Abstract
Termination of signaling of activated G protein-coupled receptors (GPCRs) is essential for maintenance of cellular homeostasis. It is well established that β-arrestin redistributes to phosphorylated GPCRs and thereby facilitates desensitization of classical G protein-dependent signaling. β-Arrestin in turn serves as a scaffold to initiate a second wave of signaling. Here, we report a molecular mechanism that regulates the termination of unconventional β-arrestin-dependent GPCR signaling. We identify protein phosphatase 1β (PP1β) as a phosphatase for the cluster of phosphorylated threonines (353TTETQRT359) within the sst2A somatostatin receptor carboxyl terminus that mediates β-arrestin binding using siRNA knock-down screening. We show that PP1β-mediated sst2A dephosphorylation is initiated directly after receptor activation at or near the plasma membrane. As a functional consequence of diminished PP1β activity, we find that somatostatin- and substance P-induced but not epidermal growth factor-induced ERK activation was aberrantly enhanced and prolonged. Thus, we demonstrate a novel mechanism for fine tuning unconventional β-arrestin-dependent GPCR signaling in that recruitment of PP1β to activated GPCRs facilitates GPCR dephosphorylation and, hence, leads to disruption of the β-arrestin-GPCR complex.
Keywords: Endocytosis, ERK, G Protein-coupled Receptor (GPCR), Protein Phosphatase, Trafficking, β-Arrestin, Dephosphorylation, Somatostatin, Somatostatin Receptor
Introduction
Desensitization of GPCR2 signaling causes a reduction of receptor response to repeated or long-lasting stimuli. It usually involves agonist-induced phosphorylation of cytoplasmic parts of the receptor by GRKs or second messenger-dependent protein kinases such as protein kinase A or protein kinase C (1). Agonist-induced phosphorylation allows binding of β-arrestin to the receptor (2). It is well established that β-arrestin promotes desensitization of G protein signaling and induces receptor internalization. More recent work has established that β-arrestin binding in turn stimulates a second wave of signaling (3, 4).
Although the regulation of agonist-induced phosphorylation has been studied in detail for many GPCRs, the molecular mechanisms and functional consequences of receptor dephosphorylation are far from understood. Earlier studies have identified retinal degeneration C as the phosphatase required for rhodopsin dephosphorylation in Drosophila melanogaster. The catalytic domain of retinal degeneration C exhibits high homology to PP1, PP2, and PP3 (5–7). Loss of retinal degeneration C causes disturbance of light-signal transduction and leads to light-dependent retinal degeneration (7). At the same time, a PP2-related phosphatase that dephosphorylates the β2-adrenergic receptor was identified and named GPCR phosphatase (8, 9). It was proposed that GPCR phosphatase is tethered to vesicular membranes and that receptors have to internalize into an acidic endosomal compartment to become dephosphorylated (8, 9). However, later it was shown that inhibition of β2-adrenergic receptor internalization with dominant negative dynamin or hypertonic sucrose did not affect the rate of receptor dephosphorylation. Similar, D1 dopamine receptor dephosphorylation was not blocked in the presence of concanavalin A, which also inhibits receptor internalization (10). More recent studies have shown that phosphatase inhibitors such as okadaic acid and calyculin A can block the dephosphorylation of a number of GPCRs including the β2-adrenergic receptor, D1 dopamine receptor, parathyroid hormone receptor 1, thromboxane A receptor, and the vasopressin receptor 1. However, a specific phosphatase has not been identified so far (10–14).
In the present study, we have examined the mechanism and function of receptor dephosphorylation using the sst2A somatostatin receptor as model. Earlier, we have defined the carboxyl-terminal 353TTETQRT359 motif as primary phosphorylation and β-arrestin-binding site of the sst2A receptor (15). Additionally, agonist-induced phosphorylation occurs on two nearby serine residues, Ser341 and Ser343 (16). We have then generated phosphosite-specific antibodies, which enabled us to study the spatial and temporal dynamics of agonist-induced sst2A phosphorylation in detail (17). Here, we have used chemical protein phosphatase inhibitors and siRNA knock-down screening to identify PP1β as the GPCR phosphatase that catalyzes rapid dephosphorylation of the residues Thr353, Thr354, Thr356, and Thr359. The dephosphorylation of these threonine residues occurs at or near the plasma membrane. Our findings suggest that PP1β-dependent GPCR dephosphorylation plays an essential role in the termination of β-arrestin-dependent signaling.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Somatostatin (SS-14) and was obtained from Bachem (Weil am Rhein, Germany). Calyculin A and okadaic acid were from Sigma. The phosphorylation-independent rabbit monoclonal anti-sst2A antibody {UMB-1}, which was generated against the following sequence, 355ETQRTLLNGDLQTSI369, was obtained from Epitomics (Burlingame, CA). The rabbit polyclonal phosphosite-specific sst2A antibodies anti-psst2A{0521} (sequence: 349RLNE(pT)(pT)E(pT)QR(pT)LLNG363), anti-Thr(P)353/Thr(P)354{0521} (sequence: 349RLNE(pT)(pT)ETQRTLLNG363), anti-Thr(P)356/Thr(P)359{0522} (sequence: 349RLNETTE(pT)QR(pT)LLNG363), and anti-Ser(P)341/Ser(P)343{3155} (sequence: 337DGER(pS)D(pS)KQDK347) were generated and extensively characterized previously (17–19). In addition, the following commercially available antibodies were used: anti-ERK1/2 (9102; Cell Signaling Technology, Boston, MA), anti-pERK (4370; Cell Signaling Technology), anti-pan-PP1 (E-9; Santa Cruz Biotechnology, Santa Cruz, CA), anti-PP1β (2029; Epitomics, Burlingame, CA), anti-PP2-pan (BD Bioscience, Franklin Lakes, NJ), anti-PP4 (C-18; Santa Cruz Biotechnology), and anti-PP5 (H-170; Santa Cruz Biotechnology).
Cell Culture and Transfection
Human embryonic kidney HEK293 cells were obtained from the German Resource Centre for Biological Material (DSMZ, Braunschweig, Germany). Cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. HEK293 cells were transfected with a plasmid encoding for a T7-tagged rat sst2A receptor or T7-tagged rat NK1 receptor using Lipofectamine 2000 according to the instructions of the manufacturer (Invitrogen). Stable transfectants were selected in the presence of 400 μg/ml G418. HEK293 cells stably expressing rat sst2A receptor were characterized using radioligand binding assays, cAMP assays, Western blot analysis, and immunocytochemistry as described previously (15, 20, 21). The level of sst2A receptor expression was ∼800 fmol/mg of membrane protein.
Immunocytochemistry
Cells were grown on poly-l-lysine-coated coverslips overnight. After the appropriate treatment with SS-14, cells were fixed with 4% paraformaldehyde and 0.2% picric acid in phosphate buffer, pH 6.9, for 40 min at room temperature and washed several times. Specimens were permeabilized and then incubated with anti-psst2A{0521} antibody followed by cyanine 3.18-conjugated secondary antibodies (Amersham Biosciences). Specimens were mounted and examined using a Zeiss LSM510 META laser scanning confocal microscope.
Western Blot Analysis
Cells were plated onto poly-l-lysine-coated 60-mm dishes and grown to 80% confluence. After the appropriate treatment with SS-14, cells were lysed in detergent buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 5 mm EDTA, 10 mm NaF, 10 mm disodium pyrophosphate, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) in the presence of protease and phosphatase inhibitors Complete mini and PhosSTOP (Roche Diagnostics). Glycosylated proteins were partially enriched using wheat germ-lectin-agarose beads as described (22–24). Proteins were eluted from the beads using SDS-sample buffer for 20 min at 60 °C and then resolved on 8% SDS-polyacrylamide gels. After electroblotting, membranes were incubated with 0.1 μg/ml anti-psst2A, anti-pS341/pS343, anti-pT353/pT354, or anti-pT356/pT359 followed by detection using an enhanced chemiluminescence detection system (Amersham Biosciences). Blots were subsequently stripped and reprobed with anti-sst2A antibody {UMB-1} to confirm equal loading of the gels.
Small Interfering RNA (siRNA) Silencing of Gene Expression
Chemically synthesized double-stranded siRNA duplexes (with 3′-dTdT overhangs) were purchased from Qiagen (Hilden, Germany) for the following human mRNA targets: PP1α (5′-AAGAGACGCTACAACATCAAA-3′), PP1β (5′-TACGAGGATGTCGTCCAGGAA-3′), PP1γ (5′-AACATCGACAGCATTATCCAA-3′), PP2α (5′-ACACCTCGTGAATACAATTTA-3′), PP2β (5′-CCGACAAATTACCCAAGTATA-3′), PP4 (5′-CGGACAATCGACCGAAAGCAA-3′), PP5 (5′-CTCGTGGAAACCACACTCAAA-3′), and a nonsilencing RNA duplex (5′-GCUUAGGAGCAUUAGUAAA-3′). HEK293 cells were transfected with HiPerFect (Qiagen) according to the instructions of the manufacturer for 3 days. Silencing was quantified by immunoblotting. All experiments showed protein levels reduced by ≥80%.
Data Analysis
ImageJ 1.39s software was used to desensitize and quantify protein bands detected on Western blots. Data were analyzed using GraphPad Prism 4.0 software. Statistical analysis was carried out with one-way ANOVA followed by the Bonferroni post test. p values of <0.05 were considered statistically significant.
RESULTS
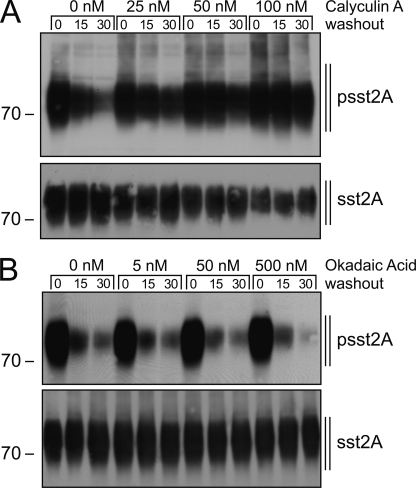
Calyculin A but Not Okadaic Acid Prevents Dephosphorylation of the 353TTETQRT359 Motif
Initial experiments showed that complete dephosphorylation of the carboxyl-terminal 353TTETQRT359 motif of the rat sst2A receptor occurred within 30 min after agonist removal. We then examined whether the phosphatase activity required for this rapid dephosphorylation was sensitive to the cell permeable phosphatase inhibitors calyculin A or okadaic acid. When HEK293 cells stably expressing the sst2A receptor were exposed to increasing concentrations of phosphatase inhibitors, sst2A dephosphorylation was inhibited in a dose-dependent manner only by calyculin A but not by okadaic acid (Fig. 1). Both calyculin A and okadaic acid can effectively block PP2, PP4, and PP5 activity. In contrast to okadaic acid, calyculin A is also a potent inhibitor of PP1 activity (25, 26). Thus, the present data suggest that PP1 dephosphorylates the 353TTETQRT359 motif of the sst2A receptor.
FIGURE 1.
Calyculin A but not okadaic acid prevents sst2A receptor dephosphorylation. HEK293 cells stably expressing rat sst2A were treated with calyculin A (A) or okadaic acid (B) for 10 min at the indicated concentrations and then exposed to 1 μm SS-14 for 5 min in the presence of calyculin A or okadaic acid. Cells were washed three times with ice-cold PBS and then were directly lysed or incubated for 15 or 30 min in culture medium (washout) at 37 °C in the presence of calyculin A or okadaic acid. The levels of phosphorylated sst2A receptors (upper panels) and total sst2A receptors (lower panels) were then determined by Western blot analysis. Note that calyculin A inhibited sst2A receptor dephosphorylation in a dose-dependent manner. Western blots shown are representative of three independent experiments for each condition. The positions of molecular mass markers are indicated on the left (kDa).
PP1β Catalyzes Rapid 353TTETQRT359 Dephosphorylation
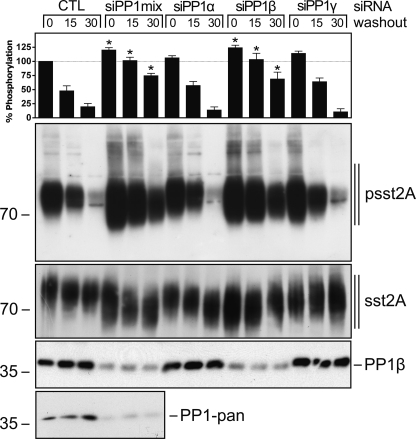
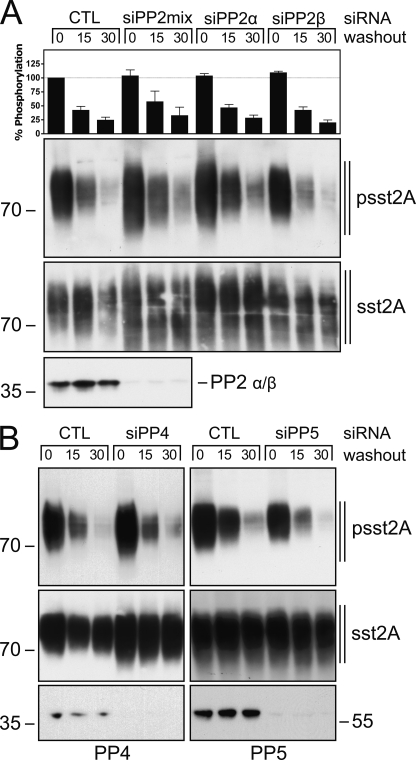
Next, we transfected sst2A-expressing HEK293 cells with specific siRNA sequences directed against the catalytic subunits α, β, and γ of PP1 and examined the time-course of 353TTETQRT359 dephosphorylation. Simultaneous knockdown of all three catalytic subunits confirmed that PP1 activity was required for efficient sst2A dephosphorylation (Fig. 2). Selective inhibition of PP1α or PP1γ expression had no effect on sst2A dephosphorylation (Fig. 2). In contrast, inhibition of PP1β expression resulted in an enhancement of 353TTETQRT359 phosphorylation in presence of agonist and a clearly delayed receptor dephosphorylation after agonist removal (Fig. 2). Given that PP2, PP4, and PP5 are also sensitive to calyculin A, we used a similar siRNA approach to evaluate their contribution to sst2A receptor dephosphorylation (Fig. 3). As depicted in Fig. 3A, siRNA knockdown of the catalytic subunits α or β of PP2 did not substantially inhibit 353TTETQRT359 dephosphorylation. Similar inhibition of PP4 or PP5 expression did not alter the time course of sst2A dephosphorylation (Fig. 3B). Thus, these findings identify PP1β as bona fide GPCR phosphatase for the β-arrestin acceptor site of the sst2A receptor. Our results also suggest that PP1β-mediated sst2A dephosphorylation is initiated shortly after receptor activation.
FIGURE 2.
PP1β catalyzes 353TTETQRT359 dephosphorylation. HEK293 cells stably expressing the rat sst2A receptor were transfected with the indicated siRNAs or a nonsilencing RNA (CTL) for 72 h and then exposed to 1 μm SS-14 for 5 min. Cells were washed three times with ice-cold PBS and then were directly lysed or incubated for 15 or 30 min in culture medium (washout) at 37 °C. The levels of phosphorylated sst2A receptors (upper panels) and total sst2A receptors (lower panels) were then determined by Western blot analysis. siRNA knockdown was confirmed using a PP1β and a PP1-pan antibody. The sst2A phosphorylation was quantified and is expressed as a percentage of the phosphorylation induced by SS-14 in CTL siRNA-transfected cells. Data correspond to the mean ± S.E. (error bars) from at least four independent experiments performed in duplicate. Data were analyzed by one-way ANOVA followed by the Bonferroni post test (*, p < 0.05). Note that PP1β knockdown resulted in enhanced receptor phosphorylation and delayed receptor dephosphorylation. The positions of molecular mass markers are indicated on the left (in kDa).
FIGURE 3.
Inhibition of PP2, PP4, or PP5 expression does not alter sst2A receptor dephosphorylation. HEK293 cells stably expressing the rat sst2A receptor were transfected with PP2 siRNAs (A), PP4 or PP5 siRNAs (B), or a nonsilencing RNA (CTL) for 72 h and then exposed to 1 μm SS-14 for 5 min. Cells were washed three times with ice-cold PBS and then were directly lysed or incubated for 15 or 30 min in culture medium (washout) at 37 °C. The levels of phosphorylated sst2A receptors (upper panels) and total sst2A receptors (lower panels) were then determined by Western blot analysis. siRNA knockdown was confirmed using pan-PP2, PP4, and PP5 antibodies. Western blots shown are representative of four independent experiments for each condition. The positions of molecular mass markers are indicated on the left (in kDa).
PP1β Catalyzes 353TTETQRT359 Dephosphorylation At or Near the Plasma Membrane
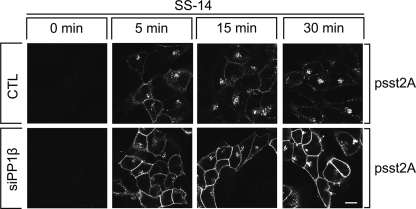
We then evaluated the effect of PP1β siRNA knockdown on the subcellular distribution of phosphorylated sst2A receptors in SS-14-treated cells. As depicted in Fig. 4, inhibition of PP1β expression facilitated detection of phosphorylated sst2A receptors at the plasma membrane already 5 min after agonist exposure. This enhanced ability to detect phosphorylated sst2A receptors at the plasma membrane persisted throughout the 30-min treatment period. These results strongly suggest that sst2A receptor dephosphorylation is initiated directly after receptor activation at or near the plasma membrane. Nevertheless, inhibition of PP1β expression did not change the rate of sst2A receptor internalization (data not shown).
FIGURE 4.
PP1β dephosphorylates sst2A receptors at the plasma membrane. HEK293 cells stably expressing the rat sst2A receptor were transfected with the nonsilencing RNA (CTL, upper panel) or PP1β siRNA (lower panel) for 72 h and then exposed to 1 μm SS-14 for 0, 5, 15, or 30 min. Cells were fixed, stained with anti-psst2A antibody, processed for immunofluorescence, and examined by confocal microscopy. Shown are representative images from one of three independent experiments performed in duplicate. Scale bar, 20 μm.
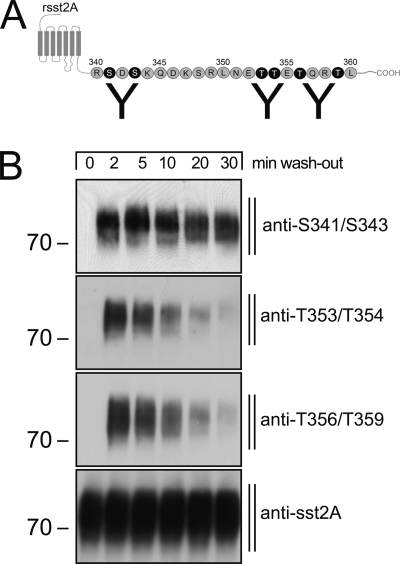
Distinct Temporal Dynamics of Ser341/Ser343 Dephosphorylation
Next, we evaluated dephosphorylation of the carboxyl-terminal serine residues, Ser341 and Ser343, in sst2A-expressing HEK293 cells. In addition, we used antibodies that selectively detect Thr(P)353/Thr(P)354 and Thr(P)356/Thr(P)359 (Fig. 5A). Again, after agonist removal, the threonine cluster is nearly completely dephosphorylated within 30 min (Fig. 5B). In contrast, phosphorylation of the serine residues Ser341/Ser343 is only slightly diminished 30 min after agonist washout (Fig. 5B).
FIGURE 5.
Distinct temporal dynamics of sst2A receptor dephosphorylation. A, schematic representation of rat indicates binding site of phosphospecific-antibodies anti-Thr(P)353/Thr(P)354, anti-Thr(P)356/Thr(P)359, and anti-Ser(P)341/Ser(P)343. B, HEK293 cells expressing the rat sst2A receptor were exposed to 1 μm SS-14 for 5 min. Cells were washed with ice-cold PBS and lysed. The same blot was sequentially stripped and immunoblotted with anti-Ser(P)341/Ser(P)343, anti-Thr(P)353/Thr(P)354, anti-Thr(P)356/Thr(P)359, and anti-sst2A antibodies. Note that Ser341/Ser343 dephosphorylation occurred at a slower rate than 353TTETQRT359 dephosphorylation. The positions of molecular mass markers are indicated on the left (in kDa).
Inhibition of 353TTETQRT359 Dephosphorylation Results in Aberrantly Enhanced and Prolonged ERK Activation
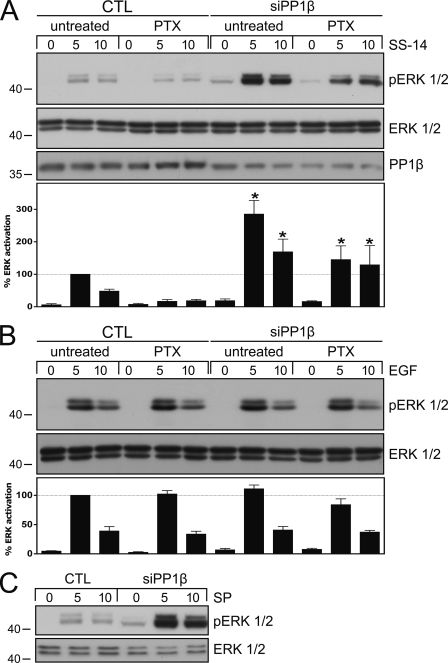
We have recently shown that phosphorylation of the 353TTETQRT359 motif is essential for β-arrestin recruitment to the sst2A receptor (17). We have also shown that sst2A receptor stimulation leads to both Gi protein-dependent and β-arrestin-dependent ERK activation (17, 27). We therefore examined ERK activation under conditions when sst2A dephosphorylation was abrogated by siRNA knockdown of PP1β. As depicted in Fig. 6A, inhibition of PP1β expression resulted in a robust increase in SS-14-stimulated ERK activation. This increase was selective as inhibition of PP1α or PP1γ expression had no effect on SS-14-mediated ERK activation (data not shown). Interestingly, the increase in SS-14-stimulated ERK activation was only partly blocked in the presence of pertussis toxin, suggesting that PP1β siRNA knockdown strongly facilitated β-arrestin-dependent ERK activation (Fig. 6A). To exclude the possibility that inhibition of PP1β expression could directly alter the ERK pathway, we stimulated our cells with epidermal growth factor (EGF) under otherwise identical conditions. As depicted in Fig. 6B, neither PP1β siRNA transfection nor pertussis toxin treatment promoted any change in the EGF-mediated ERK activation. The NK1 neurokinin receptor is similar to the sst2A somatostatin receptor in that it forms stable complexes with β-arrestin and activates ERK in a β-arrestin-dependent manner (28, 29). We therefore transfected HEK293 cells stably expressing the NK1 receptor with PP1β siRNA and examined ERK activation. Similar to that observed with the sst2A receptor, ERK activation was aberrantly enhanced and prolonged after addition of substance P (Fig. 6C), suggesting that PP1β activity was also required for termination of NK1-mediated ERK signaling.
FIGURE 6.
Inhibition of sst2A dephosphorylation results in enhanced and prolonged ERK activation. A, HEK293 cells stably expressing the rat sst2A receptor were transfected with PP1β siRNA or a nonsilencing RNA (CTL) for 72 h. Where indicated, cells were incubated with 100 ng/ml pertussis toxin (PTX) 16 h before agonist exposure. Cells were then exposed to 1 μm SS-14 for 0, 5, or 10 min. The levels of phosphorylated ERK1/2 and total ERK1/2 were then determined by Western blot analysis. siRNA knockdown was confirmed using a PP1β antibody. Bands were quantified and expressed as percent ERK activation induced by SS-14 exposure for 5 min in CTL-transfected cells (dotted line). Data correspond to the mean ± S.E. (error bars) from four independent experiments performed in duplicate. Results were analyzed by one-way ANOVA followed by the Bonferroni post-test (*, p < 0.05). B, HEK293 cells stably expressing the rat sst2A receptor were transfected with PP1β siRNA or a nonsilencing RNA (CTL) for 72 h. Where indicated, cells were incubated with 100 ng/ml pertussis toxin (PTX) 16 h before agonist exposure. Cells were then exposed to 1 ng/ml EGF for 0, 5, or 10 min. The levels of phosphorylated ERK1/2 and total ERK1/2 were then determined by Western blot analysis. Bands were quantified and expressed as percent ERK activation induced by EGF exposure for 5 min in CTL-transfected cells (dotted line). Data correspond to the mean ± S.E. from three independent experiments performed in duplicate. C, HEK293 cells stably expressing the rat NK1receptor were transfected with PP1β siRNA or a nonsilencing RNA (CTL) for 72 h. Cells were then exposed to 1 μm substance P (SP) for 0, 5, or 10 min. The levels of phosphorylated ERK1/2 and total ERK1/2 were then determined by Western blot analysis. The experiment was repeated three times. Note that inhibition of PP1β expression resulted in an aberrantly enhanced and prolonged ERK activation in SS-14- and substance P- but not in EGF-treated cells. The positions of molecular mass markers are indicated on the left (in kDa).
DISCUSSION
Desensitization of GPCR signaling is essential for maintenance of cellular homeostasis. For many GPCRs, agonist-dependent regulation involves rapid phosphorylation of a series of phosphate acceptor sites within the carboxyl-terminal tail of the receptor. This phosphorylation facilitates binding of β-arrestin, which in turn mediates desensitization of G protein-dependent signaling. In addition, β-arrestin serves as scaffold to facilitate receptor internalization and to initiate a second wave of signaling (30, 31). However, until now the mechanisms involved in the negative feedback regulation of unconventional β-arrestin-dependent signaling remained elusive. Given the fact that β-arrestin-dependent signaling is initiated by its binding to phosphorylated GPCRs, we hypothesized that receptor dephosphorylation by specific GPCR phosphatases would lead to disruption of the GPCR-β-arrestin complex and, hence, facilitate termination of β-arrestin-dependent signaling.
In the present study, we used a combination of phosphosite-specific antibodies and siRNA screening to identify PP1β as bona fide GPCR phosphatase for the sst2A somatostatin receptor. Inhibition of PP1β expression resulted in increased agonist-driven receptor phosphorylation at the 353TTETQRT359 motif and facilitated detection of phosphorylated receptors at the plasma membrane shortly after agonist exposure, indicating that PP1β catalyzes dephosphorylation directly after receptor activation at or near the plasma membrane. Remarkably, dephosphorylation of Ser341/Ser343 occurred at a much slower rate than dephosphorylation of the four threonine residues. It has been reported that sst2A internalization is a prerequisite for Ser341/Ser343 dephosphorylation, but not for Thr353/Thr354 or Thr356/Thr359 dephosphorylation (16). These findings strongly suggest that Ser341/Ser343 dephosphorylation occurs via a mechanism distinct from rapid PP1β-dependent 353TTETQRT359 dephosphorylation. Hence, GPCR dephosphorylation occurs in distinct temporal and spatial dynamics for different phosphorylation sites.
PP1 is composed of a catalytic subunit bound to one or more regulatory subunits. At present, >40 different regulatory PP1 subunits are known, which determine substrate specificity and subcellular localization of PP1s (32). It remains open to question whether a single or multiple regulatory PP1 subunits are required for efficient GPCR dephosphorylation and whether PP1β is recruited directly to phosphorylated GPCRs or via additional adapter proteins.
In previous work, we have clearly demonstrated that GPCR kinase 2/3-driven phosphorylation of the 353TTETQRT359 motif is essential for β-arrestin binding. We have also shown that sst2A receptor stimulation leads to both Gi protein-dependent and β-arrestin-dependent ERK activation (17, 27). In the present work, we found that inhibition of PP1β expression resulted in a robust increase in β-arrestin-dependent ERK activation in SS-14-treated cells. This effect was selective. It was not seen after exposure to EGF or after inhibition of PP1α or PP1γ expression under otherwise identical conditions, suggesting that diminished PP1 activity does not directly lead to a general enhancement of ERK excitability. Nevertheless, this effect was not unique. Similar to that seen with the sst2A receptor, ERK activation was also robustly enhanced and prolonged after exposure of NK1-expressing cells to substance P suggesting that not only the sst2A receptor but also other GPCRs require functional PP1β for termination of their β-arrestin-dependent ERK signaling.
In conclusion, we identify PP1β as the first GPCR phosphatase for the sst2A receptor. Our findings indicate that PP1β-mediated 353TTETQRT359 dephosphorylation is initiated directly after receptor activation at or near the plasma membrane. In contrast, Ser341/Ser343 dephosphorylation occurs at a much slower rate by a distinct yet unknown mechanism. We also discovered a novel mechanism for fine tuning unconventional β-arrestin-dependent GPCR signaling in that engagement of PP1β facilitates GPCR dephosphorylation. Dephosphorylation in turn leads to disruption of the β-arrestin-GPCR complex and thereby limits β-arrestin-dependent ERK signaling. We propose that this could be a common mechanism for many GPCRs.
Acknowledgments
We thank Heidrun Guder and Heike Stadler for excellent technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft Grant SCHU924/10-3 and Deutsche Krebshilfe Grant 108036.
- GPCR
- G protein-coupled receptor
- PP
- protein phosphatase
- SS-14
- somatostatin
- sst
- somatostatin receptor.
REFERENCES
- 1. Marchese A., Paing M. M., Temple B. R., Trejo J. (2008) Annu. Rev. Pharmacol. Toxicol. 48, 601–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krupnick J. G., Benovic J. L. (1998) Annu. Rev. Pharmacol. Toxicol. 38, 289–319 [DOI] [PubMed] [Google Scholar]
- 3. Lefkowitz R. J., Shenoy S. K. (2005) Science 308, 512–517 [DOI] [PubMed] [Google Scholar]
- 4. Defea K. A. (2011) Cell. Signal. 23, 621–629 [DOI] [PubMed] [Google Scholar]
- 5. Steele F. R., Washburn T., Rieger R., O'Tousa J. E. (1992) Cell 69, 669–676 [DOI] [PubMed] [Google Scholar]
- 6. Byk T., Bar-Yaacov M., Doza Y. N., Minke B., Selinger Z. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 1907–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vinós J., Jalink K., Hardy R. W., Britt S. G., Zuker C. S. (1997) Science 277, 687–690 [DOI] [PubMed] [Google Scholar]
- 8. Pitcher J. A., Payne E. S., Csortos C., DePaoli-Roach A. A., Lefkowitz R. J. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 8343–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krueger K. M., Daaka Y., Pitcher J. A., Lefkowitz R. J. (1997) J. Biol. Chem. 272, 5–8 [DOI] [PubMed] [Google Scholar]
- 10. Gardner B., Liu Z. F., Jiang D., Sibley D. R. (2001) Mol. Pharmacol. 59, 310–321 [DOI] [PubMed] [Google Scholar]
- 11. Chauvin S., Bencsik M., Bambino T., Nissenson R. A. (2002) Mol. Endocrinol. 16, 2720–2732 [DOI] [PubMed] [Google Scholar]
- 12. Innamorati G., Sadeghi H., Birnbaumer M. (1998) J. Biol. Chem. 273, 7155–7161 [DOI] [PubMed] [Google Scholar]
- 13. Spurney R. F. (2001) J. Pharmacol. Exp. Ther. 296, 592–599 [PubMed] [Google Scholar]
- 14. Tran T. M., Friedman J., Baameur F., Knoll B. J., Moore R. H., Clark R. B. (2007) Mol. Pharmacol. 71, 47–60 [DOI] [PubMed] [Google Scholar]
- 15. Tulipano G., Stumm R., Pfeiffer M., Kreienkamp H. J., Höllt V., Schulz S. (2004) J. Biol. Chem. 279, 21374–21382 [DOI] [PubMed] [Google Scholar]
- 16. Ghosh M., Schonbrunn A. (2011) J. Biol. Chem. 286, 13561–13573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pöll F., Lehmann D., Illing S., Ginj M., Jacobs S., Lupp A., Stumm R., Schulz S. (2010) Mol. Endocrinol. 24, 436–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fischer T., Doll C., Jacobs S., Kolodziej A., Stumm R., Schulz S. (2008) J. Clin. Endocrinol. Metab. 93, 4519–4524 [DOI] [PubMed] [Google Scholar]
- 19. Nagel F., Doll C., Pöll F., Kliewer A., Schröder H., Schulz S. (2011) Mol. Endocrinol. 25, 859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pfeiffer M., Koch T., Schröder H., Laugsch M., Höllt V., Schulz S. (2002) J. Biol. Chem. 277, 19762–19772 [DOI] [PubMed] [Google Scholar]
- 21. Pfeiffer M., Koch T., Schröder H., Klutzny M., Kirscht S., Kreienkamp H. J., Höllt V., Schulz S. (2001) J. Biol. Chem. 276, 14027–14036 [DOI] [PubMed] [Google Scholar]
- 22. Mundschenk J., Unger N., Schulz S., Höllt V., Schulz S., Steinke R., Lehnert H. (2003) J. Clin. Endocrinol. Metab. 88, 5150–5157 [DOI] [PubMed] [Google Scholar]
- 23. Plöckinger U., Albrecht S., Mawrin C., Saeger W., Buchfelder M., Petersenn S., Schulz S. (2008) J. Clin. Endocrinol. Metab. 93, 1203–1210 [DOI] [PubMed] [Google Scholar]
- 24. Schulz S., Pauli S. U., Schulz S., Händel M., Dietzmann K., Firsching R., Höllt V. (2000) Clin. Cancer Res. 6, 1865–1874 [PubMed] [Google Scholar]
- 25. Honkanen R. E., Golden T. (2002) Curr. Med. Chem. 9, 2055–2075 [DOI] [PubMed] [Google Scholar]
- 26. Cohen P. T., Philp A., Vázquez-Martin C. (2005) FEBS letters 579, 3278–3286 [DOI] [PubMed] [Google Scholar]
- 27. Lesche S., Lehmann D., Nagel F., Schmid H. A., Schulz S. (2009) J. Clin. Endocrinol. Metab. 94, 654–661 [DOI] [PubMed] [Google Scholar]
- 28. Oakley R. H., Laporte S. A., Holt J. A., Barak L. S., Caron M. G. (2001) J. Biol. Chem. 276, 19452–19460 [DOI] [PubMed] [Google Scholar]
- 29. Oakley R. H., Laporte S. A., Holt J. A., Caron M. G., Barak L. S. (2000) J. Biol. Chem. 275, 17201–17210 [DOI] [PubMed] [Google Scholar]
- 30. DeWire S. M., Ahn S., Lefkowitz R. J., Shenoy S. K. (2007) Annu. Rev. Physiol. 69, 483–510 [DOI] [PubMed] [Google Scholar]
- 31. Luttrell L. M., Gesty-Palmer D. (2010) Pharmacol. Rev. 62, 305–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Virshup D. M., Shenolikar S. (2009) Mol. Cell 33, 537–545 [DOI] [PubMed] [Google Scholar]