Abstract
G protein-activated K+ channels (Kir3 or GIRK) are activated by direct interaction with Gβγ. Gα is essential for specific signaling and regulates basal activity of GIRK (Ibasal) and kinetics of the response elicited by activation by G protein-coupled receptors (Ievoked). These regulations are believed to occur within a GIRK-Gα-Gβγ signaling complex. Fluorescent energy resonance transfer (FRET) studies showed strong GIRK-Gβγ interactions but yielded controversial results regarding the GIRK-Gαi/o interaction. We investigated the mechanisms of regulation of GIRK by Gαi/o using wild-type Gαi3 (Gαi3WT) and Gαi3 labeled at three different positions with fluorescent proteins, CFP or YFP (xFP). Gαi3xFP proteins bound the cytosolic domain of GIRK1 and interacted with Gβγ in a guanine nucleotide-dependent manner. However, only an N-terminally labeled, myristoylated Gαi3xFP (Gαi3NT) closely mimicked all aspects of Gαi3WT regulation except for a weaker regulation of Ibasal. Gαi3 labeled with YFP within the Gα helical domain preserved regulation of Ibasal but failed to restore fast Ievoked. Titrated expression of Gαi3NT and Gαi3WT confirmed that regulation of Ibasal and of the kinetics of Ievoked of GIRK1/2 are independent functions of Gαi. FRET and direct biochemical measurements indicated much stronger interaction between GIRK1 and Gβγ than between GIRK1 and Gαi3. Thus, Gαi/oβγ heterotrimer may be attached to GIRK primarily via Gβγ within the signaling complex. Our findings support the notion that Gαi/o actively regulates GIRK. Although regulation of Ibasal is a function of GαiGDP, our new findings indicate that regulation of kinetics of Ievoked is mediated by GαiGTP.
Keywords: Fluorescence Resonance Energy Transfer (FRET), G Protein-coupled Receptors (GPCR), G Proteins, Gating, Ion Channels
Introduction
G protein-coupled receptor (GPCR)3 signaling is one the most important cellular signaling cascades as witnessed by the hundreds (∼800) of genes in the human genome (1), with some cells expressing as many as a hundred different receptor types (2). An agonist-bound GPCR initiates GDP-GTP exchange at the Gα subunit, which serves as the “on-off” switch of the GPCR-initiated process and as the trigger for the release of the Gβγ dimer. Both the active GTP-bound Gα subunit (GαGTP) and Gβγ directly interact and regulate various effector molecules such as phospholipase Cβ, adenylyl cyclase, and other enzymes, as well as a number of ion channels (1, 3–6). In particular, the G protein-activated inwardly rectifying K+ (GIRK) channel is the archetypal Gβγ effector activated by direct binding of Gβγ (7). GIRK is an important mediator of inhibitory actions of Gi/o-coupled GPCRs, notably the vagal inhibition of cardiac pacing and of the inhibitory actions of a large number of neurotransmitters in the brain (8–10).
It is becoming clear that GIRK, the prototypical Gβγ effector (11), is also an effector of the Gα subunit, which appears to play multiple roles in modulation of GIRK activity. Gαi controls the gating of GIRK by acting as a servo-type regulator, operating both as a damper of persistent inhibitory signaling by reducing the basal activity of GIRK (Ibasal) and, when activated by GPCR, as the generator of the main inhibitory signal, the evoked current of GIRK (Ievoked) (12, 13). Several lines of evidence point to an active modulation of GIRK both by the nonactivated GDP-bound form of Gαi3 (Gαi3GDP) (12, 13) and by the activated GαiGTP (14–16), but the details and mechanism of Gα action are poorly understood. In line with the notion that Gαi might affect channel behavior are recent findings suggesting that the GIRK channel serves as a multiprotein scaffold in signaling complexes with Gβγ, Gαi/o, regulators of G protein signaling, and possibly certain GPCRs (12, 16–21). This multiprotein complex is believed to enable specific and fast interactions of many protein partners, such as Gαi and GIRK, thus enabling them to modulate one another. However, the composition of such complexes and the roles of individual protein partners are yet to be determined (9, 22).
To assess modes of interactions between Gαi3 and GIRK within the hypothetical signaling complex, spectroscopic approaches appear particularly attractive. Tagging with fluorescent proteins (xFPs; supplemental Fig. S1A) allows both detection of the tagged protein and assessment of its interaction in vivo with other proteins using resonance energy transfer methods, mainly Förster RET (FRET) (23) or bioluminescence RET (24). However, fusion of xFP to the Gα subunit has proved challenging. Only a handful of positions have been reported to tolerate insertion of xFPs to produce functional Gαi/o proteins (25–29). Even when normal function in one or several aspects is reported, it is often debated whether other actions are preserved after xFP tagging (25, 28). Another notable controversy, relevant to the question of GIRK-related signaling complex, pertains to the direct Gαi/o-GIRK interaction in vivo. A measurable bioluminescence resonance energy transfer between GIRK1-GFP and Gαi1-RLuc (fused with luciferase) was interpreted to suggest a stable GIRK-Gαi1 signaling complex, probably within the endoplasmic reticulum (21). However, no measurable FRET between GIRK2 and Gαo could be detected (20). These discrepancies could arise from inadequate functioning of some xFP-fused GIRKs or Gαi and stress the need for their rigorous testing.
Here, we investigated the functional coupling between Gαi and GIRK using three distinct Gαi3 constructs (Gαi3xFPs) labeled with cyan or yellow fluorescent proteins (CFP or YFP, respectively). We have found that xFP labeling of Gαi, even at positions widely regarded as producing functional Gα proteins, may impede Gα-effector interactions and regulations. Furthermore, none of the Gαi3xFPs, even the one that best mimicked the effects of Gαi3WT, produced a measurable FRET signal with GIRK1, contrasting the strong FRET between GIRK1 and Gβγ. Taken together with biochemical data, these results suggested that Gαi3 associates with GIRK primarily via Gβγ. Importantly, the use of Gαi3xFPs revealed differential regulation of basal and evoked GIRK activities by Gα, which was confirmed by titrated expression of Gαi3WT. We conclude that control of the basal activity of GIRK and the fast provision of “free” Gβγ for GIRK activation (following GPCR activation) are distinct functions of the Gαi subunit in this signaling cascade.
EXPERIMENTAL PROCEDURES
cDNA Constructs
The cDNAs used in this study were obtained or prepared using standard PCR-based procedures. All cDNA constructs were inserted in two vector types as follows: pGEX (for production of GST fusion proteins in Escherichia coli) or into high expression oocyte vectors containing 5′- and 3′-untranslated sequences of Xenopus β-globin: pGEMHE, or its derivative pGEMHJ, or pBS-MXT. Rat GIRK1 (U01071), mouse GIRK2–1 (U11859), and human Gαi3 (J03198) were used for oocyte expression and as the basis for modifications. The cDNA for GST-G1NC was kindly provided by Craig A. Doupnik, University of South Florida. GST-G1NC was constructed with a His6 linker replacing the transmembrane segment (amino acids 85–184). GST was fused to its N terminus via a 6-amino acid linker (Leu, Val, Pro, Arg, Gly, and Ser). GST-Gαi3 was prepared as described previously (31). G1NC for in vitro translation was prepared as described previously (16); its transmembrane segment (amino acids 85–184) was deleted and replaced by an 8-amino acid linker GSTASGST. GIRK1CFP was created by replacing its stop codon with an XbaI restriction site. The coding sequence of CFP was inserted between the XbaI site and a HindIII site immediately following the stop codon of the CFP. YFP-labeled Gαi3 constructs were obtained or constructed as follows. Gαi3117, where YFP was inserted in the αb-αc loop, with the first Met of YFP placed at position 117 of Gαi3, was kindly provided by Alfred G. Gilman (28). Myr-YFP-Gαi3 was designed similarly to Ref. 27, but instead of a 20-amino acid GAP43 palmitoylation signal, it started with a 17-amino acid myristoylation signal derived from the Src protein, as described previously (32), followed by the first Met of CFP or YFP and then by Gαi3 (the stop codon of xFP was removed). Gαi3-CT-YFP was constructed like GIRK1CFP, where the Gα stop codon was removed and XbaI restriction site was added instead. YFP was inserted immediately after the Gαi3 insert and terminated with a stop codon and a HindIII restriction site. PTX-insensitive Gαi3s were created using PCR, by mutating the cysteine at position 351 (4th position from the C terminus) to isoleucine, C351I.
Experimental System and Electrophysiology
Experiments were approved by the Tel Aviv University Institutional Animal Care and Use Committee (permit no. 11-05-064). Xenopus oocytes were prepared and injected with RNA, as described previously (12), and incubated for 3–5 days at 20–22 °C in ND96 solution (96 mm NaCl, 2 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 5 mm HEPES, pH 7.5), supplemented with 2.5 mm sodium pyruvate, 100 μg/ml streptomycin, and 62.75 μg/ml penicillin. Whole-cell currents were measured using standard two-electrode voltage clamp procedures at 20–22 °C, in the ND96 (low K+) solution or in a high K+ solutions (24 mm K+, isotonically replacing NaCl in ND96) as described previously (16). When designed, protomer A of pertussis toxin (PTX) was injected into oocytes 2–20 h before current recordings (33). Currents were recorded at −80 mV, filtered at 500 Hz, and sampled at 5 or 10 kHz. Data acquisition and analysis were done using pCLAMP (Molecular Devices). Calculation of activation time constant (τact) was done using a standard mono-exponential fit using the Clampfit program of the pCLAMP suite.
Pulldown Assays
GST-fused proteins were purified using standard protocols (31). [35S]Methionine-labeled proteins were synthesized in rabbit reticulocyte lysate. For pulldown experiments, the following procedure was used: Gαi3 (when present) was incubated at 30 °C for 20 min in a total volume of 50 ml of high-K+ binding buffer containing 33 μm of either GDP or GTPγS and 0.01% Lubrol (31). Next, Gβ1γ2 was added for another 20 min. The full cytosolic domain of GIRK1 (G1NC) was then added, and the total reaction volume was brought to 300 μl (with binding buffer containing 30 μm GDP or GTPγS), and the incubation was continued for 1 h at room temperature. Then 5 μl were removed and used to measure the loaded protein (“input”). Binding to glutathione-Sepharose beads and elution with 15 mm glutathione were done as described previously (31). The eluted proteins were separated on 12% SDS-polyacrylamide gels. Using this procedure, different pulldown experiments were done as follows: 3 μg of purified histidine-tagged Gβ1γ2 was used to pull down 5 μl of reticulocyte lysate containing 35S-labeled Gαi3xFP proteins or 5 μl of YFP alone (Fig. 2; no 35S-labeled G1NC was added in this experiment). 3 μg of purified GST-fused G1NC was used to pull down 5 μl of reticulocyte lysate containing 35S-labeled Gαi3WT and 5 μl of Gβ1γ2 (Fig. 3). Autoradiograms were obtained by imaging and quantitating the dried gels using PhosphorImager and the software ImageQuaNT (GE Healthcare). Western blots were performed using Gβ antibody (Santa Cruz Biotechnology) and ECL reagents from Pierce.
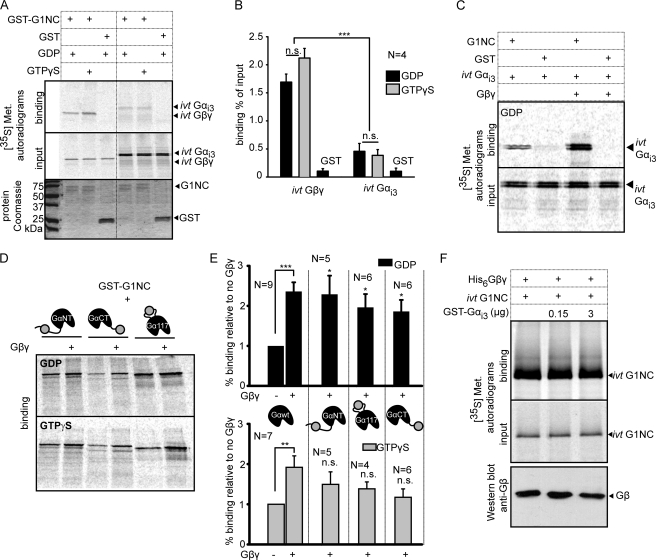
FIGURE 2.
All fluorescently labeled Gαi3xFP subunits bind Gβγ. A, purified histidine-tagged Gβ1γ2 (His6Gβγ) was used to pull down 35S-Gαi3xFP proteins or YFP alone, synthesized ivt in reticulocyte lysate, in the presence of GDP or GTPγS. Representative autoradiograms of bound proteins (upper panel, Binding) and one-sixtieth of the total loaded protein (lower panel, Input) are shown. Specificity of binding was determined by control pulldown with nickel beads (no His6Gβγ). Very little nonspecific binding of 35S-YFP to His6Gβγ or to nickel beads was detected. B, summary of binding of the ivt Gαi3xFP proteins, expressed as percent of input of each protein. Each bar represent mean ± S.E. of N experiments; N is shown above the bars. C, statistical analysis of the effect of GTPγS on the interaction between His6Gβγ and ivt synthesized Gαi3xFP proteins. Percent binding in GTPγS relative to GDP was calculated in each experiment, and mean % binding calculated from all experiments is shown for each Gαi3 construct. For each construct, the effect of GTPγS was analyzed using paired t test; *, p < 0.05; **, p < 0.01; ***, p < 0.001. The differences between the four proteins were analyzed using one-way ANOVA and found nonsignificant (n.s.; p > 0.05).
FIGURE 3.
YFP-labeled Gαi3 proteins bind GIRK1 but appear deficient in a triple GIRK1-Gα-Gβγ interaction test. A, pulldown of in vitro translated, 35S-labeled Gαi3WT and Gβ1γ2 by purified GST-fused full cytosolic domain of GIRK1, GST-G1NC. Upper and middle panels show representative autoradiograms of bound and added 35S-labeled proteins, respectively (binding and input as in Fig. 2). The lower panel shows Coomassie staining of the added proteins. GST-G1NC appears as two bands, with upper band corresponding to the correct calculated molecular size (∼72 kDa). GST-G1NC, but not GST, binds Gαi3WT or Gβ1γ2, no matter the nucleotide added (GDP or GTPγS). B, summary of binding of Gαi3WT and Gβ1γ2 by GST-G1NC, expressed in % of input. C, purified Gβ1γ2 enhances the binding of 35S-labeled Gαi3WT to GST-G1NC in the presence of GDP. Pulldown was done using glutathione-Sepharose, with GST alone as negative control. Similar result was observed in the presence of GTPγS (see summary in E). D, representative experiment showing the binding of 35S-labeled ivt Gαi3xFP proteins to GST-G1NC, in the absence or presence of purified Gβ1γ2. E, summary of the effect of Gβ1γ2 on the interaction between G1NC and 35S-labeled ivt Gαi3xFP from four to nine independent experiments. In GDP, Gβ1γ2 significantly enhances the interaction between G1NC and all 35S-labeled ivt Gαi3xFP (top), but it fails to do so in GTPγS (bottom). n.s., nonsignificant. F, Gαi3 does not enhance interaction between His6Gβ1γ2 and 35S-labeled ivt G1NC (representative experiment, out of two). His6Gβ1γ2 (3 μg) pulls down ivt G1NC in the absence or presence of two different amounts of purified GST-Gαi3WT. Upper and middle panels show binding and 1/60 input of ivt G1NC, respectively. The lower panel shows Western blot of His6Gβ1γ2.
Imaging Analysis and FRET
Fluorescent signals were collected with the Zeiss 510 META confocal microscope. Immunolabeling of GIRK1 in giant excised PM patches was done using GIRK1 antibody as described previously (12, 13, 34). In brief, the vitelline membrane was peeled off, and the oocytes were placed on glass coverslips. After sticking to the coverslip, the oocyte was removed; pieces of membranes attached to the coverslip, with their cytosolic leaflet surface exposed to the external solution, were washed and fixated, and nonspecific sites were blocked with donkey immunoglobulin G (IgG, whole molecule, 1:400, Jackson ImmunoResearch), and coverslips were incubated with GIRK1 antibody (Alomone Labs, Jerusalem), followed by incubation with secondary antibody (CY3 donkey anti-rabbit IgG, 1:400, Jackson ImmunoResearch). The CY3 fluorescence was imaged by exciting the dye at 514 nm; the emitted light was collected between 540 and 615 nm using the spectral mode of the Zeiss 510 Meta (beam splitter HFT 405/514/633).
Intact oocytes were imaged in ND96 solution in a 0.7-mm glass-bottom dish, as described previously (16). Fluorescent signals were collected from circular regions of interest of ∼75 pixels in the membrane area, close to the midline of the animal hemisphere, and from three background regions of interest outside the oocyte using a 20× air objective. CFP was excited using a 405 nm laser line, and emission spectrum was collected using the META detector. Peak emission (481–492-nm band) was chosen for the comparison of expression levels of CFP-labeled proteins. YFP was excited using a 514 nm, and peak emission (524–535 nm) was used for comparison of expression levels.
FRET experiments were performed as described previously (16, 35). Briefly, two spectra were collected from the animal hemisphere of each oocyte, with 405-nm (CFP excitation) and 514-nm (YFP excitation) laser lines. Net FRET signal of the CFP/YFP labeled channels was calculated in the YFP emission range (with the 405 nm excitation) by consecutive subtraction of a scaled CFP-only spectrum (giving the A ratio parameter) and then of the ratio A0, which report the direct excitation of YFP by the 405 nm laser, as in Equations 1 and 2,
Because of the use of different experimental settings in independent experiments, the fluorescence intensities cannot be used to construct FRET titration curves. We imaged a doubly-labeled protein expressing both CFP and YFP at a 1:1 stoichiometry (YFP-IRK1-CFP, DL-IRK1(16)) in each experiment to convert the fluorescence of CFP and YFP into their molar ratio (see supplemental Fig. S6). This was achieved by calculating the slope of the CFP to YFP fluorescence of DL-IRK1. Then this slope was used as a reference to convert CFP and YFP fluorescence, in the other groups of the same experiment, into a molar ratio, which can be collected and used from many experiments and implemented into one titration curve. Calculation of Eapp and distances between fluorophores was done as described previously (36, 37).
Statistical Analysis
Results are shown as mean ± S.E. Multiple group comparison was done using one-way analysis of variance (ANOVA) followed by Tukey all-pairwise analysis. Two group comparisons were done using two-tailed t test or paired t test when applicable. Correlations between two parameters were examined using Spearman test. Asterisks indicate statistically significant differences as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
RESULTS
To address GIRK-Gαi interactions in vivo using optical methods, we constructed xFP-labeled GIRK1 and Gαi3 and compared with their wild-type counterparts. Functional tests were done in Xenopus oocytes, where the regulation of GIRK by GPCRs, Gβγ, and Gα has been extensively characterized. GIRK currents were recorded under voltage clamp at −80 mV. The neuronal GIRK1/2 (heterotetramer composed of GIRK1 and GIRK2 subunits) shows a sizeable basal activity (Ibasal), especially at high levels of channel expression, presumably because of excess of associated Gβγ and/or lack of sufficient channel-associated Gαi/o (32). Ibasal is revealed by exchanging a physiological, low-K+ (ND96; 2 mm) solution to a high-K+ (“hK”; 24 mm) solution (Fig. 1A). When coexpressed with the muscarinic receptor 2 (m2R), GIRK is readily responsive to activation by agonist (acetylcholine; ACh). In the absence of coexpressed Gα, the ACh-evoked current (Ievoked or IACh) reports the activation of endogenous Gαi/o of the oocyte (Fig. 1A, left panel) (38).
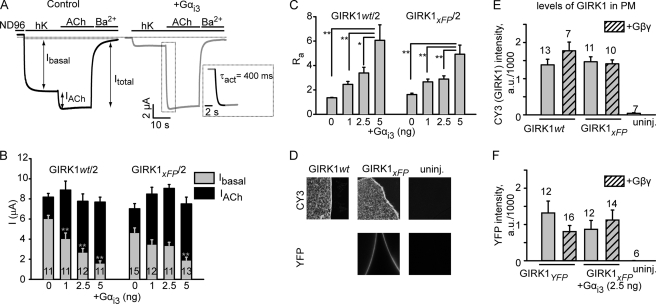
FIGURE 1.
GIRK1xFP/GIRK2WT channel behaves like GIRK1/2WT. A, representative GIRK1xFP/2WT currents measured in Xenopus oocytes. Left trace, oocyte were injected with RNAs of GIRK1CFP (1 ng), GIRK2 (1 ng), and m2R (0.5 ng). Membrane potential was −80 mV. Switching from a physiological low K+ solution (2 mm K+; ND96) to a high K+ solution (24 mm K+; hK) reveals the basal activity of the channel (Ibasal). Addition of the agonist ACh (10 μm) shows the evoked current (IACh). Ba2+ (5 mm) was then added to inhibit GIRK currents, allowing us to calculate the net Ibasal and Itotal. Right trace, coexpression of Gαi3WT (5 ng) reduces Ibasal and enhances IACh. Inset, zoom on the activation phase of IACh. Monoexponential fit (black line) used to calculate the activation time constant (τact) is shown superimposed on the actual trace (gray line). B, increasing amounts of Gαi3WT significantly reduces Ibasal of both GIRK1WT/2 and GIRK1CFP/2, and enhances IACh without reducing Itotal, hence causing a significant increase in the relative extent of activation, Ra (C). D, GIRK1WT/2 and GIRK1YFP/2 channels express at comparable levels in the plasma membrane. PM levels of GIRK1 and GIRK1YFP were assessed using the giant excised membrane patches method (top images), and GIRK1YFP was also visualized with YFP fluorescence in intact oocytes (bottom images). No GIRK1 could be detected by any of these methods in native oocytes. E, summary of the expression level of both channels. Coexpression of Gβγ does not cause any significant change in surface expression levels of GIRK1WT/2 and GIRK1xFP/2. F, coexpression of Gβγ or Gαi3WT does not cause any significant changes in GIRK1YFP PM expression level. In all figures, number of oocytes is shown within or above the bars. *, p < 0.05; **, p < 0.01 compared with the control group; a.u., arbitrary units.
Ibasal is largely Gβγ-dependent and is strongly sensitive to coexpressed Gαi/o, which reduces Ibasal in a dose-dependent manner and concomitantly increases Ievoked, thereby increasing the ratio of activation, Ra (Ra = Itotal/Ibasal, where Itotal = Ibasal + Ievoked) (Fig. 1, A–C). The reduction in Ibasal is a function of GαiGDP and reflects the formation of Gαβγ heterotrimers, which predisposes (“primes”) the channel for subsequent activation by the GPCR (12, 39). Among several Gαi/o subunits tested, we have chosen Gαi3 as the preferred donor of Gβγ and regulator of Ibasal. Coexpressed Gαi3 provides for fast kinetics of agonist response (Figs. 1A, inset, and 4), accelerating its time constant (τact) compared with endogenous Gαi/o (40). Notably, Gαi3WT does not reduce the total GIRK current (Fig. 1B), as opposed to Gβγ-“scavenging” proteins (39). In fact, Itotal may actually increase following moderate expression of Gαi3WT (40). Ibasal, Ievoked, Itotal, Ra, and τact have been chosen as the essential set of parameters to assess the functional coupling of xFP-labeled Gαi3 and GIRK1/2.
FIGURE 4.
YFP labeling of Gαi3 and PM level titration reveals the duality of Gαi3 regulation of GIRK. A, representative images of oocytes expressing the various Gαi3xFP. For presentation purposes, the contrast was digitally enhanced equally in all images for better visualization. B–D, characterization of expression and functional effects of Gαi3 and Gαi3xFP subunits: Gαi3NT, Gαi3117, and Gαi3CT. Each panel presents the result of one experiment. Each experiment was repeated at least twice. Left to right, representative GIRK1/2 currents; PM expression of Gαi3xFPs; mean values of Ibasal, IACh, and Itotal; extent of activation, Ra; τact of Ievoked for the highest mRNA concentration of the Gαi3 subunit. Number of oocytes is shown within bars. Statistical comparisons of multiple groups were done using one way ANOVA followed by pairwise Tukey test compared with control group (where no Gα subunit was coexpressed). **, p < 0.01. n.s., nonsignificant; a.u., arbitrary units.
Fluorescent Labeling of GIRK1 Does Not Perturb Regulation by Gαi3
xFP-labeled GIRK channels have been found functional with respect to trafficking to the PM and regulation by GPCRs and Gβγ (13, 16, 17, 20, 21, 41–43), but regulation by Gαi/o has not been tested. We examined the regulation by Gαi3 of heterotetrameric GIRK1/2 formed by GIRK1 labeled with CFP or YFP at the C terminus and the wild-type GIRK2 (GIRK2WT). The resulting GIRK1xFP/2 showed fast activation by ACh and inward rectification like the GIRK1/2WT (supplemental Fig. S2). The GIRK1xFP/2 channel also exhibited proper PM targeting and expression, comparable with the WT GIRK1/2 channel, as detected by antibody labeling of GIRK1 in giant excised plasma membrane patches (Fig. 1D, top panel) (34). Expression of GIRK1YFP was also monitored in whole intact oocytes (Fig. 1D, bottom panel). Like the GIRK1/2WT, the GIRK1xFP/2 showed dose-dependent regulation by Gαi3WT; Ibasal was reduced, and Ievoked was concomitantly increased, and Itotal slightly increased or remained unchanged (Fig. 1, A and B). As described for GIRK1/2WT (12), positive correlation between the increase in the dose of injected Gαi3 RNA and subsequent increase in Ra was observed (Fig. 1C). Overexpression of Gαi3WT and/or Gβγ did not significantly affect the PM levels of GIRK1xFP/2 (Fig. 1, E and F). In all, GIRK1xFP/2 is properly regulated by Gαi3WT.
YFP-labeled Gαi3 Proteins Bind GIRK1 and Gβγ but Appear Deficient in a Triple GIRK1-Gα-Gβγ Interaction Test
We next tested three different xFP-Gαi3 constructs as follows: N- and C-terminally labeled (Gαi3NT and Gαi3CT, respectively) and an internally labeled one (Gαi3117). Gαi3NT has been constructed to start with a 15-amino acid sequence containing a myristoylation signal, followed by CFP or YFP and then the full-length Gαi3. N-terminally lipid-modified and xFP-labeled Gαi subunits have been reported to successfully couple GPCRs to GIRK, reducing Ibasal and eliciting Ievoked (27). Gαi3117, in which YFP was inserted in the αb-αc loop of Gαi3 (with its methionine starting at amino acid 117) of Gαi3 via a two-amino acid linker, showed proper uncatalyzed GDP-GTP exchange in vitro and an α2-adrenergic receptor-induced dissociation (rearrangement) from Gβγ in mammalian cells (28). Gαi3CT is a novel construct, in which YFP is fused to the end of the C terminus of Gαi3 via a two-amino acid linker.
We first examined the interaction of Gαi3xFP proteins with Gβγ. 35S-Labeled Gαi3YFP proteins, synthesized in vitro in reticulocyte lysate, were pulled down by purified histidine-tagged Gβ1γ2 (His6Gβγ) in the presence of GDP or GTPγS. In the presence of GDP, all three Gαi3YFP-bound His6Gβγ at least as well as Gαi3WT, whereas 35S-labeled YFP did not bind His6Gβγ (Fig. 2, A and B). As expected, Gαi3-Gβγ binding was significantly reduced in the presence of GTPγS (Fig. 2, A and C) (44). The residual binding could be attributed to the presence of a second nucleotide-independent Gβγ-binding site in Gαi (45), but this issue was not pursued. In all, although semiquantitative, this assay showed the expected guanine nucleotide-dependent interaction of all three Gαi3YFP constructs with Gβγ.
GIRK subunits bind both Gβγ and Gαi (18, 19, 40, 46–49). Furthermore, Gβγ enhances the interaction of Gαi3 with the full cytosolic domain of GIRK1 (13, 16). Although the exact functional correlate of this effect is not known, we hypothesized that integrity of triple Gα-Gβγ-GIRK interactions within the presumptive signaling complex may be an important factor in proper GIRK regulation. To examine whether the Gαi3YFP proteins interact with GIRK1 and how this regulation is affected by Gβγ, we used a purified GST-fused full cytosolic domain of GIRK1 containing both the N- and C-terminal cytoplasmic domains but lacking its transmembrane region, GST-G1NC. GST-G1NC properly bound 35S-labeled, in vitro translated (ivt) 35S-Gαi3WT and Gβγ, in the presence of either GDP or GTPγS (Fig. 3A). Notably, the binding of GST-GIRK1 to Gβγ was 4–5-fold stronger than to Gαi3WT, as indicated by the smaller percentage of bound versus added protein (Fig. 3B). Furthermore, the binding of 35S-Gαi3WT to GST-G1NC was enhanced in the presence of purified Gβγ, ∼2.5-fold in GDP and ∼2-fold in GTPγS (Fig. 3, C and E). These data corroborate our previous report that used a reciprocal protocol, with purified GST-Gαi3 and in vitro synthesized 35S-G1NC (16).
Next, we tested the interaction of GST-G1NC with Gαi3YFP proteins and its modulation by Gβγ. GST-G1NC bound all three 35S-Gαi3YFP constructs at least as well as Gαi3WT, and it did not bind ivt 35S-YFP (Fig. 3, D and E, supplemental Fig. S3). In the presence of GDP, all of the Gαi3YFPs showed the expected Gβγ-dependent enhancement of interaction with GIRK1 (Fig. 3D, top, summary in E) but failed to do so in GTPγS (Fig. 3, D, bottom, F). These results suggest a possible flaw in function of the YFP-labeled Gαi3 subunits within the presumptive GIRK-Gα-Gβγ signaling complex.
In contrast to the effect of Gβγ on GIRK1-Gαi3 binding, addition of purified GST-Gαi3 did not affect the interaction between His6Gβγ and 35S-G1NC (Fig. 3F). These observations indicate that, within the signaling complex, Gαi3WT is probably bound to the channel via Gβγ and not vice versa (see below). In view of the absence of any effect, we did not pursue the matter with the YFP-labeled Gαi3 subunits.
YFP Labeling and PM Level Titration of Gαi3 Reveal Duality of Gαi3 Regulation of GIRK
We next examined functional regulation of GIRK1CFP/2 by YFP-labeled Gαi3 constructs (Fig. 4). In each experiment, we monitored the expression of the fluorescent subunits at the PM (Fig. 4A) along with the regulation of the GIRK1CFP/GIRK channel (Fig. 4, B–D). We first examined the effects of Gαi3NT and compared them with those of Gαi3WT measured in the same experiment. Increasing the amount of mRNA of Gαi3NT resulted in a concomitant increase in its PM expression (Fig. 4B), without markedly affecting the PM level of GIRK1CFP (supplemental Fig. S4A). In most experiments (n = 4 out of 6), Gαi3NT was unable to reduce Ibasal and did not substantially increase Ievoked in a wide range of Gα:channel mRNA stoichiometries. Accordingly, Ra was unchanged, suggesting that Gαi3NT cannot proficiently control the basal activity of the channel (Fig. 4B). Nevertheless, coexpression of Gαi3NT accelerated the activation kinetics of ACh-induced Ievoked, reducing τact to below 1 s, similarly to Gαi3WT (Fig. 4B), suggesting that Gαi3NT efficiently transduces activation from GPCR to GIRK. It thus appears that reduction in Ibasal and acceleration of agonist response may be separate functions of Gαi.
Next, we examined Gαi3117 and Gαi3CT (Fig. 4, C and D). Both proteins expressed well at the PM and, unlike Gαi3NT, reduced Ibasal. Gαi3117 did not reduce Itotal, therefore increasing Ra, although not as well as Gαi3WT. However, Gαi3117 persistently slowed down τact, to 6–8 s, substantially slower than τact seen with endogenous Gα (Fig. 4C). Strikingly, Gαi3CT decreased Ibasal, IACh, and Itotal at all expression levels (Fig. 4D), highly reminiscent of a Gβγ scavenger (13), and markedly slowed τact. In all, Gαi3CT was the least functional of all three YFP-labeled constructs. These results indicate the possibility of imperfect coupling of Gαi3117 and Gαi3CT to m2R or to GIRK.
We have previously reported that to substantially reduce Ibasal of GIRK1/2, 2.5–5-fold excess of Gαi3WT RNA over the RNA of the channel was necessary, suggesting that Gαi “titrates” the expressed channel (12). We noticed that Gαi3NT expresses less well than Gαi3117 or Gαi3CT (e.g. Fig. 4A), raising the possibility that this is the reason for the inability of Gαi3NT to reduce Ibasal. Therefore, we titrated the amount of RNA of Gαi3NT and Gαi3117 to reach similar PM expression levels, keeping the RNA dose of the channel constant (1 ng of each subunit) (Fig. 5, A and B; see complete results of two experiments in supplemental Fig. S4, B and C). When high doses of the RNA of Gαi3NT were injected and expressed at the PM equally with Gαi3117 (Fig. 5, A and B), it was able to reduce Ibasal by ∼60% and increase the Ra, although Gαi3117 reduced Ibasal even more, by ∼80% (Fig. 5C). Importantly, when Gαi3NT and Gαi3117 showed equal expressions, Gαi3NT significantly accelerated τact (as Gαi3WT), whereas Gαi3117 significantly decelerated the activation (Fig. 5D).
FIGURE 5.
Titrated expression of Gαi3 constructs reveals independence of regulation of basal current and activation kinetics by Gαi. A–D, similar levels of PM expression of Gαi3NT and Gαi3117 were achieved by injecting variable amounts of RNA (see supplemental Fig. S4, B and C, for a full summary of this and an additional similar experiment). The amount of expressed GIRK1CFP/2 was also monitored. A, representative images of oocytes expressing Gαi3NT (20 ng of RNA) and Gαi3117 (2 ng of RNA) at comparable expression levels. The summary of PM expression levels of all constructs is shown in B. Note that coexpression of Gαi3 caused only slight, statistically insignificant, changes in channel expression. C and D, effect of Gαi3WT and of equally expressed Gαi3NT and Gαi3117 on channel activity (Ibasal, IACh, Itotal; Ra is shown in the inset, and τact in D). Correction of Ibasal for minor changes in GIRK expression (see Refs. 13, 16) in different test groups did not yield major changes except for accentuating the decrease in Ibasal caused by Gαi3WT. E–H, mild expression of Gαi3WT (2.5 ng of RNA) does not affect GIRK expression (E) and does not reduce Ibasal (F), but it still significantly increases Ra (G) and accelerates activation (H). A representative experiment, out of three, in which the RNA dose of Gαi3WT was chosen or titrated to be just below the level that significantly reduces Ibasal, is shown. Number of oocytes is shown within bars. *, p < 0.05; **, p < 0.01. n.s., nonsignificant (compared with control group (where no Gα subunit was coexpressed)).
In the experiment of Fig. 5, A–D, Gαi3WT was expressed at a relatively low dose that did not decrease Ibasal, but acceleration of τact was clearly observed (Fig. 5D). However, correction for expression level changes indicated that a slight increase in PM expression of the channel caused by Gαi3WT could compensate for the decrease in Ibasal (Fig. 5C, open bars). Therefore, we performed additional experiments with low doses of Gαi3WT RNA (1–2.5 ng). One of them is shown in Fig. 5, E–H. These experiments confirmed that acceleration of τact by Gαi3WT (Fig. 5H) takes place under conditions when Ibasal is unchanged (Fig. 5F), and the expression of the channel is not altered (Fig. 5E). Thus, reduction in Ibasal and acceleration of agonist response appear to be separable functions of Gαi.
Suppression of Endogenous Gαi/o Accentuates Differences among xFP-labeled Gαi3
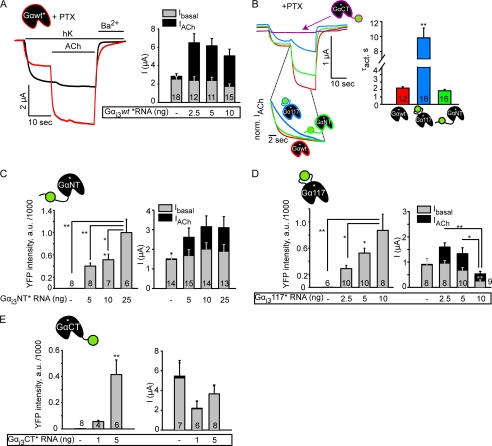
In studying the properties of heterologously expressed Gαi/o, it is customary to suppress the activation of endogenous Gαi/o with PTX. However, since PTX prevents the dissociation of Gαi/o from Gβγ and “freezes” the heterotrimeric form (50), there is a chance that it may also lock the hypothetical complex of GIRK with the inactivated Gαi/oβγ heterotrimer and interfere with actions of the expressed Gα. With this reservation, we used PTX to alleviate the interference from the endogenous Gαi/o. We constructed PTX-insensitive Gαi3*WT and Gαi3*YFP constructs (asterisk denotes the C351I mutation that prevents ADP-ribosylation by PTX (51)). The oocytes were injected with the catalytic subunit of PTX 2–20 h prior to current measurement. PTX eliminated Ievoked in the absence of coexpressed Gαi (Fig. 6A and supplemental Fig. S5B). PTX treatment also tended to reduce Ibasal and the PM levels of coexpressed Gαi3 proteins and of GIRK1xFP (supplemental Fig. S5). However, Ievoked was faithfully restored after coexpression of Gαi3*WT, although in these two experiments the reduction of Ibasal by Gαi3WT did not reach statistical significance (Fig. 6, A and B). τact was 2.2 ± 0.19 s (n = 12), slower than with PTX-sensitive Gαi3WT, probably reflecting an interference of the C351I mutation with Gα activation by the GPCR (52). In contrast, Gαi3*CT was completely unable to activate GIRK after PTX treatment (Fig. 6, B and C), suggesting that the small agonist-induced activation seen after overexpression of Gαi3CT in the absence of PTX (Fig. 4D) was due to endogenous Gαi/o; the slowing of τact probably resulted from competition between the two Gαi pools (53).
FIGURE 6.
Suppression of endogenous Gαi/o with PTX accentuates the differences between the xFP-labeled Gαi3. A, representative currents showing the effect of PTX treatment. PTX almost completely abolished IACh (black trace), whereas coexpression of PTX-insensitive Gαi3WT (denoted Gαi3WT*) completely restores IACh (red trace). B, representative current traces and summary of τact showing the activation of GIRK using PTX-insensitive YFP-labeled Gαi3 subunits. Gαi3CT* does not mediate channel activation via m2R. The lower set of traces in B shows normalized ACh-evoked currents, demonstrating differences in kinetics of activation (icons show the color code). C–E, summary of PM expression of the PTX-insensitive YFP-labeled Gαi3 subunits: Gαi3NT*, Gαi3117*, and Gαi3CT*, respectively, and their functional effects on GIRK currents. *, p < 0.05; **, p < 0.01. n.s., nonsignificant (compared with control group (where no Gαsubunit was coexpressed)). a.u., arbitrary units.
Both Gαi3*NT and Gαi3*117 showed robust RNA dose-dependent expression in the PM and restored Ievoked (Fig. 6, B–D). Although Gαi3*NT did not reduce Ibasal, it properly restored the fast activation of Ievoked, with τact identical to that obtained with Gαi3*WT (Fig. 6, B and D). It is probable that the lack of reduction in Ibasal stems from its relatively low expression pattern along with the tendency of PTX to further reduce its expression (supplemental Fig. S5A). In contrast, both Gαi3*CT and Gαi3*117 reduced Ibasal; at high expression levels Gαi3*117 also robustly reduced Ievoked and Itotal. Notably, Ievoked with Gαi3*117 activated very slowly with a τact of ∼10 s, as observed with nonmutated Gαi3 proteins (Fig. 5E). In all, of the three Gαi3*YFP constructs, Gαi3*NT best conveys the activation from GPCR to GIRK. Gαi3*117 is able to regulate Ibasal but is deficient in providing the proper fast activation. The striking differences in actions of Gαi3NT and Gαi3117 further support the notion that regulation of GIRKs basal activity and fast donation of Gβγ for GPCR-induced GIRK activation are separate functions of Gαi/o.
FRET Indicates an Intimate Contact of GIRK1 with Gβγ but Not with Gαi3xFP
Although none of xFP-labeled Gαs fully reproduced all GIRK regulations and interactions seen with Gαi3WT, Gαi3NT and Gαi3117 were functional enough to attempt the assessment of Gαi3xFP-GIRK interaction by FRET. GIRK1CFP/2 and Gαi3-YFP were expressed in oocytes, and spectral FRET analysis was performed using Zeiss 510 meta confocal microscope (Fig. 7, supplemental Fig. S6) (16, 35). CFP-labeled and G protein-insensitive inwardly rectifying K+ channel IRK1 (Kir2.1) was used as the negative control. We titrated the expression of each of the proteins to construct FRET saturation curves (36, 54). To overcome variability inherent to fluorescence measurements, we utilized a previously reported doubly xFP-labeled YFP-IRK1-CFP expressing CFP and YFP at a 1:1 molar ratio, thus enabling us to calibrate the readings of CFP and YFP and to assess their actual molar ratio in each experiment (supplemental Fig. S6B) (16). The obtained molar ratio is independent of the experimental settings and allows combining the results of multiple experiments into one saturation curve. The “saturating” value of FRET reflects the distance between the centers of the fluorophores, CFP and YFP (36), assuming an absence of anisotropic effects (17). We estimated that the maximal density of the expressed fluorescent proteins was usually below 100 molecules/μm2,4 which is mild compared with many hundreds of molecules/μm2 usually exploited in FRET experiments in mammalian cells (55). This strategy helps to avoid “crowding” effects that may lead to FRET among weakly interacting molecules due to random collisions (54).
FIGURE 7.
CFP-labeled GIRK shows FRET with YFP-labeled Gβγ but not with YFP-labeled Gαi3 subunits. A, emission spectra of groups expressing GIRK1CFP/2 (black trace) or GIRK1CFP/2 and Gαi3NT (light gray trace) when the donor CFP was excited with the 405 nm laser. Note that the spectra almost overlap. In contrast, B shows the emission spectra of GIRK1CFP/2 or GIRK1CFP/2 and GβγYFP. Note the large increase in emission in the YFP emission range (520–580 nm), revealing the energy transfer between the donor and acceptor molecules. C–F, apparent FRET efficiency (Eapp) of resonance transfer between GIRK1CFP and Gαi3117 (C), GIRK1CFP and Gαi3NT (D), CFPQ-GIRK1 and Gαi3117 (E), and GIRK1CFP and GβγYFP (F). IRK1CFP was used for the assessment of nonspecific FRET signal (negative control). Measurements from multiple experiments were incorporated in a single curve, by using donor/acceptor molar ratio (see “Experimental Procedures”). Curves were fitted to a hyperbolic function.
We detected very small, if any, FRET signals between GIRK1CFP/2 and Gαi3117-YFP or Gαi3NT-YFP, even at very high donor/acceptor molar ratios. There were no significant differences between the FRET signals obtained by either GIRK1CFP or IRK1CFP (our negative control) and the Gαi3-YFPs (Fig. 7, A, C and D). We also extended our FRET assay to an N-terminally labeled GIRK1 (CFP-GIRK1) and similarly found no evidence for a specific FRET signal (Fig. 7E). We also tested the interaction between GIRK1CFP/2 and GβγYFP, in which Gγ2 was labeled (see under “Experimental Procedures”). Confirming previous studies by Reuveny and co-workers (17) and Hebert and co-workers (21), GIRK1CFP and GβγYFP gave a saturable and specific FRET signal in Xenopus oocytes, much higher that the nonspecific signals obtained for GIRK and Gα or IRK and Gβγ (Fig. 7, B and F). The apparent maximal FRET efficiency obtained by fitting a Michaelis-Menten-type equation to the data, Eapp(max), was 14.5%. This corresponds to a distance of ∼65 Å assuming random fluorophore orientation (36), similar to a previous estimate of ∼60 Å obtained with GIRK1/4 (17). Our findings strengthen the notion that Gβγ is in close proximity to GIRK even prior to activation of the receptor. If Gαi3 is present in the signaling complex, it should lie at a greater distance (>10 nm).
DISCUSSION
We investigated the role and mechanisms of regulation of the neuronal GIRK1/2 channel by Gαi using wild-type and three xFP-fused Gαi3 constructs. An extensive array of functional assays showed that none of the Gαi3xFPs can fully reproduce all regulatory effects of the wild-type Gαi3. Of the three Gαi3xFP, only the N-terminally labeled, myristoylated Gαi3NT mimicked all aspects of regulation, but it was less efficient in reducing the basal activity (Ibasal). The distinct functional properties of the different Gαi3xFPs and dose-dependent differences in effects of Gαi3WT revealed that regulations of basal and agonist-evoked activity of GIRK1/2 are separate and independent functions of Gαi. These functions are probably carried out by the two different guanine nucleotide-associated forms of Gα, although GαiGDP regulates Ibasal, GαiGTP accelerates the Gβγ-induced channel opening following activation of the GPCR. A combination of direct biochemical binding and FRET measurements indicated that, within the GIRK-G protein signaling complex, Gα is attached to GIRK via Gβγ. The findings reported here provide new structural and functional insight into the mechanisms of GPCR-G protein-GIRK signaling pathway and support the idea that GIRK is an effector of both Gβγ and Gαi.
xFP Tagging of Gα May Disrupt Regulation of Effectors
Proteins tagged with derivatives of GFP (xFPs) are generally reported to retain native function, despite their large size (∼27 kDa; see supplemental Fig. S1) (56). We find that C- or N-terminal xFP-tagged GIRK1 produces functional channels properly gated not only by GPCRs and Gβγ, as shown previously (17, 41), but also by GαiWT. However, this was not the case with xFP-tagged Gαi3. Our results reveal striking incompetence of two of the three xFP-labeled constructs used here in adequately restoring GPCR-GIRK coupling. Only the N-terminally myristoylated xFP-fused Gαi3NT produced activation as fast as Gαi3WT. GPCR-induced activation with Gαi3117 was substantially slower, and it was completely absent in the C-terminally xFP-labeled Gαi3CT. The latter is probably due to defective activation by the GPCR, which crucially depends on interaction of GPCR with an intact C terminus of Gα (1). Gαi3CT probably competes with the endogenous Gα for m2R and Gβγ, thus reducing both free [Gβγ] (and thus Ibasal and Itotal) and the amount of GPCR available for interaction with Gi/o.
Of particular interest is the flaw in coupling to GIRK exhibited by Gαi3117, as evidenced in kinetic slowing of agonist-induced activation (compared with the activation via the endogenous Gαi/o of the oocyte), which stands in contrast to the acceleration caused by Gαi3WT and Gαi3NT. Incidentally, the kinetics of activation of GIRK by Gαi3117 and similar constructs have not been tested in the past, whereas other aspects of their function have been described as normal. Gibson and Gilman (28) have demonstrated that Gαi3117 and analogous constructs of Gαi1 and Gαi2 have adequate uncatalyzed GDP-GTP exchange in vitro and robust coupling to several isoforms of α2 adrenergic receptors, as monitored by rearrangements of Gα and Gβγ reported by FRET. Fast FRET or bioluminescence resonance energy transfer measurements indicated rapid (<1 s) GPCR-induced rearrangements of Gα and Gβγ, using constructs of Gαo and Gαi1 labeled with xFPs at positions analogous to 117 in Gαi3, as well as Gαi/o labeled in the αB-αD loop (position 91 in Gαi1) (25, 29). Thus, the coupling of Gαi3117 to GPCR and to Gβγ appears intact, and the slowing of GIRK activation probably reflects a problem in the Gαi3117-GIRK dialog, in line with the idea that Gαi actively regulates GIRK gating (12, 39). Indeed, interaction with GIRK and specific activation of GIRK by Gαi/o-coupled GPCRs involve the helical domain of Gαi/o (57). We propose that xFP insertions in the Gα helical domain obstruct Gαi/o-GIRK interaction via the interface between the helical domain of Gαi/o and the putative Gα-interaction site in GIRK1, interfering with normal regulation of GIRK by Gαi/o.
Regulation of Basal and Evoked Activities of GIRK Are Separate Functions of Gαi
Heterologously expressed GIRK1/2 (or GIRK1/4) channels have substantial Gβγ-dependent basal activity, and Gα is necessary to keep the basal activity low and to ensure robust activation by agonists (12, 58). The reduction of Ibasal is carried out by Gαi/oGDP, by forming Gαβγ heterotrimers, which presumably remain attached to the channel (18, 19, 39). The formation of this minimal signaling complex, by itself, is expected to accelerate activation of the channel by agonists (59), with no need to assume that the activated Gαi/oGTP contributes to activation. The reasoning is proximity (59) and stoichiometry; a GIRK channel has four Gβγ-binding sites (60, 61), and extent of activation is a graded function of number of bound Gβγ molecules (30, 62). If some of the heterologously expressed GIRK channels are associated with less than four Gαi/oβγ heterotrimers, addition of lacking Gαi/o could enhance and accelerate activation. Accordingly, in our previous work, we attributed the acceleration of τact by coexpressed Gαi3 to formation of Gαi3βγ-GIRK complexes (40). If this is the case, it is possible that Gαi3117 does not form such complexes (slow dissociation from Gβγ can be excluded; see above).
However, the minimal hypothesis implicating GαiGDP alone in the accelerating effect of coexpressed Gαi appears insufficient to account for our new observations. First, we find that the acceleration of τact by Gαi3WT and Gαi3NT takes place in the absence of a reduction in Ibasal, which is a hallmark of formation of Gαi/oβγ heterotrimers functionally associated with the channel (13). In fact, the two constructs that best reduce Ibasal, Gαi3117 and Gαi3CT, are deficient in GPCR-driven coupling to GIRK, which further emphasizes the separation of the two functions of Gαi. Second, in protein interaction assays, Gαi3xFPGDP constructs bound GIRK1 at least as well as the wild-type Gαi3, both in the absence and presence of Gβγ (Figs. 2 and 3), suggesting that the formation of Gαiβγ-GIRK complex by these proteins is retained.
In view of these considerations, we hypothesize that acceleration of τact is carried out by GαiGTP, which acts synergistically with Gβγ in GIRK1/2 activation. So far no direct positive regulation of mammalian GIRKs by “active” GαGTP has been demonstrated. Rather, an inhibitory regulation of GIRK1/4 by Gαi1GTPγS (but not Gαi3GTPγS) has been observed at very low expression levels of the channel, suggesting a mechanism involving interference with endogenous Gαi/o (12, 14). However, a synergistic, mutually obligatory activation by GαiGTP and Gβγ in artificial membranes has been recently found in a chimeric channel composed of a transmembrane domain of a bacterial K+ channel and a truncated cytosolic domain of mammalian GIRK1 (15). Furthermore, coexpression of a constitutively active (GTP-bound) mutant of Gαi3 significantly modified Gβγ-induced conformational changes in the cytosolic domains of GIRK1 and GIRK2 subunits within the GIRK1/2 heterotetrameric channel, also suggesting more than additive effects of Gαi3GTP and Gβγ (16). In accelerating τact, Gαi/oGTP could regulate GIRK by a direct interaction, as supported by biochemical evidence (e.g. Fig. 3); however, an indirect effect cannot be ruled out. Thus, regulation of GIRK channels by Gαi/oGTP appears plausible but requires further study.
Gαi/oβγ Heterotrimers Contact GIRK1 Primarily via Gβγ
The idea that GIRK channel forms a signaling complex with the G protein has become widely accepted (for review, see Refs. 9, 22), but structural and molecular details are poorly understood. The interaction of GIRK1 subunit with Gαi is enhanced by Gβγ, supporting the notion that heterotrimeric Gαiβγ is anchored to GIRK1 (13, 16, 19). It has been proposed that, at least in GIRK2 subunit, anchoring occurs via Gαi (18), suggesting a tight contact between GIRK and Gαi/o preceding GPCR activation. However, studies that sought support for this hypothesis using resonance energy transfer yielded inconsistent results (see “Introduction”) (20, 21). Because both studies (20, 21) used Gα constructs with luciferase or xFP insertions within the helical domain of Gαi, we initially hypothesized that the defect in functional interaction of these proteins with GIRK could account for the inconsistent results. We therefore used the N-terminally labeled Gαi3, which carried the closest resemblance to Gαi3WT in its regulation of GIRK1/2. Yet, neither Gαi3NT nor Gαi3117 produced a significant FRET signal in our experiments. Under the same conditions, a strong FRET between Gβγ and GIRK1 was observed, confirming previous publications (17, 21).
What could be the reason for the lack of FRET between Gαi3 and GIRK1? Below we are listing four types of possibilities, starting from the least plausible. 1) When GIRK and Gαi3 are bound to each other, the distance between the xFP labels exceeds the limit of FRET resolution (∼100 Å), or the angle between the donor and acceptor dipoles is unfavorable for FRET. We deem this as unlikely, given the moderate sizes of these proteins (supplemental Fig. S1) and the fact that placing xFP labels on either N or C terminus of GIRK1, or N terminus of Gαi3, or in the helical domain did not make a difference. At present, we cannot rule out other obstructions to FRET such as quenching of some of the fluorescence by the protein environment of GIRK-bound Gαi3xFP. 2) Subtle differences between Gαi3NT and Gαi3WT bring about such a dramatic change in GIRK1-Gαi3 interaction that the close interaction between the channel and Gαiβγ, via Gαi, is lost. This is also unlikely; our functional and biochemical data do not lend support for this notion. 3) The hypothetical Gαiβγ-GIRK complex is not stable but dynamic, with relatively low affinity of interaction that is not captured in our FRET assay. This possibility cannot be ruled out, despite the strong functional and biochemical evidence in support of a stable complex (22).
We favor a fourth alternative explanation; within the GIRK1/2-Gαi/oβγ signaling complex, Gαi/o is anchored to the channel primarily via Gβγ. The molecular sizes of Gα and Gβγ (supplemental Fig. S1), and the estimated 60–65 Å distance between the centers of xFPs associated with GIRK1 and Gγ, suggest that the distance between GIRK1- and Gαi-attached fluorophores may be big enough to thwart FRET if Gαi is associated with GIRK1 through its binding with Gβγ. This possibility is further supported by several lines of new and published data. 1) Our previous functional and immunocytochemical studies suggested that heterologously expressed GIRK1/2 is preferentially associated with Gβγ, whereas, under certain conditions, Gαi/o appears to be missing from the signaling complex (32, 39). These findings are in line with the above assumption. 2) The binding of Gαi3 to GIRK1 is several times weaker than that of Gβγ (Fig. 3). Lack of strong binding of GαGTP, compared with the strong binding of Gβγ, to immunoprecipitated subunits of cardiac GIRK channel has been reported previously (47). It is important to emphasize that here we used the full cytosolic domain of GIRK1, rather than purified separate Gβγ- or Gα-binding segments, because the actual Gβγ-binding surface of GIRK1 (and probably other GIRK subunits) is composed of several N- and C-terminal cytosolic segments (48). Importantly, the enhancing effect of Gβγ on GIRK1-Gαi3 binding occurs only in the context of a full cytosolic domain of GIRK1, but not with separate N- or C-terminal domains (16). This is the first time that a direct comparison of Gαi3 and Gβγ binding to full cytosolic domain of GIRK1 is done. The pulldown of Gαi3 and Gβγ, although semiquantitative, was performed under identical conditions and in the same experiment, enhancing our confidence in the result. 3) Gβγ enhances the interaction of GIRK1 with Gαi3, but Gαi3 does not affect the interaction of GIRK1 with Gβγ (Fig. 3); the opposite could be expected if Gβγ were anchored via Gαi/o. In all, our findings support a model in which the GIRK is the scaffold of the signaling complex that anchors Gβγ, whereas Gαi/o is associated with the complex via its binding to Gβγ.
Supplementary Material
Acknowledgments
We thank Isabella Tselnicker for critical reading of the manuscript and Dr. C. Doupnik for the generous gift of GST-G1NC construct.
This work was supported, in whole or in part, by National Institutes of Health Grant GM60419 (to C. W. D.). This work was also supported by United States Israel Binational Science Foundation Grant 2009255 (to N. D. and C. W. D.) and Israel Science Foundation Grants 1396/05 and 49/08 (to N. D.) and 386/09 (to T. I.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
S. Berlin and N. Dascal, unpublished observations.
- GPCR
- G protein-coupled receptor
- GIRK
- G protein-activated K+ channel
- ACh
- acetylcholine
- GST
- glutathione S-transferase
- ivt
- in vitro translated
- CT
- C terminus
- NT
- N terminus
- CFP
- cerulean fluorescent protein
- m2R
- muscarinic m2 receptor
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- PTX
- pertussis toxin
- PM
- plasma membrane.
REFERENCES
- 1. Oldham W. M., Hamm H. E. (2008) Nat. Rev. Mol. Cell Biol. 9, 60–71 [DOI] [PubMed] [Google Scholar]
- 2. Hakak Y., Shrestha D., Goegel M. C., Behan D. P., Chalmers D. T. (2003) FEBS Lett. 550, 11–17 [DOI] [PubMed] [Google Scholar]
- 3. Milligan G., Kostenis E. (2006) Br. J. Pharmacol. 147, S46–S55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ikeda S. R. (1996) Nature 380, 255–258 [DOI] [PubMed] [Google Scholar]
- 5. Yevenes G. E., Moraga-Cid G., Avila A., Guzmán L., Figueroa M., Peoples R. W., Aguayo L. G. (2010) J. Biol. Chem. 285, 30203–30213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dascal N. (2001) Trends Endocrinol. Metab. 12, 391–398 [DOI] [PubMed] [Google Scholar]
- 7. Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. (1987) Nature 325, 321–326 [DOI] [PubMed] [Google Scholar]
- 8. Dascal N. (1997) Cell. Signal. 9, 551–573 [DOI] [PubMed] [Google Scholar]
- 9. Lüscher C., Slesinger P. A. (2010) Nat. Rev. Neurosci. 11, 301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hibino H., Inanobe A., Furutani K., Murakami S., Findlay I., Kurachi Y. (2010) Physiol. Rev. 90, 291–366 [DOI] [PubMed] [Google Scholar]
- 11. Wickman K., Clapham D. E. (1995) Physiol. Rev. 75, 865–885 [DOI] [PubMed] [Google Scholar]
- 12. Peleg S., Varon D., Ivanina T., Dessauer C. W., Dascal N. (2002) Neuron 33, 87–99 [DOI] [PubMed] [Google Scholar]
- 13. Rubinstein M., Peleg S., Berlin S., Brass D., Keren-Raifman T., Dessauer C. W., Ivanina T., Dascal N. (2009) J. Physiol. 587, 3473–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schreibmayer W., Dessauer C. W., Vorobiov D., Gilman A. G., Lester H. A., Davidson N., Dascal N. (1996) Nature 380, 624–627 [DOI] [PubMed] [Google Scholar]
- 15. Leal-Pinto E., Gómez-Llorente Y., Sundaram S., Tang Q. Y., Ivanova-Nikolova T., Mahajan R., Baki L., Zhang Z., Chavez J., Ubarretxena-Belandia I., Logothetis D. E. (2010) J. Biol. Chem. 285, 39790–39800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berlin S., Keren-Raifman T., Castel R., Rubinstein M., Dessauer C. W., Ivanina T., Dascal N. (2010) J. Biol. Chem. 285, 6179–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riven I., Iwanir S., Reuveny E. (2006) Neuron 51, 561–573 [DOI] [PubMed] [Google Scholar]
- 18. Clancy S. M., Fowler C. E., Finley M., Suen K. F., Arrabit C., Berton F., Kosaza T., Casey P. J., Slesinger P. A. (2005) Mol. Cell. Neurosci. 28, 375–389 [DOI] [PubMed] [Google Scholar]
- 19. Huang C. L., Slesinger P. A., Casey P. J., Jan Y. N., Jan L. Y. (1995) Neuron 15, 1133–1143 [DOI] [PubMed] [Google Scholar]
- 20. Fowler C. E., Aryal P., Suen K. F., Slesinger P. A. (2007) J. Physiol. 580, 51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rebois R. V., Robitaille M., Galés C., Dupré D. J., Baragli A., Trieu P., Ethier N., Bouvier M., Hébert T. E. (2006) J. Cell Sci. 119, 2807–2818 [DOI] [PubMed] [Google Scholar]
- 22. Zylbergold P., Ramakrishnan N., Hebert T. E. (2010) Channels 4, 411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vogel S. S., Thaler C., Koushik S. V. (2006) Sci. STKE 2006, re2. [DOI] [PubMed] [Google Scholar]
- 24. Hébert T. E., Galés C., Rebois R. V. (2006) Cell Biochem. Biophys. 45, 85–109 [DOI] [PubMed] [Google Scholar]
- 25. Bünemann M., Frank M., Lohse M. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 16077–16082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janetopoulos C., Jin T., Devreotes P. (2001) Science 291, 2408–2411 [DOI] [PubMed] [Google Scholar]
- 27. Leaney J. L., Benians A., Graves F. M., Tinker A. (2002) J. Biol. Chem. 277, 28803–28809 [DOI] [PubMed] [Google Scholar]
- 28. Gibson S. K., Gilman A. G. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 212–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galés C., Van Durm J. J., Schaak S., Pontier S., Percherancier Y., Audet M., Paris H., Bouvier M. (2006) Nat. Struct. Mol. Biol. 13, 778–786 [DOI] [PubMed] [Google Scholar]
- 30. Sadja R., Alagem N., Reuveny E. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 10783–10788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rishal I., Keren-Raifman T., Yakubovich D., Ivanina T., Dessauer C. W., Slepak V. Z., Dascal N. (2003) J. Biol. Chem. 278, 3840–3845 [DOI] [PubMed] [Google Scholar]
- 32. Rishal I., Porozov Y., Yakubovich D., Varon D., Dascal N. (2005) J. Biol. Chem. 280, 16685–16694 [DOI] [PubMed] [Google Scholar]
- 33. Sharon D., Vorobiov D., Dascal N. (1997) J. Gen. Physiol. 109, 477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singer-Lahat D., Dascal N., Mittelman L., Peleg S., Lotan I. (2000) Pflugers Arch. 440, 627–633 [DOI] [PubMed] [Google Scholar]
- 35. Zheng J., Varnum M. D., Zagotta W. N. (2003) J. Neurosci. 23, 8167–8175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bykova E. A., Zhang X. D., Chen T. Y., Zheng J. (2006) Nat. Struct. Mol. Biol. 13, 1115–1119 [DOI] [PubMed] [Google Scholar]
- 37. Zheng J., Zagotta W. N. (2003) Science's STKE 2003, PL7. [DOI] [PubMed] [Google Scholar]
- 38. Dascal N., Schreibmayer W., Lim N. F., Wang W., Chavkin C., DiMagno L., Labarca C., Kieffer B. L., Gaveriaux-Ruff C., Trollinger D., Lester H. A., Davidson N. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 10235–10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rubinstein M., Peleg S., Berlin S., Brass D., Dascal N. (2007) J. Physiol. 581, 17–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ivanina T., Varon D., Peleg S., Rishal I., Porozov Y., Dessauer C. W., Keren-Raifman T., Dascal N. (2004) J. Biol. Chem. 279, 17260–17268 [DOI] [PubMed] [Google Scholar]
- 41. Ivanina T., Neusch C., Li Y. X., Tong Y., Labarca C., Mosher D. F., Lester H. A. (2000) FEBS Lett. 466, 327–332 [DOI] [PubMed] [Google Scholar]
- 42. Chan K. W., Sui J. L., Vivaudou M., Logothetis D. E. (1997) J. Biol. Chem. 272, 6548–6555 [DOI] [PubMed] [Google Scholar]
- 43. Mirshahi T., Logothetis D. E. (2004) J. Biol. Chem. 279, 11890–11897 [DOI] [PubMed] [Google Scholar]
- 44. Lambright D. G., Sondek J., Bohm A., Skiba N. P., Hamm H. E., Sigler P. B. (1996) Nature 379, 311–319 [DOI] [PubMed] [Google Scholar]
- 45. Wang J., Sengupta P., Guo Y., Golebiewska U., Scarlata S. (2009) J. Biol. Chem. 284, 16906–16913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kunkel M. T., Peralta E. G. (1995) Cell 83, 443–449 [DOI] [PubMed] [Google Scholar]
- 47. Krapivinsky G., Krapivinsky L., Wickman K., Clapham D. E. (1995) J. Biol. Chem. 270, 29059–29062 [DOI] [PubMed] [Google Scholar]
- 48. Ivanina T., Rishal I., Varon D., Mullner C., Frohnwieser-Steinecke B., Schreibmayer W., Dessauer C. W., Dascal N. (2003) J. Biol. Chem. 278, 29174–29183 [DOI] [PubMed] [Google Scholar]
- 49. Finley M., Arrabit C., Fowler C., Suen K. F., Slesinger P. A. (2004) J. Physiol. 555, 643–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sunyer T., Monastirsky B., Codina J., Birnbaumer L. (1989) Mol. Endocrinol. 3, 1115–1124 [DOI] [PubMed] [Google Scholar]
- 51. West R. E., Jr., Moss J., Vaughan M., Liu T., Liu T. Y. (1985) J. Biol. Chem. 260, 14428–14430 [PubMed] [Google Scholar]
- 52. Waldhoer M., Wise A., Milligan G., Freissmuth M., Nanoff C. (1999) J. Biol. Chem. 274, 30571–30579 [DOI] [PubMed] [Google Scholar]
- 53. Vorobiov D., Bera A. K., Keren-Raifman T., Barzilai R., Dascal N. (2000) J. Biol. Chem. 275, 4166–4170 [DOI] [PubMed] [Google Scholar]
- 54. James J. R., Oliveira M. I., Carmo A. M., Iaboni A., Davis S. J. (2006) Nat. Methods 3, 1001–1006 [DOI] [PubMed] [Google Scholar]
- 55. Falkenburger B. H., Jensen J. B., Hille B. (2010) J. Gen. Physiol. 135, 99–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang F., Moss L. G., Phillips G. N., Jr. (1996) Nat. Biotechnol. 14, 1246–1251 [DOI] [PubMed] [Google Scholar]
- 57. Rusinova R., Mirshahi T., Logothetis D. E. (2007) J. Biol. Chem. 282, 34019–34030 [DOI] [PubMed] [Google Scholar]
- 58. He C., Zhang H., Mirshahi T., Logothetis D. E. (1999) J. Biol. Chem. 274, 12517–12524 [DOI] [PubMed] [Google Scholar]
- 59. Hille B. (1992) Neuron 9, 187–195 [DOI] [PubMed] [Google Scholar]
- 60. Yamada M., Jahangir A., Hosoya Y., Inanobe A., Katada T., Kurachi Y. (1993) J. Biol. Chem. 268, 24551–24554 [PubMed] [Google Scholar]
- 61. Corey S., Clapham D. E. (2001) J. Biol. Chem. 276, 11409–11413 [DOI] [PubMed] [Google Scholar]
- 62. Ivanova-Nikolova T. T., Breitwieser G. E. (1997) J. Gen. Physiol. 109, 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.