of 7α-hydroxycholesterol was added in the extraction step as an internal standard. LC conditions were as follow: solvent A contained 95% (v/v) H2O and 5% (v/v) CH3CN, and solvent B contained 95% (v/v) CH3CN and 5% (v/v) CH3OH. The column was maintained at an initial condition of 90% (v/v) solvent B for 1.5 min with a flow rate of 350 μl/min, followed by a linear gradient increasing to 100% (v/v) solvent B over 1.5 min. This condition was maintained for 3.5 min and then returned to the initial condition over 0.1 min and maintained until the end of a 9.2-min run. The column temperature was maintained at 40 °C. The injection volume was 15 μl. Absorbance at 236 nm (7-ketocholesterol) and 205 nm (7α-hydroxycholesterol as an internal standard) was monitored. Data collection and quantitative analysis were conducted with Waters MassLynx version 4.1 and QuanLynx version 4.1 software, respectively.
Measurement of 7-Dehydrocholesterol Oxidation Activity with E. coli Bicistronic Membranes Expressing Human P450 1A1 and P450 1B1
Enzyme reaction mixtures for P450 1A1 or P450 1B1 reactions contained 0.1 μm P450 1A1 or P450 1B1 (with a similar level of NADPH:P450 reductase from coexpression) (47) and 7-dehydrocholesterol in 3.1 mm HPβCD in 100 mm potassium phosphate buffer (pH 7.4) in a typical reaction volume of 500 μl. Assays were conducted at 37 °C for up to 60 min, with incubations initiated by the addition of an NADPH-generating system (46). Following extraction, LC-MS analyses (APCI in the positive ion mode) were conducted as described above.
Measurement of [25,26,26,26,27,27,27-d7]-7-Dehydrocholesterol Oxidation in Human Liver Microsomes
The amount of cholesterol in a pool of 10 human liver microsomal samples (seven males and three females) was first quantified as described (40). Aliquots of the pool of the 10 human liver microsomal samples containing exactly 50 nmol of cholesterol were added to a 1.0-ml reaction mixture containing 100 nmol of [25,26,26,26,27,27,27-d7]-7-dehydrocholesterol and 3.1 mm HPβCD in 50 mm potassium phosphate buffer (pH 7.4). The assays were conducted at 37 °C for 60 min. Incubations were initiated by the addition of an NADPH-generating system (46). Reactions were quenched with 2.0 ml of CH2Cl2 and mixed with a Vortex device. Following extraction, LC-MS analyses (APCI in the positive ion mode) were conducted as described above.
Measurement of P450 7A1 Oxidation of Lathosterol
Enzyme reaction mixtures typically contained 0.2 μm P450 7A1, 3.0 μm NADPH:P450 reductase, 60 μm l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine, lathosterol in 3.1 mm HPβCD, and 0.41 mm Tween 20 in 50 mm potassium phosphate buffer (pH 7.4). Incubations were conducted (at 37 °C) for 3 min, initiated by the addition of an NADPH-generating system (46). Reactions were quenched with 2.0 ml of CH2Cl2 and mixed with a Vortex device. Following extraction of samples, LC-MS analyses (APCI in the positive ion mode) were conducted as described above.
To calculate the rates of 7-ketocholestanol and cholestanol 7α,8α-epoxide formation, 50 nmol of 4β-hydroxycholesterol was added in the extraction step as an internal standard. MS analyses were performed with APCI in the positive ion mode. Quantitation was based on multiple reaction monitoring (7-ketocholestanol, cholestanol 7α,8α-epoxide, and 4β-hydroxycholesterol: m/z 385 → 367, collision energy of 8 V). The data were collected and quantified using Finnigan Xcalibur version 1.0 software.
Measurement of P450 7A1 Oxidation of [7-d1]-7-Dehydrocholesterol
Enzyme reaction mixtures contained 0.2 μm P450 7A1, 3.0 μm NADPH:P450 reductase, 60 μm l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine, [7-d1]-7-dehydrocholesterol in 3.1 mm HPβCD, and 0.41 mm Tween 20 in 50 mm potassium phosphate buffer (pH 7.4). The assays were conducted at 37 °C for 3 min. Incubations were initiated by the addition of an NADPH-generating system (46). Reactions were quenched with 2.0 ml of CH2Cl2 and mixed with a Vortex device. Following extraction of samples, LC-MS analyses (APCI in the positive ion mode) were conducted as described above.
Stability of Cholestanol 7α,8α-Epoxide and Cholesterol 7α,8α-Epoxide
Reaction mixtures contained 60 μm l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine with or without 1.0 μm P450 7A1 in 50 mm potassium phosphate buffer (pH 7.4). The assays were conducted at 37 °C for 30 min (cholesterol 7α,8α-epoxide) or 60 min (cholestanol 7α,8α-epoxide). Incubations were initiated by the addition of 10 μm cholestanol 7α,8α-epoxide or cholesterol 7α,8α-epoxide solution in CH3OH (1% (v/v) CH3OH in reaction mixtures). Reactions were quenched with 2.0 ml of CH2Cl2 containing 5 nmol of cholesterol as an internal standard and mixed with a Vortex device. Following extraction, LC-MS analyses (APCI in the positive ion mode) were conducted as described above with a TSQ Quantum mass spectrometer (for cholestanol 7α,8α-epoxide). The ions at m/z 367 (M + H+ − 2H2O; tR = 5.0 min for cholestanol 7α,8α-epoxide) and m/z 369 (M + H+ − H2O; tR = 7.2 min for cholesterol) were used to quantify cholestanol 7α,8α-epoxide. In the case of cholesterol 7α,8α-epoxide, the Orbitrap mass spectrometer was used (with UPLC) in the positive ion electrospray mode, and HCO2H (0.1%, v/v) was added to the mobile phase. The tR of the epoxide was 3.83 min for the epoxide, and the ion at m/z 401 was monitored.
RESULTS
7-Ketocholesterol Formation from 7-Dehydrocholesterol in Human Liver Microsomes
7-Ketocholesterol formation activity was measured with the substrate [25,26,26,26,27,27,27-d7]-7-dehydrocholesterol (100 μm) in a pool of 10 human liver microsomal samples in the presence of endogenous cholesterol (50 μm measured). The [d7]-7-dehydrocholesterol substrate was used because of the presence of endogenous 7-ketocholesterol in the microsomes. The product was defined by co-migration with an authentic standard and an identical fragmentation pattern, as elaborated further under studies with P450 7A1 (see below). The measured rate was 0.014 ± 0.002 pmol/min/mg of protein, which is 100-fold less than that of cholesterol 7α-hydroxylation in human liver microsomes (2.5 ± 1.6 pmol/min/mg of protein) (40). The estimated rate of 7-dehydrocholesterol oxidation in liver microsomes may be compromised by the competition of the endogenous cholesterol, in that the measured Km for (free) cholesterol for P450 7A1 is ∼1 μm (40) and an IC50 of 1 μm (for 7-ketocholesterol) has been reported for cholesterol 7α-hydroxylation (20).
7-Dehydrocholesterol Oxidation Activities of P450 7A1, P450 1A1, P450 1B1, and P450 3A4
P450 3A4 (known to have cholesterol 4β-hydroxylation activity (48–50)), P450 1A1, and P450 1B1 did not show any catalytic activity toward 7-dehydrocholesterol. On the other hand, P450 7A1, the cholesterol 7α-hydroxylase, catalyzed the conversion of 7-dehydrocholesterol to two products (Fig. 2A). The most intense product peak at tR = 4.82 min showed m/z 401 and 383, and the tR and mass spectra (including fragmentation) were identical to those of 7-ketocholesterol (Fig. 2, A–D). One minor oxidation product (m/z 401, 383, and 365 at tR = 2.74 min) was also detected. This compound was subsequently shown to be cholesterol 7α,8α-epoxide by co-chromatography with the synthetic material and HRMS (Fig. 3).
FIGURE 2.
LC-MS chromatograms and mass spectra of products formed from 7-dehydrocholesterol by P450 7A1. 7-Dehydrocholesterol (20 μm) was incubated with a reconstituted system including 0.2 μm P450 7A1, 3 μm NADPH:P450 reductase, 60 μm l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine, 3.1 mm HPβCD (0.45%, w/v), and 0.41 mm Tween 20 (0.05%, v/v) in 50 mm potassium phosphate buffer (pH 7.4) for 60 min. A, extracted ion chromatogram (EIC) of the m/z 383 ion of the products of 7-dehydrocholesterol. B, extracted ion chromatogram of the m/z 383 ion of the standard 7-ketocholesterol. C, mass spectrum of the product (tR = 4.82 min). D, mass spectrum of the standard 7-ketocholesterol (tR = 4.78 min). E, mass spectrum of the minor product of 7-dehydrocholesterol (tR = 2.74 min).
FIGURE 3.
LC-HRMS chromatogram of products formed from 7-dehydrocholesterol by P450 7A1. The incubations and conditions are similar to those described in the legend to Fig. 2, except that the products were analyzed using an Orbitrap mass spectrometer (HRMS) under slightly different UPLC conditions. The tR values for synthetic cholesterol 7α,8α-epoxide and 7-ketocholesterol were 3.83 and 5.43 min, respectively (corresponding to the peaks at tR = 2.74 and 4.78 min in Fig. 2). The calculated mass of the MH+ ion of both compounds is 401.3420 (1.7 ppm error).
Rates of 7-ketocholesterol formation by P450 7A1 were measured in the presence of 3.1 mm HPβCD plus 0.41 mm Tween 20. Cholesterol binding to Tween 20 and HPβCD has been reported, with Kd values of 2.5 and 1.5 μm, respectively (51). A model including these two equilibria (i.e. 7-dehydrocholesterol + Tween 20 ⇄ 7-dehydrocholesterol·Tween 20 and 7-dehydrocholesterol + HPβCD ⇄ 7-dehydrocholesterol·HPβCD) was used to fit the data using the program Dynafit (52), and we assume here that the Kd values of 7-dehydrocholesterol for both Tween 20 and HPβCD are similar to those of cholesterol.3 Based on this correction for the free concentration of 7-dehydrocholesterol, the calculated kcat and Km(7-dehydrocholesterol) values were 2.2 ± 0.1 min−1 and 1.1 ± 0.1 μm, respectively (Fig. 4), yielding an estimated catalytic efficiency of 2.0 × 106 m−1 min−1 (3 × 104 m−1 s−1).
FIGURE 4.

Rates of 7-ketocholesterol formation from 7-dehydrocholesterol by recombinant P450 7A1. The line shown is a fitting result (Dynafit (52)) for the formation of 7-ketocholesterol by P450 7A1 in the presence of 3.1 mm HPβCD and 0.41 mm Tween 20. kcat = 2.2 ± 0.1 min−1, and Km = 1.1 ± 0.1 μm (based on the calculated free concentration of 7-dehydrocholesterol).
Oxidation of Lathosterol to 7-Ketocholestanol and Cholestanol 7α,8α-Epoxide by P450 7A1
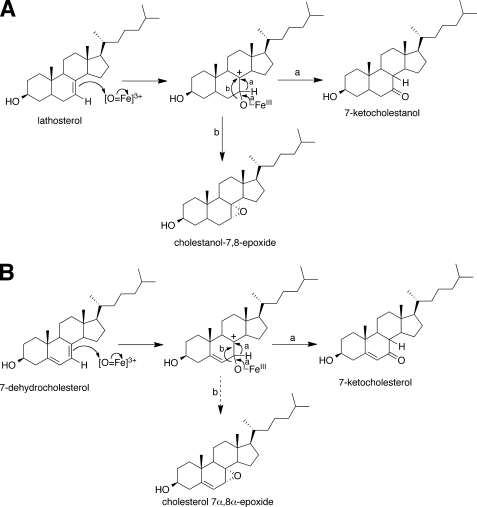
Three product peaks were detected in the reaction of P450 7A1 with lathosterol (Fig. 5A). The product peak at tR = 4.99 min showed ions at m/z 403, 385, and 367 (Fig. 5, A and C). A synthetic standard of cholestanol 7α,8α-epoxide also showed the same tR and mass spectrum (Fig. 5, B and D), indicating that this product is cholestanol 7α,8α-epoxide. The second intense product peak (tR = 4.70 min) showed m/z characteristics identical to 7-ketocholestanol (Fig. 5, A, B, E, and F), indicating that this is 7-ketocholestanol. The minor product peak (tR = 4.49 min) showed m/z 385 and 367 (Fig. 5, A and G), and the structure of this product is unknown.
FIGURE 5.
LC-MS chromatograms and mass spectra of products formed from lathosterol by P450 7A1. Lathosterol (20 μm) was incubated with a reconstituted system including 0.2 μm P450 7A1, 3 μm NADPH:P450 reductase, 60 μm l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine, 3.1 mm HPβCD (0.45%, w/v), and 0.41 mm Tween 20 (0.05%, v/v) in 50 mm potassium phosphate buffer (pH 7.4) for 3 min. A, extracted ion chromatogram (EIC) of the ion at m/z 385 (product of lathosterol). B, extracted ion chromatogram of the ion at m/z 385 (standards 7-ketocholestanol (tR = 4.70 min) and cholestanol 7α,8α-epoxide (tR = 5.00 min)). C, mass spectrum of the product of lathosterol (tR = 4.99 min). D, mass spectrum of the standard cholestanol 7α,8α-epoxide (tR = 5.00 min). E, mass spectrum of the product of lathosterol (tR 4.70 min). F, mass spectrum of the standard 7-ketocholestanol (tR = 4.70 min). G, mass spectrum of the unknown product of lathosterol (tR = 4.49 min).
Rates of 7-ketocholestanol and cholestanol 7α,8α-epoxide formation by P450 7A1 were measured in the presence of 0.41 mm Tween 20 and 3.1 mm HPβCD. The model including these two equilibria (i.e. lathosterol + Tween 20 ⇄ lathosterol·Tween 20 and lathosterol + HPβCD ⇄ lathosterol·HPβCD) was used to fit the data using the program Dynafit (52), assuming that Kd values of lathosterol for both compounds are similar to those of cholesterol.4 Based on such estimates of the free concentration of lathosterol, the calculated kcat and Km(lathosterol) values for 7-ketocholestanol formation were 3.7 ± 0.2 min−1 and 1.8 ± 0.3 μm, respectively (supplemental Fig. S4), yielding an estimated catalytic efficiency of 2.1 × 106 m−1 min−1 (3 × 104 m−1 s−1). The calculated kcat and Km(lathosterol) values for cholestanol 7α,8α-epoxide formation were 7.1 ± 0.4 min−1 and 2.1 ± 0.3 μm, respectively (supplemental Fig. S4), yielding an estimated catalytic efficiency of 3.4 × 106 m−1 min−1 (5 × 104 m−1 s−1).
Stability of Cholestanol 7α,8α-Epoxide and 7-Cholesterol 7α,8α-Epoxide in the Absence and Presence of P450 7A1
Cholestanol 7α,8α-epoxide was incubated with or without P450 7A1 in 50 mm potassium phosphate buffer (pH 7.4) for 60 min. No degradation of this compound was detected with or without P450 7A1 (1.0 μm). 7-Ketocholestanol (m/z 403, 385, and 367 at tR = 4.70 min) was not detected in the sample including P450 7A1 (Fig. 5F and supplemental Fig. S5).
Cholesterol 7α,8α-epoxide was also incubated with or without P450 7A1 in 50 mm potassium phosphate buffer (pH 7.4) for 30 min, and no degradation of this compound was detected with or without P450 7A1 (1.0 μm) using LC-HRMS (supplemental Fig. S3). In particular, no 7-ketocholesterol was formed. These results indicate that cholestanol 7α,8α-epoxide and cholesterol 7α,8α-epoxide are stable under the experimental conditions used and are not converted to 7-ketocholestanol and 7-ketocholesterol, respectively, by P450 7A1.
Oxidation of [7-d1]-7-Dehydrocholesterol by P450 7A1
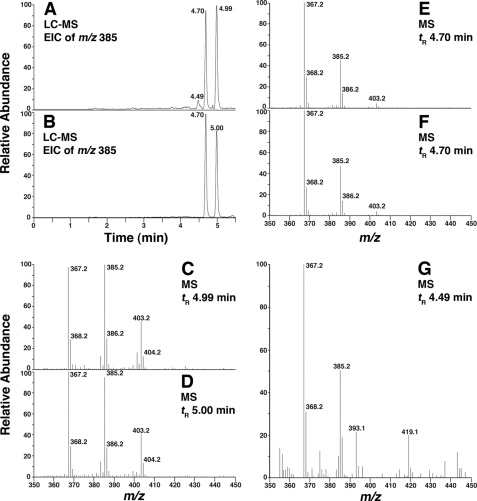
The oxidation of [7-d1]-7-dehydrocholesterol by P450 7A1 was examined. The formation of d1-7-ketocholesterol (m/z 402 and 384 at tR = 4.67 min) was confirmed and indicates a deuterium shift from C-7, presumably to C-8 in the product (Fig. 6).
FIGURE 6.
LC-MS chromatograms and mass spectra of products formed by P450 7A1 from d0- and [7-d1]-7-dehydrocholesterol. d0- or [7-d1]-7-dehydrocholesterol (20 μm) was incubated with a reconstituted system including 0.2 μm P450 7A1, 3 μm NADPH:P450 reductase, 60 μm l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine, 3.1 mm HPβCD (0.45%, w/v), and 0.41 mm Tween 20 (0.05%, v/v) in 50 mm potassium phosphate buffer (pH 7.4) for 3 min. A, extracted ion chromatogram (EIC) of the m/z 401 ion of the products of d0-7-dehydrocholesterol. B, extracted ion chromatogram of the m/z 402 ion of the products of [7-d1]-7-dehydrocholesterol. C, mass spectrum of the product of d0-7-dehydrocholesterol (tR = 4.67 min). D, mass spectrum of the product of [7-d1]-7-dehydrocholesterol (tR = 4.66 min).
DISCUSSION
In this study, recombinant human P450 7A1 was shown to catalyze the conversion of 7-dehydrocholesterol (the last compound in the pathway to cholesterol) to 7-ketocholesterol. The oxidation proceeds with formation of cholesterol 7,8-epoxide only as a minor product, and an epoxide is not an intermediate in the reaction (Fig. 1B). The conversion of 7-dehydrocholesterol to 7-ketocholesterol was also demonstrated in human liver microsomes.
7-Ketocholesterol and 7-keto bile acids have been known in the literature for some time (15, 16, 25, 32). Cholesterol is generally considered to be the precursor of these 7-keto compounds, but the mechanism of formation has been unclear. Song et al. (36) purified a 7α-hydroxycholesterol dehydrogenase from hamster liver microsomes. This enzyme had catalytic activity toward a number of hydroxysteroids, including bile acids. The catalytic efficiency reported (∼5 × 104 m−1 s−1) is similar to the value we found for human P450 7A1 (3 × 104 m−1 s−1) (Fig. 4), although an issue in comparisons is how abundant these enzymes are in the liver. Antibody and activity measurements showed that the dehydrogenase was apparently absent in rat liver and present only at low levels in some humans (36). Although we have not tested the specificity of P450 7A1 with other Δ7-dehydrosteroids, we would presume that the specificity of P450 7A1 is rather limited, in that it is generally assumed to work only with cholesterol and very related compounds (i.e. 7-dehydrocholesterol; see above).
Another potential source of 7-ketocholesterol is lipid peroxidation. Free radical oxidation of cholesterol occurs by initial hydrogen atom abstraction at C-7, and 7α- and 7β-cholesterol hydroperoxides are the predominant products formed after chain propagation by oxygen addition and hydrogen atom transfer to the peroxyl radical. Secondary hydroperoxides such as 7-hydroperoxycholesterol readily undergo dehydration, providing a route to 7-ketocholesterol (32), and the 7-keto compound can also be formed from 7α- and 7β-hydroperoxycholesterol by dehydration through a radical mechanism by abstracting the remaining hydrogen atom at C-7 (53). The “Russell mechanism” is another reaction pathway that can give rise to 7-ketocholesterol, where a radical chain termination reaction occurs by the reaction of two peroxyl radicals, followed by the decomposition of the resulting tetraoxide (54, 55).
7-Ketocholesterol has been studied extensively, in terms of its biological properties, as outlined in the Introduction. Individuals with the rare disease cerebrotendinous xanthomatosis have high blood levels of 7-ketocholesterol (25), which may also be due to elevated levels of 7-dehydrocholesterol in the plasma of these patients (56). Recently, 7-ketocholesterol was reported to be one of the oxysterol biomarkers for Niemann-Pick C1 disease (29). High blood levels of 7-ketocholesterol are also found in rhesus monkeys with hemorrhagic fever (30) or following high salt loading of hypertensive baboons (31). 7-Ketocholesterol is a strong inhibitor of P450 7A1 and also up-regulates synthesis of this protein (20). 7-Keto bile acids are also found in humans and may derive from 7-ketocholesterol (57).
Recently, 7-ketocholesterol was found to accumulate in AY9944 (an inhibitor of DHCR7)-treated rats (37), where 7-dehydrocholesterol is the dominant sterol precursor. Although it seems unusual to observe higher levels of 7-ketocholesterol with decreased levels of cholesterol, our results here on 7-dehydrocholesterol being an alternative precursor to 7-ketocholesterol provide a reasonable explanation for this observation. We have demonstrated that the human liver microsomal enzyme P450 7A1 also converts 7-dehydrocholesterol to 7-ketocholesterol, and accumulation of 7-ketocholesterol in the blood of SLOS patients would be expected.
P450 7A1 was shown here to directly oxidize 7-dehydrocholesterol to 7-ketocholesterol without an intermediate epoxide (Figs. 2 and 3 and supplemental Fig. S6). Mechanisms are proposed in Fig. 1. Such a reaction has precedence in previous work from one of our laboratories, where (rat) P450 2B1 converted trans-1-phenyl-1-butene to two ketones (38). Subsequently, Mansuy et al. (39) showed the conversion of styrene to phenylacetaldehyde in NADPH-fortified rat liver microsomes, apparently catalyzed by a P450. In both cases, the epoxide was formed as the major product, but the epoxides were stable and were shown not to be intermediates in carbonyl formation. A comparison can be made with hydroxylation of aryl compounds and the 1,2-“NIH shift” (i.e. p to m shift of hydrogen due to a 4-keto intermediate) (58), although in the case of aryl compounds, the epoxides are often unstable, and demonstrating a “non-epoxide” mechanism is difficult. Precedent also exists for 1,2-halide migration in the conversion of vinyl halides to carbonyls without the intermediary of epoxides (38, 59).
In the above cases, some epoxide is always formed and is dominant in the case of the hydride shifts. We first examined lathosterol oxidation (Fig. 5) as a model, in that the synthesis of the 7,8-epoxide is more straightforward than in the case of 7-dehydrocholesterol (supplemental Figs. S1 and S2). Cholestanol 7α,8α-epoxide and 7-ketocholestanol (Fig. 5) were formed in a ratio of ∼2:1, respectively (supplemental Fig. S4). The epoxide was stable and did not convert to the 7-keto product, even in the presence of P450 7A1 (supplemental Fig. S5). Oxidation of 7-dehydrocholesterol yielded a single major product and only trace 7,8-epoxide (Figs. 2 and 3). Again, this 7,8-epoxide was stable and was shown not to rearrange to the 7-keto product (supplemental Fig. S6). This appears to be the only identified example of a dominant conversion of an olefin to a carbonyl (with only minor epoxide formation). Exactly why P450 7A1 senses the shape of 7-dehydrocholesterol to form primarily the carbonyl but gives a mixture of epoxide and 7-keto product with lathosterol is unclear but is undoubtedly due to the structural differences in the sterol A and B rings.
The mechanisms for oxidation (Fig. 1) follow from the mechanism we proposed for trans-1-phenyl-1-butene (38). Beyond this work and that of Mansuy et al. (37), we are unaware of more precedents or previous searches for substrates that undergo such reactions. Presumably, more P450 reactions exist that involve rather exclusive conversion of an olefin to a carbonyl, although these cannot be predicted.
In summary, we have demonstrated the direct formation of 7-ketocholesterol from 7-dehydrocholesterol. The enzyme mechanism (Fig. 1) is not novel in itself (38), but the formation of only trace epoxide (Figs. 2 and 3) in such a reaction appears to be. In this case, the reaction involves a physiologically important substrate, 7-dehydrocholesterol, which has been shown to accumulate to high levels in some individuals with a deficiency in the 7-dehydrocholesterol reductase, the enzyme that catalyzes the last step in cholesterol synthesis (i.e. SLOS). The product, 7-ketocholesterol, is associated with several disease states and has been shown to accumulate in an animal model for SLOS (37). We propose that, in the absence of other enzymes that form 7-ketocholesterol, the enzyme reaction demonstrated here may be a source of 7-keto bile acids.
Supplementary Material

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” Figs. S1–S6, and additional abbreviations and references.
The clogP (calculated logarithm of the octanol-water partition coefficient) value of 7-dehydrocholesterol is 7.93, and that of cholesterol is 7.96 (ChemBioDraw Ultra version 12.0), suggesting similar partitioning with HPβCD and Tween 20.
The clogP value of lathosterol is 8.16, and that of cholesterol is 7.96 (ChemBioDraw Ultra version 12.0).
- SLOS
- Smith-Lemli-Opitz syndrome
- HPβCD
- 2-hydroxypropyl-β-cyclodextrin
- HRMS
- high resolution mass spectrometry
- UPLC
- ultra performance liquid chromatography
- APCI
- atmospheric pressure chemical ionization.
REFERENCES
- 1. Keeney D. S., Waterman M. R. (1993) Pharmacol. Ther. 58, 301–317 [DOI] [PubMed] [Google Scholar]
- 2. Guengerich F. P. (2005) in Cytochrome P450: Structure, Mechanism, and Biochemistry (Ortiz de Montellano P. R. ed) 3rd Ed., pp. 377–530, Kluwer Academic/Plenum Press, New York [Google Scholar]
- 3. Tint G. S., Irons M., Elias E. R., Batta A. K., Frieden R., Chen T. S., Salen G. (1994) N. Engl. J. Med. 330, 107–113 [DOI] [PubMed] [Google Scholar]
- 4. Kelley R. I. (1995) Clin. Chim. Acta 236, 45–58 [DOI] [PubMed] [Google Scholar]
- 5. MacLaughlin J., Holick M. F. (1985) J. Clin. Invest. 76, 1536–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holick M. F., Frommer J. E., McNeill S. C., Richtand N. M., Henley J. W., Potts J. T., Jr. (1977) Biochem. Biophys. Res. Commun. 76, 107–114 [DOI] [PubMed] [Google Scholar]
- 7. Tint G. S., Seller M., Hughes-Benzie R., Batta A. K., Shefer S., Genest D., Irons M., Elias E., Salen G. (1995) J. Lipid Res. 36, 89–95 [PubMed] [Google Scholar]
- 8. Kelley R. I., Hennekam R. C. (2000) J. Med. Genet. 37, 321–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haas D., Garbade S. F., Vohwinkel C., Muschol N., Trefz F. K., Penzien J. M., Zschocke J., Hoffmann G. F., Burgard P. (2007) J. Inherit. Metab. Dis. 30, 375–387 [DOI] [PubMed] [Google Scholar]
- 10. Porter F. D., Herman G. E. (2011) J. Lipid Res. 52, 6–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu L., Davis T. A., Porter N. A. (2009) J. Am. Chem. Soc. 131, 13037–13044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu L., Korade Z., Porter N. A. (2010) J. Am. Chem. Soc. 132, 2222–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu L., Korade Z., Rosado D. A., Jr., Liu W., Lamberson C. R., Porter N. A. (2011) J. Lipid Res. 52, 1222–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korade Z., Xu L., Shelton R., Porter N. A. (2010) J. Lipid Res. 51, 3259–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown A. J., Jessup W. (1999) Atherosclerosis 142, 1–28 [DOI] [PubMed] [Google Scholar]
- 16. Schroepfer G. J., Jr. (2000) Physiol. Rev. 80, 361–554 [DOI] [PubMed] [Google Scholar]
- 17. Javitt N. B. (2008) Steroids 73, 149–157 [DOI] [PubMed] [Google Scholar]
- 18. Björkhem I., Cedazo-Minguez A., Leoni V., Meaney S. (2009) Mol. Aspects Med. 30, 171–179 [DOI] [PubMed] [Google Scholar]
- 19. Vejux A., Lizard G. (2009) Mol. Aspects Med. 30, 153–170 [DOI] [PubMed] [Google Scholar]
- 20. Breuer O., Sudjana-Sugiaman E., Eggertsen G., Chiang J. Y., Björkhem I. (1993) Eur. J. Biochem. 215, 705–710 [DOI] [PubMed] [Google Scholar]
- 21. Brown M. S., Goldstein J. L. (1974) J. Biol. Chem. 249, 7306–7314 [PubMed] [Google Scholar]
- 22. Hughes H., Mathews B., Lenz M. L., Guyton J. R. (1994) Arterioscler. Thromb. 14, 1177–1185 [DOI] [PubMed] [Google Scholar]
- 23. Vejux A., Malvitte L., Lizard G. (2008) Braz. J. Med. Biol. Res. 41, 545–556 [DOI] [PubMed] [Google Scholar]
- 24. Moreira E. F., Larrayoz I. M., Lee J. W., Rodríguez I. R. (2009) Invest. Ophthalmol. Vis. Sci. 50, 523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Björkhem I. (1986) Anal. Biochem. 154, 497–501 [DOI] [PubMed] [Google Scholar]
- 26. Breuer O., Björkhem I. (1990) Steroids 55, 185–192 [DOI] [PubMed] [Google Scholar]
- 27. Dzeletovic S., Breuer O., Lund E., Diczfalusy U. (1995) Anal. Biochem. 225, 73–80 [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez I. R., Fliesler S. J. (2009) Photochem. Photobiol. 85, 1116–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Porter F. D., Scherrer D. E., Lanier M. H., Langmade S. J., Molugu V., Gale S. E., Olzeski D., Sidhu R., Dietzen D. J., Fu R., Wassif C. A., Yanjanin N. M., Marso S. P., House J., Vite C., Schaffer J. E., Ory D. S. (2010) Sci. Transl. Med. 2, 56ra81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gontscharow N. P., Wehrberger K., Schubert K. (1971) J. Steroid Biochem. 2, 389–391 [Google Scholar]
- 31. Gontscharow N. P., Simarina A. J., Jefremova S. K., Schön R., Schubert K. (1975) Endokrinologie 64, 213–216 [PubMed] [Google Scholar]
- 32. Smith L. L. (1987) Chem. Phys. Lipids 44, 87–125 [DOI] [PubMed] [Google Scholar]
- 33. Smith L. L., Johnson B. H. (1989) Free Radic. Biol. Med. 7, 285–332 [DOI] [PubMed] [Google Scholar]
- 34. Brown A. J., Leong S. L., Dean R. T., Jessup W. (1997) J. Lipid Res. 38, 1730–1745 [PubMed] [Google Scholar]
- 35. Murphy R. C., Johnson K. M. (2008) J. Biol. Chem. 283, 15521–15525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song W., Chen J., Dean W. L., Redinger R. N., Prough R. A. (1998) J. Biol. Chem. 273, 16223–16228 [DOI] [PubMed] [Google Scholar]
- 37. Xu L., Liu W., Sheflin L. G., Fliesler S. J., Porter N. A. (2011) J. Lipid Res., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liebler D. C., Guengerich F. P. (1983) Biochemistry 22, 5482–5489 [DOI] [PubMed] [Google Scholar]
- 39. Mansuy D., Leclaire J., Fontecave M., Momenteau M. (1984) Biochem. Biophys. Res. Commun. 119, 319–325 [DOI] [PubMed] [Google Scholar]
- 40. Shinkyo R., Guengerich F. P. (2011) J. Biol. Chem. 286, 4632–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gillam E. M., Baba T., Kim B. R., Ohmori S., Guengerich F. P. (1993) Arch. Biochem. Biophys. 305, 123–131 [DOI] [PubMed] [Google Scholar]
- 42. Hosea N. A., Miller G. P., Guengerich F. P. (2000) Biochemistry 39, 5929–5939 [DOI] [PubMed] [Google Scholar]
- 43. Hanna I. H., Teiber J. F., Kokones K. L., Hollenberg P. F. (1998) Arch. Biochem. Biophys. 350, 324–332 [DOI] [PubMed] [Google Scholar]
- 44. Guengerich F. P. (2005) Arch. Biochem. Biophys. 440, 204–211 [DOI] [PubMed] [Google Scholar]
- 45. Shaw P. M., Hosea N. A., Thompson D. V., Lenius J. M., Guengerich F. P. (1997) Arch. Biochem. Biophys. 348, 107–115 [DOI] [PubMed] [Google Scholar]
- 46. Guengerich F. P., Bartleson C. J. (2007) in Principles and Methods of Toxicology (Hayes A. W. ed) 5th Ed., pp. 1981–2048, CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 47. Parikh A., Gillam E. M., Guengerich F. P. (1997) Nat. Biotechnol. 15, 784–788 [DOI] [PubMed] [Google Scholar]
- 48. Bodin K., Bretillon L., Aden Y., Bertilsson L., Broomé U., Einarsson C., Diczfalusy U. (2001) J. Biol. Chem. 276, 38685–38689 [DOI] [PubMed] [Google Scholar]
- 49. Bodin K., Andersson U., Rystedt E., Ellis E., Norlin M., Pikuleva I., Eggertsen G., Björkhem I., Diczfalusy U. (2002) J. Biol. Chem. 277, 31534–31540 [DOI] [PubMed] [Google Scholar]
- 50. Shinkyo R., Guengerich F. P. (2011) J. Biol. Chem. 286, 18426–18433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mast N., Pikuleva I. A. (2005) J. Lipid Res. 46, 1561–1568 [DOI] [PubMed] [Google Scholar]
- 52. Kuzmic P. (1996) Anal. Biochem. 237, 260–273 [DOI] [PubMed] [Google Scholar]
- 53. Chang Y. H., Abdalla D. S., Sevanian A. (1997) Free Radic. Biol. Med. 23, 202–214 [DOI] [PubMed] [Google Scholar]
- 54. Smith L. L. (1981) Cholesterol Autoxidation, Plenum Press, New York [Google Scholar]
- 55. Howard J. A., Ingold K. U. (1968) J. Am. Chem. Soc. 90, 1056–1058 [Google Scholar]
- 56. de Sain-van der Velden M. G., Verrips A., Prinsen B. H., de Barse M., Berger R., Visser G. (2008) J. Inherit. Metab. Dis. 10.1007/s10545-008-0963-1 [DOI] [PubMed] [Google Scholar]
- 57. Strandvik B., Wahlén E., Wikström S. A. (1994) Scand. J. Clin. Lab. Invest. 54, 1–10 [DOI] [PubMed] [Google Scholar]
- 58. Guroff G., Daly J. W., Jerina D. M., Renson J., Witkop B., Udenfriend S. (1967) Science 157, 1524–1530 [DOI] [PubMed] [Google Scholar]
- 59. Miller R. E., Guengerich F. P. (1982) Biochemistry 21, 1090–1097 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.