Background: Class Ib ribonucleotide reductase of the severe pathogen Bacillus anthracis can be loaded with manganese or iron.
Results: The manganese form was 10-fold more active than the iron form in the presence of the physiological protein NrdH-redoxin.
Conclusion: The manganese form is important in the life cycle of B. anthracis.
Significance: The physiologically relevant form of ribonucleotide reductase controls B. anthracis proliferation and survival.
Keywords: Enzyme Catalysis, FMN, Ribonucleotide Reductase, Surface Plasmon Resonance (SPR), Thioredoxin Reductase, Anthrax, Flavodoxin, Gas-phase Electrophoretic Mobility Macromolecular Analysis (GEMMA)
Abstract
Bacillus anthracis is a severe mammalian pathogen encoding a class Ib ribonucleotide reductase (RNR). RNR is a universal enzyme that provides the four essential deoxyribonucleotides needed for DNA replication and repair. Almost all Bacillus spp. encode both class Ib and class III RNR operons, but the B. anthracis class III operon was reported to encode a pseudogene, and conceivably class Ib RNR is necessary for spore germination and proliferation of B. anthracis upon infection. The class Ib RNR operon in B. anthracis encodes genes for the catalytic NrdE protein, the tyrosyl radical metalloprotein NrdF, and the flavodoxin protein NrdI. The tyrosyl radical in NrdF is stabilized by an adjacent Mn2III site (Mn-NrdF) formed by the action of the NrdI protein or by a Fe2III site (Fe-NrdF) formed spontaneously from Fe2+ and O2. In this study, we show that the properties of B. anthracis Mn-NrdF and Fe-NrdF are in general similar for interaction with NrdE and NrdI. Intriguingly, the enzyme activity of Mn-NrdF was approximately an order of magnitude higher than that of Fe-NrdF in the presence of the class Ib-specific physiological reductant NrdH, strongly suggesting that the Mn-NrdF form is important in the life cycle of B. anthracis. Whether the Fe-NrdF form only exists in vitro or whether the NrdF protein in B. anthracis is a true cambialistic enzyme that can work with either manganese or iron remains to be established.
Introduction
The essential enzyme ribonucleotide reductase (RNR)4 catalyzes a key reaction in the synthesis of the necessary deoxyribonucleotide (dNTP) building blocks for DNA synthesis and is essential in all cells and organisms, except a few parasites or obligate intracellular endosymbionts (1–3). Three major classes denoted I, II, and III are known within the RNR enzyme family and differ primarily in cofactor requirement, quaternary structure, free radical chemistry, and oxygen dependence. All RNRs employ a radical chemistry reaction mechanism, and almost all are regulated via sophisticated allosteric mechanisms keeping a balanced supply of all dNTPs. Despite extensive amino acid sequence differences, the three-dimensional core structure is preserved and points to a common evolutionary history for all three RNR classes (4–6). Bacillus anthracis, the causative agent of anthrax disease, encodes an oxygen-dependent class I RNR and an oxygen-sensitive class III RNR in its genome. However, the gene for the class III-specific activase was reported to be a pseudogene (7). We have earlier shown that specific inhibition of the B. anthracis class I RNR via the radical scavenger compound N-methylhydroxylamine efficiently knocks out growth of the organism (8).
The vast majority of class I RNRs consist of a large α-component that harbors the active site and two types of binding sites for allosteric nucleoside triphosphate effectors and a small β-component that harbors a stable tyrosyl radical close to a diiron-oxo site. Common to all studied class I RNRs is that they are able to form an α2β2 holoenzyme complex, which in prokaryotes seems to be the main active form. Species-dependent larger complexes can also be induced such as the allosterically inhibited α4β4 form in Escherichia coli (9). Class I RNRs also encompass a few subclasses; the class Ib RNRs are restricted to a few phyla among eubacteria and to some bacteriophages, whereas the canonical class I RNRs (sometimes called class Ia) are found in almost all eukaryotes, in several eubacterial phyla, in a few archaea, and in some dsDNA viruses (2). Class Ib RNRs are distinguished by a special operon structure that in addition to the α- and β-polypeptides encoded by the nrdE and nrdF genes also encodes a flavodoxin protein in the nrdI gene, and in most operons, the nrdH gene encoding a low molecular mass redoxin protein that is specific for reduction of the class Ib RNR. The NrdE protein differs from the canonical class I α-component by having only one type of binding site for allosteric effectors, and the NrdF protein was recently shown to differ from the canonical β-component in its capacity to stabilize the tyrosyl radical by either forming a dimanganese-oxo center or a diiron-oxo center (1, 10, 11). Recent studies have also identified a role for the hitherto enigmatic flavodoxin protein NrdI (12–14), which is required for the manganese-dependent activation of NrdF in vitro (15, 16), but not for the iron-dependent in vitro activation of NrdF. Proficient genetic studies in E. coli showed that the Ib RNR is used under oxidative stress or iron limitation, when the canonical class Ia RNR is non-functional (17).
The class Ib RNR operon structure in the Bacillus cereus group (B. anthracis, B. cereus, Bacillus mycoides, Bacillus thuringiensis, and Bacillus weihenstephanensis spp.) encodes the nrdI, nrdE, and nrdF genes (cf. supplemental Fig. S1 for the B. anthracis operon), but the class Ib-specific nrdH gene is encoded elsewhere on the chromosome (7). Likewise, the gene for thioredoxin reductase that transfers electrons from NADPH to NrdH-redoxin is located elsewhere on the chromosome (7). In this study, we show that the manganese-dependent class Ib RNR in B. anthracis has up to 10-fold higher enzyme activity as compared with the iron-dependent form of the enzyme in presence of the physiological reductant NrdH. Apart from this critical and conceivably physiologically important difference, the Mn-NrdF and Fe-NrdF forms of B. anthracis class Ib RNR have similar characteristics in vitro. Our results conceivably point to the essential role of the manganese-dependent form of class Ib RNR in B. anthracis and the B. cereus group, and it remains to be shown whether the iron-dependent form also has a role in vivo.
EXPERIMENTAL PROCEDURES
Materials
DNA manipulations were carried out using standard protocols (18). E. coli strains DH5α (Stratagene) and Rosetta (DE3) (Novagen) were used for cloning and protein expression experiments, respectively. Wild type B. anthracis Sterne 7700 pXO1−/pXO2− (lacking both virulence plasmids) was obtained from the Swedish Defense Research Agency. E. coli strains were routinely grown in LB medium at 37 °C, and B. anthracis was grown in brain heart infusion medium (BD Biosciences) at 37 °C. When required, antibiotics and chromogenic substrates were added at the following concentrations: 50 μg/ml ampicillin, 20 μg/ml chloramphenicol, 50 μg/ml kanamycin, and 30 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal).
Construction of a B. anthracis NrdE Expression Vector
To amplify the NrdE protein, the homing endonuclease gene present in the group I intron in the B. anthracis nrdE gene (19) had to be removed due to its toxicity in cloned nrdE constructs. In two consecutive PCR reactions using B. anthracis genomic DNA (8) as template, the first pair of primers 1 (5′-ACCATGGTGCGACACATTGAACTG-3′) and 2 (5′-AAAAAACTCGAGATTCCACTTCACCCAAG-3′) was used to amplify a 985-bp fragment comprising exon 1 and the 5′ part of the intron. A second pair of primers 3 (5′-AAAAAACTCGAGTACTATCCAATAACT-3′) and 4 (5′-AGGATCCTATTAAACAGTACAAGCC-3′) was used to amplify a 1502-bp fragment comprising the 3′ part of the intron and exon 2 (supplemental Fig. S1). Both fragments were gel-purified, digested with XhoI, ligated using T4 DNA ligase (Fermentas), and used as templates for a second PCR reaction using only primers 1 and 4. The entire nrdE sequence without the endonuclease-coding region was cloned into pGEM-T-easy (Promega) and sequenced to ensure that no mutations were introduced by the PCR reactions. After digestion with NcoI and BamHI, the nrdE gene was cloned in the pET28a vector (Novagen) and named pETS146.
Expression and Purification of Recombinant B. anthracis NrdE, ApoNrdF, and NrdI Proteins
NrdE protein was produced in E. coli Rosetta (DE3) using the pET system (pETS146) according to standard procedures (Novagen). The growth temperature was 30 °C, and induction was performed for 4 h with 0.7 mm isopropyl-β-d-thiogalactopyranoside. The protein crude extract was prepared as described earlier for the NrdF protein (8). The most abundant band was the NrdE protein (predicted molecular mass 79.8 kDa), demonstrating that the B. anthracis intron is efficiently spliced during overproduction in E. coli (supplemental Fig. S2). NrdE was enriched by chromatography on a HiLoad® 16/10 Phenyl Sepharose® column (GE Healthcare) by applying a 0.75–0 m gradient of ammonium sulfate in buffer containing 50 mm Tris-HCl, pH 7.5, and 2 mm DTT (buffer A). Fractions were analyzed by SDS gel electrophoresis, and those with the B. anthracis NrdE band were concentrated and extensively washed with buffer A using a Centricon-50 tube (Millipore). All steps were carried out on ice or at 4 °C. From a typical experiment, we obtained 13–16 mg of pure NrdE protein from 5 liters of bacterial cell culture with a purity of ≥95% (supplemental Fig. S2). B. anthracis NrdF protein was purified using Rosetta (DE3) cells containing pETS145 and was obtained as apoprotein (lacking a metal center) as described previously (8). B. anthracis NrdI protein was purified using Rosetta (DE3) cells containing pETS153 as described previously (14).
Expression and Purification of Recombinant B. cereus NrdH, Thioredoxin Reductase, NrdE, NrdF, and NrdI
B. cereus ATCC 14579 NrdH-redoxin (BC3987), thioredoxin reductase (BC5159), NrdE (BC1354), NrdF (BC1355), and NrdI (BC1353) were expressed and purified as described previously (13, 20–22). In brief, genes were cloned into the pET22b vector and transformed into BL21 (DE3) cells. Transformed BL21 (DE3) cells containing pET22b-NrdI, -NrdH, -NrdE, or -NrdF were grown in TB medium containing 100 μg/ml ampicillin. Protein expression was induced by adding 0.8–1 mm isopropyl-1-thio-β-d-galactopyranoside, and cells were harvested by centrifugation after 12–16 h at 20 °C. The frozen cell paste was disintegrated using an X-press® (23), extracted with a solution containing 50 mm Tris-HCl, pH 7.5, and 10 mm EDTA (buffer B), and cleared from nucleic acids by streptomycin sulfate (2.5%) precipitation. Proteins were concentrated by ammonium sulfate precipitation using 0.29 mg/ml for NrdF and 0.43 mg/ml for NrdE, NrdH, and NrdI before desalting and purification by strong anion exchange chromatography (HiTrap Q HP) with a 0–0.5 m KCl gradient in buffer B. When required, a final purification step with Superdex-200 equilibrated in buffer B was used. For NrdE, all steps also included 2 mm DTT. B. cereus NrdH (78 residues) is 98% identical to the corresponding B. anthracis protein (BAS3897, annotated “glutaredoxin family protein”) and differs at two positions: A28V and D59N, none of which is close to the active site of NrdH.
Metal Reconstitution of ApoNrdF with Manganese or Iron
ApoNrdF was incubated with four Mn2+/dimer at room temperature for at least 5 min under anaerobic conditions, after which reduced NrdI was added and oxygen was admitted after 5–10 min. The ratio of NrdF:NrdI was ∼1:1 to enable monitoring of the tyrosyl radical formed after reaction with oxygen in the resulting light absorption spectrum. Reduction of NrdIox was performed by the addition of dithionite. Reduction of a mixture of Mn2II-NrdF and NrdIox with dithionite worked equally well and was used in some experiments. Oxygen was either added by bubbling pure oxygen through the sample with a Hamilton syringe or simply by opening the reaction vessel to air. In vitro incorporation of iron to the NrdF protein was performed as described earlier (8).
Separation of Mn-NrdF/Tyr• and NrdIox after Reconstitution
Mn-NrdF was separated from NrdI using a MonoQ column (GE Healthcare) and a 0–1 m KCl gradient in buffer A. NrdI eluted in the beginning of the gradient and Mn-NrdF eluted in two peaks, one lacking the radical and one containing 0.5 Tyr• per Mn-NrdF dimer (supplemental Fig. S3).
UV-visible Spectroscopy of Metal Reconstituted NrdF
UV-visible spectra were recorded on a PerkinElmer Life Sciences Lambda 35 spectrophotometer typically in 100-μl volume and with 10 μm NrdF dimer. If necessary, the spectra were baseline-corrected to zero absorbance at 750 nm, and for the isolated tyrosyl radical spectrum (see Fig. 1, trace C, upper graph), glitches in the spectrum were removed manually for better appearance.
FIGURE 1.
UV-visible absorption of B. anthracis Mn-NrdF and Fe-NrdF. The upper panel shows activated Mn-NrdF in the presence of NrdIox (trace A), only NrdIox (trace B), and enzymatically active Mn-NrdF after separation on anion exchange chromatography (trace C). The lower panel shows Fe-NrdF activated in the presence of NrdIox (trace A) and in the absence of NrdI (trace B), and (trace C) tyrosyl radical spectrum (after subtraction of spectrum obtained by incubation with radical scavenger hydroxylamine). The spectra in the upper panel have been normalized to 2 μm NrdF dimer, and in the lower panel, they have been normalized to 10 μm. In the lower panel, all spectra correspond to a radical content of 5.9 μm Tyr•. A.U. denotes absorbance units.
The tyrosyl radicals in the different preparations were determined from the dropline procedure using ϵ = 2.11 mm−1cm−1 (12). In mixtures with NrdI, the spectrum of NrdIox was subtracted from the composite spectrum before radical determination.
RNR Enzymatic Activity
Initial enzyme assays with B. anthracis NrdE and NrdF using conditions described for other class Ib proteins (24–27) gave very low specific activity, indicating that assay conditions were suboptimal. Interestingly, the DTT requirement had a surprisingly sharp optimum at ∼8 mm and distinct inhibition at higher DTT concentrations (supplemental Fig. S4A). The pH profile was notably narrow, with optimal activity at pH 7.5 (supplemental Fig. S4B). Attempts to further increase the enzyme activity (additions of B. anthracis crude extract and microaerobic and anaerobic assays) failed (data not shown). With optimized assay conditions, B. anthracis RNR activity was determined in 50-μl reactions containing 0.5–1 μm Mn-NrdF (reconstituted with 1:1 NrdI), 4 μm NrdE, 50 mm Tris-HCl, pH 7.5, 20 mm magnesium acetate, 0.2 mm dATP and 0.8 mm [3H]CDP (37,956 cpm/(min × nmol)). Reducing systems were 10 mm DTT, 5 μm B. cereus NrdH + 10 mm DTT, or 5 μm B. cereus NrdH + 0.5 μm thioredoxin reductase + 0.4 mm NADPH. Following 10 min of incubation at 37 °C, the reaction was terminated by adding 0.5 ml of 1 m perchloric acid, and the formed dCDP was analyzed as described (28). One enzyme unit (EU) is equivalent to 1 nmol of dCDP produced per min. Specific activity is expressed as EU per mg of NrdF protein.
GEMMA Analysis
All proteins were equilibrated on G-25 columns (GE Healthcare) into a buffer containing 20 mm ammonium acetate, pH 7.8 (plus 2 mm DTT and 0.005% (v/v) Tween 20 for NrdE protein solutions). Protein concentrations were 0.02–0.04 mg/ml (NrdE), 0.005–0.02 mg/ml (NrdF), 0.006–0.012 mg/ml (NrdI), and 0.004–0.008 mg/ml (NrdH). Substrate and/or effector nucleotides (1:1 nucleotide/magnesium acetate ratio) were included at 25–50 μm. The GEMMA system contained the following components: a 3480 electrospray aerosol generator, a 3080 electrostatic classifier, a 3085 differential mobility analyzer, and a 3025A ultrafine condensation particle counter (TSI Corp., Shoreview, MN). The parameters used in the GEMMA system were similar to those described previously (29). A capillary pressure drop of 1.4–2.0 p.s.i. was used to minimize a broad nucleotide peak in the beginning of the graphs and nonspecific binding of nucleotides to the generated protein particles (the nucleotide-induced molecular mass determination error was thereby minimized to a few percentages). When several experiments were plotted in the same graph, the baseline of each experiment was plotted 300–800 units apart for clarity.
Gel Filtration
To estimate the molecular mass of the NrdE and NrdF proteins alone or in combination at high protein concentrations (1.5–5 mg/ml NrdE and 0.75–5 mg/ml NrdF) and with high nucleotide concentration (0.2 mm dATP), we used a 2-ml column of Superdex-200 (GE Healthcare) chromatographed at 0.1 ml/min in buffer containing 50 mm Tris-HCl, pH 7.5, 8 mm DTT, 20 mm magnesium acetate, and 100 mm NaCl. Molecular mass markers (bovine serum albumin, 66 kDa; alcohol dehydrogenase, 150 kDa; β-amylase, 200 kDa; apoferritin, 443 kDa; thyroglobulin, 669 kDa, all purchased from Sigma) were run on the same column to obtain a standard curve for determination of molecular masses.
Surface Plasmon Resonance Analysis of the NrdF/NrdI Interaction
The detailed procedure has been published (30) but is briefly described here. The surface plasmon resonance (SPR) protein/protein interaction studies were performed using a Biacore 3000 biosensor instrument (GE Healthcare). A sterile-filtered and degassed mixture of 50 mm HEPES, pH 7.4, 0.15 m NaCl, 1 mm EDTA, 10 mm MgCl2, 2 mm DTT, and 0.005% (v/v) surfactant P20 was used as running buffer. B. anthracis NrdF was biotinylated with EZ-Link Sulfo-NHS-LC-LC-Biotin from Pierce Biotechnology Inc. and retained 88% of its enzymatic activity. The biotinylated NrdF protein (ligand) was subsequently immobilized to streptavidin-coated sensor chips (GE Healthcare). A biotin-deactivated flow cell was used as a reference cell in the experiments. NrdI protein solutions of different concentrations (analyte) were injected at a flow rate of 30 μl/min over the immobilized NrdF protein, and the binding was monitored. Bound NrdI protein was removed from the immobilized NrdF protein by injection of 15 μl of 0.5 m KCl followed by dissociation in running buffer. Data were analyzed using steady-state affinity calculations within the BIAevaluation 3.2 software (GE Healthcare).
RESULTS
Reconstitution of B. anthracis ApoNrdF with Manganese Requires Flavodoxin Protein NrdI
ApoNrdF (NrdF without bound metal ions) was reconstituted with either Mn2+ or Fe2+. Initially, we attempted to oxidize bound MnII with O2 or peroxide, but such experiments were not successful until the RNR-specific flavodoxin protein NrdI was included. After reduction of a Mn2II-NrdF/NrdI mixture with a slight excess of dithionite followed by O2 oxidation, a distinct band appeared at 408 nm in the visible spectrum, indicating that a tyrosyl radical had formed concomitant with oxidation of the reduced NrdIhq to NrdIox with an oxidized flavin absorption (Fig. 1, trace A, upper graph). The activated Mn-NrdF/Tyr• protein (Fig. 1, trace C, upper graph) could be separated from NrdIox (Fig. 1, trace B, upper graph) on a Mono-Q anion exchange column, and a radical content of 0.50 per Mn-NrdF dimer was calculated. Incubation of the apoNrdF protein with ferrous ions in the presence of O2 gave a reconstituted Fe-NrdF/Tyr• protein (Fig. 1, trace B, lower graph) with a radical content of 0.59 per Fe-NrdF dimer. The tyrosyl radical spectrum shown (Fig. 1, trace C, lower graph) was achieved by subtraction of a hydroxylamine-treated sample from the spectrum in trace B (Fig. 1, lower graph). Inclusion of reduced NrdIhq during iron reconstitution of apoNrdF had no effect on the radical yield (Fig. 1, trace A, lower graph).
Weak Interactions between NrdF and NrdI
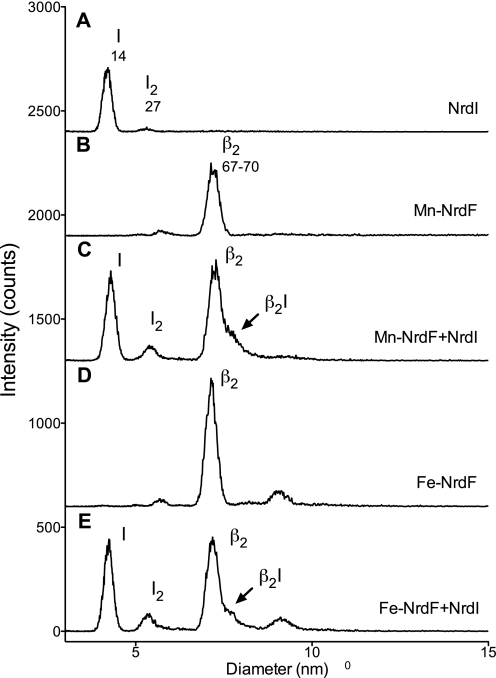
We used SPR with immobilized NrdF proteins to determine the affinity of NrdI for Mn-NrdF as compared with Fe-NrdF and apoNrdF. All NrdF/NrdI interactions were weak, but Mn-NrdF formed a slightly tighter interaction with NrdI as compared with the other NrdF forms (Table 1 and supplemental Fig. S5). The weak NrdF/NrdI interactions were also verified by GEMMA. NrdI was predominantly a monomer (Fig. 2, trace A), whereas both Mn-NrdF/Tyr• and Fe-NrdF/Tyr• were predominantly dimers (Fig. 2, traces B and D). The experimental (GEMMA and gel filtration) and theoretical molecular masses for the different forms are shown in Table 2. When NrdI was mixed with either Mn-NrdF or Fe-NrdF, a minor peak plausibly corresponding to a complex of a NrdF dimer and a NrdI monomer was formed (Fig. 2, traces C and E). However, most of the protein components in these mixtures were not engaged in an NrdF/NrdI complex, as expected from the weak interaction between NrdF and NrdI measured by SPR.
TABLE 1.
SPR measured interactions of B. anthracis NrdF forms with NrdI
| NrdF form | KD of NrdF-NrdI |
|---|---|
| μm | |
| Mn-NrdF | 22.6 ± 0.32 |
| Fe-NrdF | 29.6 ± 2.12 |
| Apo-NrdF | 28.7 ± 2.19 |
FIGURE 2.
Interactions between B. anthracis NrdF and NrdI analyzed by GEMMA. The NrdI concentration was 0.006 mg/ml in trace A and 0.012 mg/ml in traces C and E. The NrdF concentrations were 0.01 mg/ml in traces B–E. The composition and predicted sizes of the species (in kDa) are indicated.
TABLE 2.
B. anthracis NrdE, NrdI, Fe-NrdF, and Mn-NrdF interactions and quaternary states as measured by gel filtration and GEMMA
| Protein | Additionsa | Gel filtrationb (molecular mass) | GEMMAc (molecular mass) | Composition (theoretical molecular mass in kDa in parentheses) |
|---|---|---|---|---|
| kDa | kDa | |||
| NrdE | None | 82 ± 9 | 73 | α (80) |
| 196 ± 6 | α2 (160) | |||
| NrdE | dATP | NDd | 81 | α (80) |
| 173 | α2 (160) | |||
| Mn-NrdF | None | ND | 66–72 | β2 (74) |
| Fe-NrdF | None | 83 ± 6 | 66 | β2 (74) |
| NrdI | None | ND | 14 | I (13) |
| 27 | I2 (27) | |||
| Mn-NrdF + NrdI | None | ND | ND | β2I (87) |
| Fe-NrdF + NrdI | None | ND | ND | β2I (87) |
| NrdE + Mn-NrdF | dATP | ND | 70 | α/β2 (80/74) |
| 206 | α2β2 (234) | |||
| NrdE + Fe-NrdF | dATP | 80 ± 11 | 81 | α/β2 (80/74) |
| 276 ± 12 | 239 | α2β2 (234) |
a dATP concentration was 0.2 mm in gel filtration and 50 μm in GEMMA.
b Protein concentrations were 0.75, 1, 2.5, 5 mg/ml NrdF and 1.5, 2, 2.5, 5 mg/ml NrdE.
c Protein concentrations were 0.02 mg/ml (NrdE), 0.01 mg/ml (NrdF), and 0.006–0.012 mg/ml (NrdI).
d ND, not determined.
Mn-NrdF and Fe-NrdF Form α2β2 Complexes with NrdE
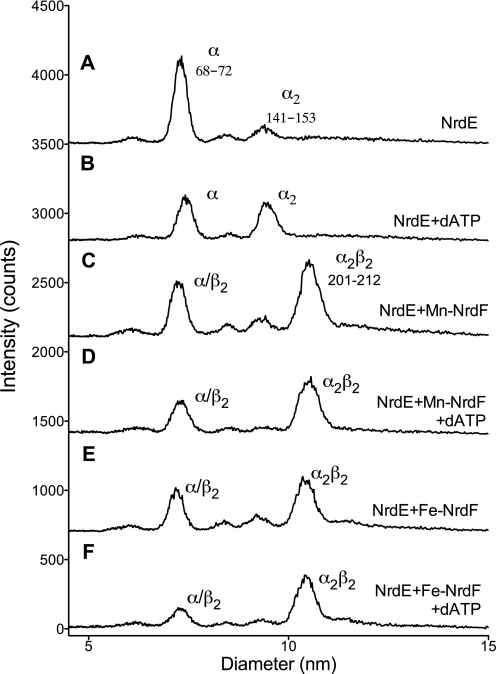
The interaction between NrdE and either of the NrdF forms was studied primarily by SPR and GEMMA. No significant difference was found in the interaction strength between the NrdE protein and the different NrdF forms in SPR (Table 3 and supplemental Fig. S6). The GEMMA analyses showed that NrdE is predominantly a monomer in the absence of effector nucleotide but that the dimer-to-monomer ratio increases in the presence of the positive allosteric effector dATP (Fig. 3). Dimerization of NrdE was also promoted by the allosteric effectors dGTP and dTTP, but not by 50 μm substrate (supplemental Fig. S7). When NrdE was mixed with NrdF, a fraction of the protein components formed a complex corresponding to an α2β2 composition (Fig. 3, traces C–F). Importantly, as indicated by the SPR data, both Mn-NrdF and Fe-NrdF behaved very similar in these mixtures.
TABLE 3.
SPR measured interactions of B. anthracis NrdF forms with NrdE
| NrdF form | KD NrdE-NrdF |
|---|---|
| μm | |
| Mn-NrdF | 0.178 ± 0.043 |
| Fe-NrdF | 0.195 ± 0.046 |
| Apo-NrdF | 0.189 ± 0.026 |
FIGURE 3.
GEMMA of B. anthracis Mn-NrdF and Fe-NrdF interactions with NrdE ± dATP. The NrdE concentration was 0.02 mg/ml, the NrdF concentration was 0.01 mg/ml, and the dATP concentration was 50 μm. The composition and predicted sizes of the species (in kDa) are indicated.
In gel filtration experiments where higher protein and dATP concentrations can be used, we confirmed the monomer/dimer equilibrium for NrdE, the dimer form of NrdF, and the formation of an α2β2 holoenzyme complex in the presence of dATP (Table 2). Densitometric analysis of an SDS-PAGE where the eluted fractions from the 276-kDa α2β2 peak had been separated showed equimolar amounts of NrdE and NrdF proteins (data not shown), and larger complexes were not observed either in gel filtration or in GEMMA. When Mn-NrdF was titrated into a mixture of NrdE + dTTP, α2β2 complex formation was nearly saturated at a molar NrdE-NrdF ratio of 2:1, indicating that some of the B. anthracis NrdE was unable to interact with NrdF (supplemental Fig. S8).
NrdH Mediates High Enzyme Activity of the Mn-NrdF Holoenzyme Complex
Interestingly, the specific activity of the Mn-NrdF form was markedly higher than that of the Fe-NrdF form in the conventional assay with DTT as a chemical reductant (Table 4). The relative enzyme activity per radical was in fact almost five times higher for Mn-NrdF as compared with Fe-NrdF. Even more intriguing is our finding that the physiological reductant NrdH-redoxin promotes an additional 2-fold higher activity for the Mn-NrdF form, but not for the Fe-NrdF form. This effect can be ascribed specifically to the NrdH protein because replacement of DTT as reductant for NrdH with the complete physiological reducing system NrdH, thioredoxin reductase, and NADPH behaved identically to the NrdH + DTT system. The Fe-NrdF protein had the same specific activity with all three reducing systems tested, whereas the Mn-NrdF protein is more active in the presence of NrdH as compared with DTT. Thus, the manganese form has a 10-fold higher activity as compared with the iron form when assayed with NrdH as reductant. Importantly, the presence of equimolar amounts of NrdI did not affect the specific activities (Table 4). To find out whether higher NrdI concentrations affected the specific activity, we titrated NrdI and only found a minor activity decrease at high NrdI excess (supplemental Fig. S9).
TABLE 4.
Enzyme activity of Mn-NrdF and Fe-NrdF from B. anthracis and B. cereus in presence or absence of NrdI and with different reducing systems
| NrdF/NrdI in assay | Reductant |
B. anthracis |
B. cereus (relative enzyme activity per radical (specific activity/Tyr•)b | |
|---|---|---|---|---|
| Specific enzyme activity | Relative enzyme activity per radical (specific activity/Tyr•)a | |||
| EU/mg | ||||
| Mn-NrdF + NrdI | NADPH + NrdH + TRc | 56 ± 5 | 1.00d | 1.00e |
| Mn-NrdF | NADPH + NrdH + TRc | 70 ± 9 | 0.95 | NDf |
| Fe-NrdF + NrdI | NADPH + NrdH + TRc | 7.2 ± 0.8 | 0.07 | 0.12 |
| Fe-NrdF | NADPH + NrdH + TRc | 7.2 ± 0.3 | 0.07 | 0.13 |
| Mn-NrdF + NrdI | DTT + NrdHg | 55 ± 11 | 0.99 | 0.94 |
| Mn-NrdF | DTT + NrdHg | 63 ± 4 | 0.84 | ND |
| Fe-NrdF + NrdI | DTT + NrdHg | 9.6 ± 0.1 | 0.09 | 0.13 |
| Fe-NrdF | DTT + NrdHg | 8.7 ± 0.1 | 0.08 | 0.13 |
| Mn-NrdF + NrdI | DTT | 27 ± 0.7 | 0.49 | 0.45 |
| Mn-NrdF | DTT | 40 ± 7 | 0.53 | ND |
| Fe-NrdF + NrdI | DTT | 8.9 ± 0.2 | 0.08 | ND |
| Fe-NrdF | DTT | 8.4 ± 0.7 | 0.08 | ND |
a Radical content was 0.30 Tyr•/Mn-NrdF dimer in presence of NrdI, 0.4 Tyr•/Mn-NrdF dimer in absence of NrdI and 0.57 Tyr•/Fe-NrdF dimer.
b Radical content was 0.33 Tyr•/Mn-NrdF dimer and 0.7 Tyr•/Fe-NrdF dimer.
c Assayed in the presence of Bacillus cereus NrdH and thioredoxin reductase (TR).
d Specific activity per radical 186 ± 16 EU/mg of Tyr•.
e Specific activity 112 ± 7 EU/mg, activity per radical 331 ± 19 EU/mg of Tyr•.
f ND, not determined.
g Assayed in the presence of Bacillus cereus NrdH.
The NrdH and thioredoxin reductase were derived from the closely related B. cereus species. When assayed in parallel, the Bacillus systems had very similar relative activities (Table 4). Furthermore the B. anthracis and B. cereus NrdE and NrdF proteins cross-reacted with preserved activities (data not shown), and the B. cereus NrdE batch had a higher activity as compared with the B. anthracis NrdE protein regardless of the NrdF protein origin.
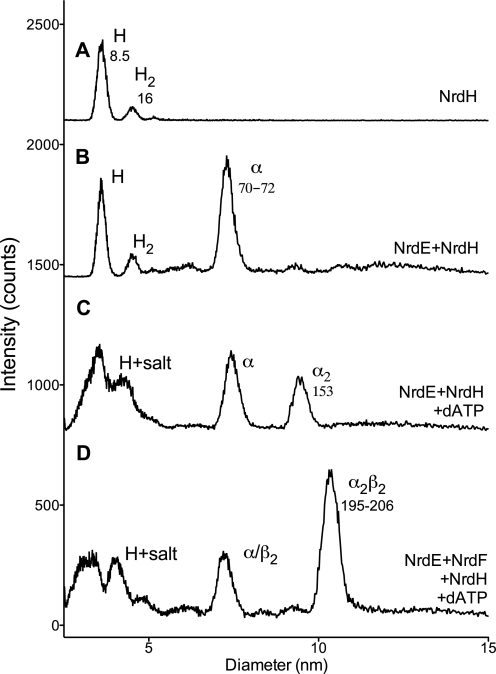
To establish whether NrdH interacts strongly with NrdE or the NrdE-Mn-NrdF holoenzyme complex, we again used GEMMA (Fig. 4 and Table 5). NrdH is predominantly a monomer in solution (Fig. 4, trace A). No strong or long lived interaction with NrdE could be detected in the absence or presence of the positive allosteric effector dATP (Fig. 4, traces B and C). Likewise, when NrdH is combined with both NrdE and Mn-NrdF, there is no additional peak present (Fig. 4, trace D), indicating that NrdH does not form a strong or long lived interaction with the NrdEF holoenzyme complex.
FIGURE 4.
GEMMA of NrdH interaction with NrdE ± dATP and NrdF. B. cereus NrdH concentration was 0.004 mg/ml in trace A and 0.008 mg/ml in traces B–D. B. anthracis NrdE was 0.04 mg/ml, B. anthracis Mn-NrdF was 0.02 mg/ml, and dATP was 50 μm. The composition and predicted sizes of the species (in kDa) are indicated.
TABLE 5.
Interactions and quaternary states of NrdH with NrdE and NrdF as measured by GEMMA
| Protein | Additionsa | GEMMA (molecular mass)b | Composition (theoretical molecular mass in kDa in parentheses) |
|---|---|---|---|
| kDa | |||
| NrdH | None | 8.5 | H (9) |
| 16 | H2 (18) | ||
| NrdE + NrdH | None | 8.5 | H (9) |
| 70–72 | α (80) | ||
| NrdE + NrdH | dATP | 70–74 | α (80) |
| 153 | α2 (160) | ||
| NrdE + NrdH + Mn-NrdF | dATP | 70–72 | α/β2 (80/74) |
| 195–206 | α2β2 (234) |
a Protein concentrations were: 0.004–0.008 mg/ml (B. cereus NrdH), 0.04 mg/ml (B. anthracis NrdE), and 0.02 mg/ml (B. anthracis NrdF).
b When included, dATP was used at a concentration of 50 μm.
DISCUSSION
Canonical class I RNRs are encoded in almost all eukaryotic genomes, in quite a number of bacteria, in a few archaea, and in several dsDNA viruses (2, 3). Class I RNRs form tetrameric or higher oligomeric complexes of two subunits: NrdA with the active site and binding sites for allosteric effectors and NrdB with a stable tyrosyl radial adjacent to a diiron-oxo center (1, 10). Two subclasses are known: class Ib, which has been shown recently to harbor the stable tyrosyl radical close to either a dimanganese-oxo center or a diiron-oxo center (15, 16, 31), and class Ic, which harbors the unpaired electron needed to initiate catalysis in a mixed valence MnIV-FeIII center and which has a phenylalanine residue at the site of the tyrosine residue in the canonical class I RNR (32, 33). This illustrates the diversity of ways that an essential unpaired electron can be safely stored in a metalloprotein matrix. The focus of this study is the class Ib RNR operon in B. anthracis, which is encoded by the genes for the catalytic NrdE protein and the radical-containing NrdF protein in an operon that also encodes the flavoprotein NrdI (7, 8, 14). We have earlier shown that the interaction of the NrdE/Fe-NrdF holoenzyme complex is one of the strongest among studied RNRs (30). The essentiality of the NrdI protein was first demonstrated in Streptococcus pyogenes (12), and recently, the E. coli NrdI was found essential for generation of the Mn2III/Tyr• cofactor in E. coli NrdF (15, 16, 34). In addition to the Ib operon, E. coli also encodes a canonical class I operon and is not dependent upon the class Ib operon during normal growth. Oxidative stress and severe iron limitation may promote the use of class Ib RNR, suggesting that class Ib RNR is essential for growth of E. coli under specific conditions (17, 35, 36). The situation is different in B. anthracis, the B. cereus group, and almost all Bacillus spp., which encode a class Ib RNR operon and a class III RNR operon (2). As class III RNRs are strictly anaerobic enzymes, B. anthracis and the other Bacillus spp. rely solely on their class Ib RNR for dNTP production during aerobic growth (8) In addition, the B. anthracis class III operon contains the nrdD gene followed by an nrdG pseudogene (7), and because the NrdG activase is required for generation of the essential radical in NrdD, the class III enzyme in B. anthracis may be non-functional. Recently, Zhang and Stubbe (37) isolated enzymatically active Mn-NrdF from Bacillus subtilis, but these studies did not take into account that all B. subtilis spp. encode two class Ib operons (2).
Little is known about the metal ion homeostasis in the B. cereus group, and it is appropriate to ask whether the B. anthracis Ib RNR is cambialistic, i.e. can work both as a manganese-dependent enzyme and as an iron-dependent enzyme. We have therefore purified apoNrdF from B. anthracis, reconstituted it with either manganese or iron, and compared the properties of Mn-NrdF and Fe-NrdF in regard to interaction with the NrdE component and specific enzyme activity in complex with NrdE. The interaction strength between the NrdE and NrdF subunits was not affected by the metal center, and similar dissociation constants and subunit compositions (α2β2) were recorded in the absence or presence of iron or manganese in the NrdF protein. As the class Ib RNR enzymes lack the dATP inhibition and higher oligomer form-mediating ATP cone domain, it was expected that we did not find higher oligomers than α2β2. However, a striking difference between the B. anthracis NrdF forms was that in the presence of the glutaredoxin-like NrdH protein, Mn-NrdF had up to 10-fold higher enzyme activity as compared with Fe-NrdF. We have also validated these results for B. cereus Ib RNR.
In many organisms, the glutaredoxin-like NrdH is encoded in the Ib RNR operon together with the genes for NrdI, NrdE, and NrdF, suggesting that NrdH is highly specific for reduction of class Ib RNR. This is the case for E. coli as well as Corynebacterium ammoniagenes and one of the two Ib operons in B. subtilis (38–40). However, in B. anthracis and the B. cereus group, the nrdH gene is encoded elsewhere on the chromosome, but a unique specificity for the Ib RNR is plausibly preserved. Our unexpected finding that Mn-NrdF is an order of magnitude more active than Fe-NrdF in vitro using conditions that mimic the in vivo situation suggests that Mn-NrdF is important in vivo. Whether the Fe-NrdF form only exists in vitro or whether the NrdF protein in B. anthracis is a true cambialistic enzyme remains to be established.
An advantage with the B. anthracis Ib RNR as compared with the E. coli Ib RNR is that the interaction between NrdF and NrdI is weak (KD = 23–30 μm), and it was therefore possible to separate NrdF with the Mn2III/Tyr• cofactor from NrdIox after reconstitution. In contrast to what has been reported for the E. coli class Ib RNR (16), we did not observe any negative effect of NrdI during reconstitution of B. anthracis Fe-NrdF or on the enzyme activity of iron- or manganese-activated NrdF. Interestingly, the affinity of NrdI to reconstituted Mn-NrdF was slightly higher as compared with apo- or Fe-NrdF. The recent cloning and expression of the C. ammoniagenes nrdF gene in its natural genetic background (31) suggests that the NrdF-to-NrdI interaction here is also weak as Mn-NrdF was isolated without NrdI. The unique characteristics of class Ib enzymes can be exploited in drug development programs targeting RNRs of microbial pathogens, e.g. from B. anthracis and the B. cereus group. B. anthracis is an extremely pathogenic bacterium with high proliferation rates during infection. After spore germination, it can easily reach 108 cfu/ml in 24 h (41). We have shown in previous work that compounds inhibiting class Ib RNR from B. anthracis have bactericidal effects (8, 42). For this reason, it is of particular interest to characterize its RNR components as future targets for antimicrobial therapies.
Supplementary Material
Acknowledgments
We are grateful to MariAnn Westman for technical assistance and Marta Hammerstad for purifying some of the B. cereus proteins used in this study. B. anthracis Sterne was a kind gift of Åke Forsberg and Ulla Eriksson, Swedish Defence Research Agency Umeå.
This work was supported by grants from the Swedish Cancer Foundation and the Swedish Research Council (to B.-M. S.), from the Fondo de Investigación Sanitaria from the Spanish Ministerio de Ciencia e Innovación (Grant PI081062), the ERA-NET PathoGenoMics, and the Ramón y Cajal program (to E. T.), from the EMBIO/MLS at the University of Oslo to (to Å. K. R.), from the Carl Trygger Foundation (to A. H.), and from the Research Council of Norway (Grants 177661/V30) and Norwegian Cancer Society (to K. K. A.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S9.
- RNR
- ribonucleotide reductase
- Fe-NrdF
- iron-containing NrdF
- Mn-NrdF
- manganese-containing NrdF
- GEMMA
- gas-phase electrophoretic mobility macromolecular analysis
- EU
- enzyme unit(s).
REFERENCES
- 1. Nordlund P., Reichard P. (2006) Annu. Rev. Biochem. 75, 681–706 [DOI] [PubMed] [Google Scholar]
- 2. Lundin D., Torrents E., Poole A. M., Sjöberg B. M. (2009) BMC Genomics 10, 589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torrents E., Sahlin M., Sjöberg B. M. (2008) in Ribonucleotide reductase (Andersson K. K. ed) pp. 17–77, Nova Science Publishers, Inc., Hauppauge, NY [Google Scholar]
- 4. Uhlin U., Eklund H. (1994) Nature 370, 533–539 [DOI] [PubMed] [Google Scholar]
- 5. Logan D. T., Andersson J., Sjöberg B. M., Nordlund P. (1999) Science 283, 1499–1504 [DOI] [PubMed] [Google Scholar]
- 6. Sintchak M. D., Arjara G., Kellogg B. A., Stubbe J., Drennan C. L. (2002) Nat. Struct. Biol. 9, 293–300 [DOI] [PubMed] [Google Scholar]
- 7. Read T. D., Peterson S. N., Tourasse N., Baillie L. W., Paulsen I. T., Nelson K. E., Tettelin H., Fouts D. E., Eisen J. A., Gill S. R., Holtzapple E. K., Okstad O. A., Helgason E., Rilstone J., Wu M., Kolonay J. F., Beanan M. J., Dodson R. J., Brinkac L. M., Gwinn M., DeBoy R. T., Madpu R., Daugherty S. C., Durkin A. S., Haft D. H., Nelson W. C., Peterson J. D., Pop M., Khouri H. M., Radune D., Benton J. L., Mahamoud Y., Jiang L., Hance I. R., Weidman J. F., Berry K. J., Plaut R. D., Wolf A. M., Watkins K. L., Nierman W. C., Hazen A., Cline R., Redmond C., Thwaite J. E., White O., Salzberg S. L., Thomason B., Friedlander A. M., Koehler T. M., Hanna P. C., Kolstø A. B., Fraser C. M. (2003) Nature 423, 81–86 [DOI] [PubMed] [Google Scholar]
- 8. Torrents E., Sahlin M., Biglino D., Gräslund A., Sjöberg B. M. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 17946–17951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rofougaran R., Crona M., Vodnala M., Sjöberg B. M., Hofer A. (2008) J. Biol. Chem. 283, 35310–35318 [DOI] [PubMed] [Google Scholar]
- 10. Sjöberg B. M. (2010) Science 329, 1475–1476 [DOI] [PubMed] [Google Scholar]
- 11. Cotruvo J. A., Stubbe J. (2011) Annu. Rev. Biochem. 80, 733–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roca I., Torrents E., Sahlin M., Gibert I., Sjöberg B. M. (2008) J. Bacteriol. 190, 4849–4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Røhr A. K., Hersleth H. P., Andersson K. K. (2010) Angew. Chem. Int. Ed. Engl. 49, 2324–2327 [DOI] [PubMed] [Google Scholar]
- 14. Johansson R., Torrents E., Lundin D., Sprenger J., Sahlin M., Sjöberg B. M., Logan D. T. (2010) FEBS J. 277, 4265–4277 [DOI] [PubMed] [Google Scholar]
- 15. Boal A. K., Cotruvo J. A., Jr., Stubbe J., Rosenzweig A. C. (2010) Science 329, 1526–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cotruvo J. A., Jr., Stubbe J. (2010) Biochemistry 49, 1297–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martin J. E., Imlay J. A. (2011) Mol. Microbiol. 80, 319–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory Press [Google Scholar]
- 19. Nord D., Torrents E., Sjöberg B. M. (2007) J. Bacteriol. 189, 5293–5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Røhr Å. K. (2010) Structural and Spectroscopic Studies of the Flavoprotein NrdI, Thioredoxin BC3987, and Ribonucleotide Reductase Diiron-Protein R2. Ph.D thesis, University of Oslo, Oslo, Norway [Google Scholar]
- 21. Tomter A. B., Bell C. B., 3rd, Røhr A. K., Andersson K. K., Solomon E. I. (2008) Biochemistry 47, 11300–11309 [DOI] [PubMed] [Google Scholar]
- 22. Tomter A. B. (2010) Spectroscopic Studies of the Ribonucleotide Reductase R2-Subunits from Mammals, Virus, and Bacteria. Ph.D thesis, University of Oslo, Oslo, Norway [Google Scholar]
- 23. Magnusson K. E., Edebo L. (1976) Biotechnol. Bioeng. 18, 865–883 [DOI] [PubMed] [Google Scholar]
- 24. Jordan A., Pontis E., Atta M., Krook M., Gibert I., Barbé J., Reichard P. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 12892–12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jordan A., Pontis E., Aslund F., Hellman U., Gibert I., Reichard P. (1996) J. Biol. Chem. 271, 8779–8785 [DOI] [PubMed] [Google Scholar]
- 26. Yang F., Curran S. C., Li L. S., Avarbock D., Graf J. D., Chua M. M., Lu G., Salem J., Rubin H. (1997) J. Bacteriol. 179, 6408–6415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fieschi F., Torrents E., Toulokhonova L., Jordan A., Hellman U., Barbe J., Gibert I., Karlsson M., Sjöberg B. M. (1998) J. Biol. Chem. 273, 4329–4337 [DOI] [PubMed] [Google Scholar]
- 28. Thelander L., Sjöberg B. M., Eriksson S. (1978) Methods Enzymol. 51, 227–237 [DOI] [PubMed] [Google Scholar]
- 29. Rofougaran R., Vodnala M., Hofer A. (2006) J. Biol. Chem. 281, 27705–27711 [DOI] [PubMed] [Google Scholar]
- 30. Crona M., Furrer E., Torrents E., Edgell D. R., Sjöberg B. M. (2010) Protein Eng. Des. Sel. 23, 633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cox N., Ogata H., Stolle P., Reijerse E., Auling G., Lubitz W. (2010) J. Am. Chem. Soc. 132, 11197–11213 [DOI] [PubMed] [Google Scholar]
- 32. Jiang W., Yun D., Saleh L., Barr E. W., Xing G., Hoffart L. M., Maslak M. A., Krebs C., Bollinger J. M., Jr. (2007) Science 316, 1188–1191 [DOI] [PubMed] [Google Scholar]
- 33. Voevodskaya N., Galander M., Högbom M., Stenmark P., McClarty G., Gräslund A., Lendzian F. (2007) Biochim. Biophys. Acta 1774, 1254–1263 [DOI] [PubMed] [Google Scholar]
- 34. Cotruvo J. A., Stubbe J. (2011) Biochemistry 50, 1672–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grass G., Franke S., Taudte N., Nies D. H., Kucharski L. M., Maguire M. E., Rensing C. (2005) J. Bacteriol. 187, 1604–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anjem A., Varghese S., Imlay J. A. (2009) Mol. Microbiol. 72, 844–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y., Stubbe J. (2011) Biochemistry 50, 5615–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jordan A., Aslund F., Pontis E., Reichard P., Holmgren A. (1997) J. Biol. Chem. 272, 18044–18050 [DOI] [PubMed] [Google Scholar]
- 39. Torrents E., Roca I., Gibert I. (2003) Microbiology 149, 1011–1020 [DOI] [PubMed] [Google Scholar]
- 40. Härtig E., Hartmann A., Schätzle M., Albertini A. M., Jahn D. (2006) Appl. Environ. Microbiol. 72, 5260–5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mock M., Fouet A. (2001) Annu. Rev. Microbiol. 55, 647–671 [DOI] [PubMed] [Google Scholar]
- 42. Torrents E., Sjöberg B. M. (2010) Biol. Chem. 391, 229–234 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.