Abstract
Decreased expression of prosurvival and progrowth-stimulatory pathways, in addition to an environment that inhibits neuronal growth, contribute to the limited regenerative capacity in the central nervous system following injury or neurodegeneration. Membrane/lipid rafts, plasmalemmal microdomains enriched in cholesterol, sphingolipids, and the protein caveolin (Cav) are essential for synaptic development/stabilization and neuronal signaling. Cav-1 concentrates glutamate and neurotrophin receptors and prosurvival kinases and regulates cAMP formation. Here, we show that primary neurons that express a synapsin-driven Cav-1 vector (SynCav1) have increased raft formation, neurotransmitter and neurotrophin receptor expression, NMDA- and BDNF-mediated prosurvival kinase activation, agonist-stimulated cAMP formation, and dendritic growth. Moreover, expression of SynCav1 in Cav-1 KO neurons restores NMDA- and BDNF-mediated signaling and enhances dendritic growth. The enhanced dendritic growth occurred even in the presence of inhibitory cytokines (TNFα, IL-1β) and myelin-associated glycoproteins (MAG, Nogo). Targeting of Cav-1 to neurons thus enhances prosurvival and progrowth signaling and may be a novel means to repair the injured and neurodegenerative brain.
Keywords: Caveolin, Membrane/Lipid Rafts, Neuronal Signaling, Dendritic Arborization
Keywords: Gene Therapy, Lipid Raft, Neurobiology, Neurotransmitter Receptors, Neurotrophins, Caveolin, Dendritic Arborization, Lipid Rafts, Neuronal Signaling
Introduction
Multiple signaling pathways have been identified that promote growth and survival of neurons and thereby facilitate the formation of synaptic connections that are essential for learning, memory, and the development of the CNS (1–3). Neurotransmitter and neurotrophic receptors, non-receptor tyrosine kinases, and other signaling mediators aggregate to mold and shape postsynaptic densities to permit high-fidelity signal transduction and the regulation of neuronal function (4–6). A major non-protein component of synapses is cholesterol, which can be a limiting factor in synapse development, synaptic activity, and transmitter release (7).
Increasing evidence shows that membrane/lipid rafts, discrete regions of the plasma membrane enriched in cholesterol, glycosphingolipids, and sphingomyelin organize prosurvival and progrowth neuronal signaling pathways (8–10), regulate cAMP formation (11), and are essential for synapse development, stabilization, and maintenance (7, 12). Caveolin (Cav),2 a cholesterol binding protein and scaffolding protein found within membrane/lipid rafts (13), organizes and targets certain neuronal growth-promoting proteins, such as components of the neurotransmitter and neurotrophic receptor signaling pathways, to membrane/lipid rafts. These include NMDA receptors, AMPA receptors, Trk receptors, GPC receptors, and Src family kinases (9, 14–16). These receptors and signaling molecules can enhance cAMP formation, an essential second messenger for promoting neuronal growth and dendritic arborization (17–21), and are found within membrane/lipid rafts in growth cones (6). In the setting of traumatic brain injury and neurodegenerative disorders, interventions that activate signaling pathways to stimulate cAMP production thus have the potential to improve functional recovery (19, 22).
A major problem following brain injury (e.g. stroke or trauma) and neurodegeneration is limited functional recovery as a consequence of a reduction in signaling that promotes neuronal growth and survival (22–25). This loss of “protective signaling” increases neuronal loss, impairs brain repair, and increases functional deficits. Therapeutic interventions, such as addition of growth factors or approaches to increase cAMP, are relatively ineffective because of the loss of key receptors and their downstream signaling molecules. Therefore, interventions that restore progrowth and prosurvival signaling within neurons have the potential not only to reduce neuronal loss and enhance endogenous brain repair but also to increase the efficacy of pharmacologic agents designed to improve functional outcome (26).
In this report, we show that overexpression of neuron-targeted Cav-1 in primary neurons enhances expression of membrane/lipid rafts and neurotransmitter and neurotrophin receptors and increases progrowth signaling, cAMP production, and dendritic growth and arborization. Conversely, siRNA-mediated loss of Cav-1 decreases membrane/lipid rafts and expression of neurotransmitter and neurotrophin receptors, blunts NMDA- and BDNF-mediated signaling, and attenuates agonist-stimulated cAMP production. Reexpression of Cav-1 in Cav-1 KO primary neurons restores prosurvival signaling and promotes neuronal growth and arborization, even in the presence of inhibitory cytokines and myelin-associated glycoproteins. These growth-promoting effects of neuron-targeted Cav-1 expression suggest that it might be useful as a therapeutic intervention to limit neurodegeneration and to enhance repair of the injured CNS.
EXPERIMENTAL PROCEDURES
All studies performed on animals were approved by the Veterans Affairs San Diego Institutional Animal Care and Use Committee and conform to the relevant National Institutes of Health guidelines.
Chemicals and Antibodies
Antibodies used for immunoblotting, immunoprecipitation, and immunofluorescence were as follows: caveolin-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; Cell Signaling Technology, Inc., San Diego, CA; and Abcam, Inc., Cambridge, MA), postsynaptic density 95 (PSD95, Abcam, Inc. and Affinity Bioreagents, Rockford, IL), N-methyl-D-aspartate receptors (NMDAR2A and NMDAR2B, Abcam, Inc.), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA receptor, Abcam, Inc.), neurotrophic tyrosine kinase receptor type 2 (TrkBR, Cell Signaling Technology, Inc.), b3-tubulin (Abcam, Inc.) and microtubule-associated protein 2 (MAP2, Abcam, Inc.), phospho-ERK1/2 (Neuromics, Edina, MN) and total ERK1/2 (Stressgen), phospho and total Src (Cell Signaling Technology, Inc.), Ca2+/calmodulin-dependent protein kinases II (P-CaMKII, Cell Signaling Technology, Inc.), GAPDH (Imgenex, San Diego, CA), and cholera toxin B (CT-B, Molecular Probes, Inc./Invitrogen). Primary antibodies were visualized using secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Inc.) and ECL reagent (Amersham Biosciences). All displayed bands were compared with molecular weight standards (Santa Cruz Biotechnology, Inc.). The amount of protein per sample was determined using a dye-binding protein assay (Bio-Rad). For immunofluorescence, FITC and Texas Red secondary antibodies were obtained from Molecular Probes, Inc. All agonists were purchased commercially: BDNF (50 ng/ml, Invitrogen), NMDA (10 mm, Calbiochem), dopamine 1 receptor agonist (10 mm, SKF 81297 hydrobromide, Tocris), 5-hydroxytryptophan receptor 6 (10 mm, EMD 386088 hydrobromide, Tocris), forskolin (10 mm, F6886, Sigma-Aldrich), methyl-β-cyclodextrin (MβCD, 3 mm, C4555, Sigma-Aldrich), and cholesterol (50 mg/ml, C4951, Sigma-Aldrich). The phosphodiesterase 4 (PDE4) inhibitor rolipram was purchased from Sigma-Aldrich (10 mm, R6520).
Isolation and Culture of Primary Neurons
Primary neurons were isolated from the brains (cortex and hippocampus) of postnatal days 1–3 C57BL/6J wild-type and Cav-1−/− mice using the papain dissociation system from Worthington as described previously (9, 27). Neurons were cultured in Neurobasal A media supplemented with B27 (2%), 250 mm GLUTMax1, and penicillin/streptomycin (1%) and grown on poly-D-lysine/laminin (2 mg/cm2)-coated plates at 37 °C in 5% CO2 for days as indicated. Anti-mitotic agents (cytosine arabinoside or fluorodeoxyuridine) was added for the first 3 days following isolation to prevent non-neuronal cells from proliferating. Assessment with neuronal markers revealed that > 90% of the cells were neurons. After 3 days, the antimitotic agents were removed to reduce neuronal toxicity associated with these agents. Because neuronal cell death occurs during culture, we added basic fibroblast growth factor or platelet-derived growth factor (PDGF for mice) to maintain neuronal viability and plasticity.
Adenovirus Expressing Short Hairpin Small Interference RNA for Caveolin 1 (AdvshRNACav1)
The expression of caveolin-1 in primary neurons was suppressed by using an adenoviral vector encoding a short hairpin loop for small interfering RNA to Cav-1. The sequences of caveolin 1 siRNA that are generated intracellularly are as follows: Sense, GGAAAUUGAUCUGGUCAACtt and antisense, GUUGACCAGAUCAAUUUCCtt. Cells were treated with varying doses of the vector (6 × 107 pfu/ml) for 72 h. Functional knockdown of protein expression was assessed by immunoblotting and immunofluorescence microscopy.
cAMP RIA
We assayed cAMP accumulation by incubating cells for 30 min with the PDE4 inhibitor rolipram (10 mm) followed by GPCR agonists for 15 min. To terminate the reaction, assay medium was aspirated, and 250 ml of ice-cold TCA 7.5%, w/v) was added. cAMP content in TCA extracts was determined by radioimmunoassay (28, 29). Production of cAMP was normalized to the amount of protein (determined using a dye-binding protein assay (Bio-Rad)) per sample.
Biochemical Characterization of Membrane/Lipid Rafts
Sucrose density fractionation was performed on primary neurons as described previously (9). Neurons were lysed in 500 mm Na2CO3 (pH 11.0) to extract peripheral membrane proteins. Cells were homogenized using three 10-s bursts of a tissue grinder and then sonicated with three cycles of 20-s bursts of sonication and a 1-min incubation on ice. Approximately 1 ml of homogenate (2 mg for both SynGFP and SynCav1 samples) were mixed with 1 ml of 90% sucrose in 25 mm MES buffered solution, 150 mm NaCl (MBS (pH 6.5)) to form 45% sucrose and loaded at the bottom of an ultracentrifuge tube. A discontinuous sucrose gradient was generated by layering 6 ml of 35% sucrose prepared in MBS (250 mm Na2CO3) followed by 4 ml of 5% sucrose (in MBS/Na2CO3). Gradients were centrifuged at 280,000 × g using a SW41Ti rotor (Beckman) for 4 h at 4 °C. Samples were removed in 1-ml aliquots to form 12 fractions. Because membrane/lipid rafts are concentrated at the 5/35% interface (fractions 4 and 5), only fractions 4–12 were prepared for cholesterol analysis and immunoblotting. Quantification of cholesterol was performed using the Amplex® Red cholesterol assay (Invitrogen, catalog no. A12216). Approximately 50 ml of normalized samples, in triplicate, were placed in 96-well tissue culture clear flat-bottom plates (Corning, catalog no. 3997), where 50 ml of working solution containing Amplex Red was added as per directions. Plates were incubated at 37 °C and were protected from light. Plates were then placed into an Infinite m200 PRO (Tecan) plate reader and were read at 571 nm and 585 nm for absorption and fluorescence emission, respectively. Data were placed on a standard curve using known amounts of cholesterol controls for analysis.
Immunoblot Analysis
Samples were separated by SDS-polyacrylamide gel electrophoresis using 10% or 4–12% acrylamide gels (Invitrogen) and transferred to polyvinylidene difluoride membranes (Millipore) by electroelution. Membranes were blocked in 20 mm PBS Tween (1%) containing 3% BSA and incubated with primary antibodies overnight at 4 °C. Primary antibodies were visualized using secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Inc.) and ECL reagent (Amersham Biosciences). Bands were compared with molecular weight standards (Santa Cruz Biotechnology, Inc.). The amount of protein per fraction were determined using a dye-binding protein assay (Bio-Rad).
Generation of the SynCav1 Construct
To link the neuron-specific synapsin (Syn) promoter with the Cav-1 cDNA, an XbaI-SalI DNA fragment containing the Syn promoter was inserted into the NheI-SalI sites of pEGFP-N1 (Clontech). The resulting plasmid was designated pSyn-EGFP. A 685-bp Cav-1 cDNA was isolated from the pCRII-TOPO vector (Invitrogen) by digestion with PmeI-NotI and inserted into the SmaI-NotI site of the pSyn-EGFP to generate pSyn-Cav-1, in which the EGFP gene was replaced with Cav-1 cDNA. The Syn-promoter-Cav-1 cassette was isolated from pSyn-Cav-1 and inserted into the BamHI site of the HIV1 vector backbone plasmid pHIV7 (30). The resulting plasmid was designated pHIV1-Syn-Cav-1. Lentivirus vectors were produced by transient cotransfection of HEK293T cells maintained in DMEM with 10% FCS. HEK293T cells in 150-mm dishes were cotransfected by the polyethylenimine method with each HIV1 vector plasmid, pLP1 (pGag-Pol), pLP2 (pRev) (Invitrogen), and pCMV-G (31). Conditioned medium was collected at days 1, 2, and 3 post-transfection, filtered through a 0.45-μm filter, and concentrated by centrifugation at 7000 rpm for 16 h at 4 °C in a Sorvall GS-3 rotor. The resulting pellets were resuspended in buffer containing 10 mm Tris HCl (pH 7.8), 1 mm MgCl2, and 3% sucrose. HIV1-CMV-GFP vector (1 × 109 IU/ml) was used as the standard. HEK293 cells in a 6-well plate were incubated with different titers of viruses in the presence of polybrene (4 μg/ml). Infected cells were passaged every 4 days, and their DNA was prepared at day 14 post-infection using the DNeasy blood and tissue kit (Qiagen). Real-time quantitative PCR was performed using a primer set selected from the woodchuck hepatitis virus (WHV) posttranscriptional regulatory element (WPRE) sequence contained in the HIV1 vector backbone.
Immunofluorescence Confocal Microscopy
Primary neurons were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature, incubated with 100 mm glycine (pH 7.4) for 10 min to quench aldehyde groups, permeabilized in buffered Triton X-100 (0.1%) for 10 min, blocked with 1% BSA/PBS/Tween (0.05%) for 20 min, and then incubated with primary antibodies in 1% BSA/PBS/Tween (0.05%) for 24–48 h at 4 °C. Excess antibody was removed by washing with PBS/Tween (0.1%) for 15 min followed by incubation with FITC or Alexa-conjugated secondary antibody (1:250) for 1 h. To remove excess secondary antibody, tissue or cells were washed six times at 5-min intervals with PBS/Tween (0.1%) and incubated for 20 min with the nuclear stain DAPI (1:5000) diluted in PBS. Cells were washed for 10 min with PBS and mounted in gelvatol for microscopic imaging. Confocal images were captured with an Olympus confocal microscope system (Applied Precision, Inc., Issaquah, WA) that included a Photometrics changed coupled device mounted on a Nikon TE-200 inverted epifluorescence microscope. Between 30 and 80 optical sections spaced by approximately 0.1–0.3 mm were captured. Exposure times were set so that the camera response was in the linear range for each fluorophore. Data sets were analyzed using FluoroView. Quantitation of dendritic branching, length, area, and volume was performed using Autoneuron, which measures three-dimensional image volume stacks (MBF Bioscience). Statistical analysis was performed using Prism. All parametric data were analyzed by unpaired t tests or ANOVA Bonferroni's multiple comparison as appropriate. Post-hoc comparisons were made by Student Neuman Keuls tests. Significance was set at p < 0.05. Statistical analysis was performed using Prism 4 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Neuron-targeted Overexpression of Cav-1 Enhances Expression of Prosurvival Signaling Components and Augments Signaling in Primary Neurons
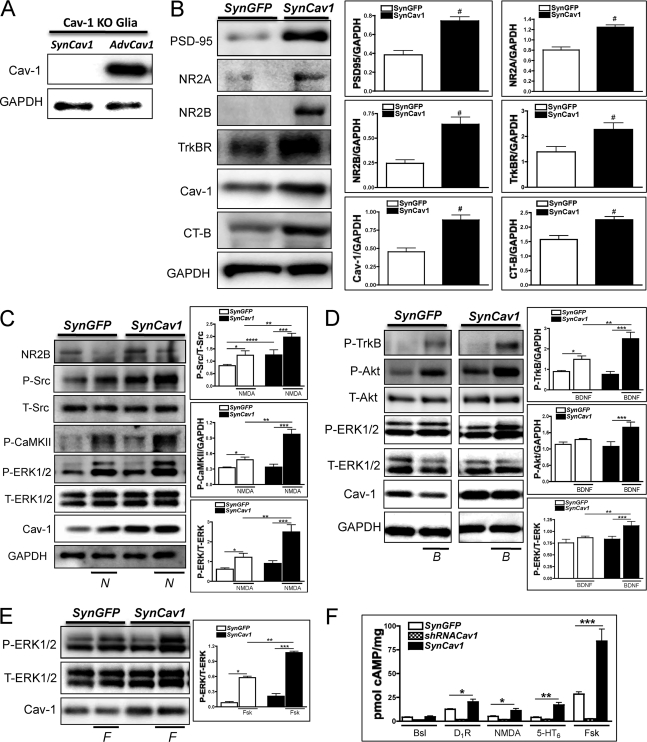
We have shown previously that siRNA-mediated knockdown of Cav-1 disrupts NMDA-mediated signaling and blunts neuroprotection following oxygen glucose deprivation (9). Moreover, Cav-1 KO mice exhibit reduced synaptic signaling and scaffolding proteins in hippocampal synaptosomes, and this is associated with an inability to be preconditioned against lethal ischemia (32). We therefore tested whether neuron-targeted Cav-1 expression would enhance membrane/lipid raft formation and expression of synaptic receptors and, in addition, would promote prosurvival signaling. SynCav1, a vector that contains the synapsin promoter upstream of Cav, specifically targets Cav-1 expression to neurons (32) (Fig. 1). To show that this vector specifically expresses Cav-1 in neurons, we isolated glia from Cav-1 KO mice that had been incubated with SynCav1 for 72 h. As a positive control, we incubated glia from Cav-1 mice with a non-tissue-specific adenoviral vector containing the Cav-1 gene. Glia from Cav-1 KO mice expressed Cav-1 if incubated with the nonspecific AdvCav1 vector but not if incubated with SynCav1, indicating the neuronal specificity of SynCav1 (Fig. 1A). Treatment of primary neurons from WT mice with SynCav-1-containing lentivirus significantly enhanced expression of membrane/lipid rafts (as assessed by CT-B), PSD-95, NR2A, NR2B, TrkB, and AMPAR compared with control virus (SynGFP) (n = 6 experiments, p < 0.05, unpaired t test) (Fig. 1B). To confirm that SynCav1-induced increase in expression of prosurvival proteins (e.g. NR2A, NR2B, TrkB) results in functional signaling, we wanted to confirm that this increase results in functional prosurvival signaling at synaptic rather than extrasynaptic sites (33). We thus transfected neurons with SynCav1 for 72 h and then stimulated them with NMDA (N, 10 μm), BDNF (B, 50 ng/ml), or Fsk (F, 10 μm) for 10 min. In SynCav1-incubated neurons, stimulation with NMDA enhanced the activation of the prosurvival kinases P-Src, P-CaMKII, and P-ERK1/2 compared with NMDA treatment of SynGFP-incubated neurons (n = 4 experiments, p < 0.05, unpaired t test) (Fig. 1C). BDNF enhanced P-TrkB, P-Akt, and P-ERK1/2 in SynCav1-incubated neurons (n = 4 experiments, p < 0.05, unpaired t test) (Fig. 1D). Forskolin enhanced P-ERK1/2 in neurons incubated with SynCav1 (n = 4 experiments, p < 0.05, unpaired t test) (Fig. 1E). Incubation with SynCav1 significantly increased cAMP formation in response to agonism of the dopamine 1 receptor (D1R), NMDAR, serotonin receptor (5-HT6), or with stimulation of adenylyl cyclase with forskolin (n = 4 experiments, p < 0.05, unpaired t test). AdvshRNACav1-incubated neurons showed a loss of D1R-, NMDAR-, 5-HT6-, and Fsk-stimulated cAMP formation (Fig. 1F). These results thus demonstrate that neuron-targeted Cav-1 overexpression in primary neurons enhances expression of prosurvival receptors and membrane/lipid rafts and, in addition, enhances activity of progrowth signaling molecules (e.g. activation of prosurvival kinases and cAMP synthesis).
FIGURE 1.
Neuron-targeted expression of Cav-1 enhances expression of pro-survival signaling components in wild-type primary neurons. A, Cav-1 KO glia were incubated with SynCav1 or a non-tissue-specific adenoviral vector containing the Cav-1 gene (AdvCav1). Cav-1 KO glia show reexpression of Cav-1 with the nonspecific AdvCav1 but not SynCav1, indicating SynCav1 neuronal specificity. B, primary neurons were transfected with SynGFP or SynCav1 (2 × 109 viral particles) for 72 h and then subjected to immunoblot analysis. SynCav1 enhanced protein expression of PSD-95, NR2A, NR2B, TrkBR, and CT-B (lipid raft marker). n = 6; #, p < 0.05. C, NMDA (10 μm, 10 min) treatment enhanced P-ERK1/2, P-CaMKII, and P-Src (n = 4–9, p < 0.05) in SynCav1-expessing neurons. *, SynGFP-NMDA versus SynGFP-basal; **, SynCav1-NMDA versus SynGFP-NMDA; ***, SynCav1-basal versus SynGFP-basal; ****, SynCav1-basal versus SynGFP-basal. D, BDNF (50 ng/ml, 10 min) treatment enhanced P-TrkB, P-Akt, and P-ERK1/2 (n = 4, p < 0.05) in SynCav1-expressing neurons. *, SynGFP-bdnf versus SynGFP-basal; **, SynCav1-bdnf versus SynGFP-bdnf; ***, SynCav1-bdnf versus SynCav1-basal. E, forskolin (F) (10 μm, 10 min) treatment enhanced P-ERK1/2 (n = 4, p < 0.05) in SynCav1-expressing neurons. *, SynGFP-fsk versus SynGFP-basal; **, SynCav1-fsk versus SynGFP-fsk; ***, SynCav1-fsk versus SynCav1-basal. F, neurons were transfected with SynGFP, SynCav1, or AdvshRNACav1 for 72 h followed by treatment with a dopamine 1 receptor agonist (D1R) (10 μm), NMDA, serotonin receptor 6 agonist (10 μm, 5-HT6), or Fsk (10 μm), and cAMP was measured by radioimmunoassay (28, 29). Neurons were pretreated with the PDE4 inhibitor rolipram (10 μm). Stimulation of D1R, NMDAR, 5-HT7, and adenylyl cyclase (AC) significantly increased cAMP formation in the SynCav1-transfected neurons (n = 4, p < 0.05; **, p < 0.005; ***, p < 0.0005). Conversely, agonist-stimulated cAMP formation is blunted in shRNACav1-transfected neurons. Bsl, basal.
Neuron-targeted Overexpression of Cav-1 Enhances Dendrite Number and Length and Expression of Membrane/Lipid Rafts
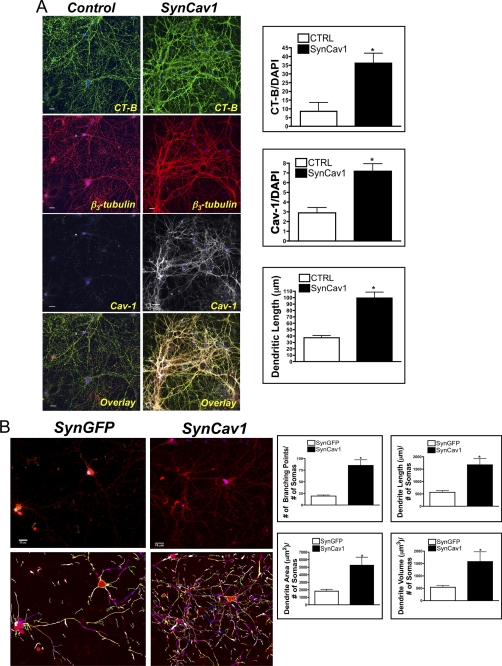
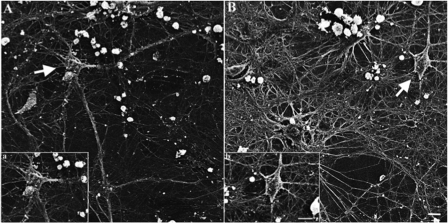
Because progrowth signaling components were elevated with in SynCav1-transfected neurons, we tested whether such neurons have enhanced growth and arborization of their dendrites. We found that primary neurons incubated with SynCav1 for 21 days have enhanced membrane/lipid raft expression (as indicated by CT-B) and a 3- to 4-fold increase in dendritic length (n = 4 experiments, p < 0.05, unpaired t test) (Fig. 2A). This treatment also enhanced the branching, length, area, and volume of dendrites of SynCav1-incubated neurons compared with such features in SynGFP-incubated neurons (n = 4 experiments, p < 0.05, unpaired t test), as quantitated using Autoneuron (MBF Bioscience) (Fig. 2B). Analysis using scanning electron microscopy revealed that SynCav1- incubated neurons (Fig. 3, B, b) display enhanced dendritic arborization compared with SynGFP-incubated neurons (A, a). Thus, neuron-targeted Cav-1 enhances prosurvival receptor expression and signaling molecules that promote dendritic growth in primary neurons.
FIGURE 2.
Neuron-targeted expression of Cav-1 enhances membrane/lipid rafts and dendrite number and length. A, primary neurons (days in vitro, 4) were transfected with SynCav1 (109 viral particles/ml), and cells were grown for 21 days and then stained with CT-B (488) (green, top panels), the dendritic shaft marker β3-tubulin (red, center panels), and for Cav-1 (white, bottom panels; *, p < 0.05. Scale bar = 10 μm). CTRL, control. B, primary neurons were incubated with SynCav1 or SynGFP for 21 days. Dendritic branching, length, and area were then measured using Autoneuron, a tracing algorithm that measures three-dimensional image volume stacks. Top panels, neurons stained for the neuronal F-actin binding protein drebrin (red). Bottom panels, Autoneuron tracing of the drebrin stain. *, p < 0.05, Scale bar = 10 μm.
FIGURE 3.
Neuron-targeted expression of Cav-1 enhances dendritic growth. Primary neurons were incubated with SynCav1 or SynGFP for 21 days and imaged by scanning electron microscopy. A, SynGFP neurons (a). B, SynCav1-incubated neurons (b). Scale bar = 10 μm. Images were acquired on a Hitachi S-270 scanning electron with a Gatan digital camera.
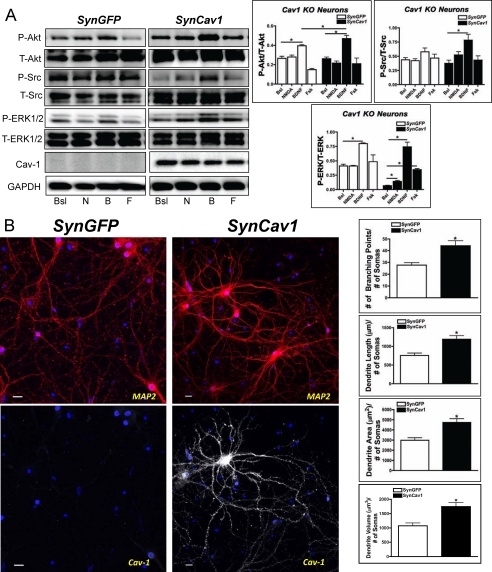
Neuron-targeted Reexpression of Cav-1 in Cav-1 KO Neurons Restores Prosurvival Signaling and Enhances Dendritic and Axonal Growth
To assess the ability of Cav-1 to restore prosurvival signaling and function in Cav-1 KO neurons, we incubated neurons from Cav-1 KO mice with lentiviruses that contained SynCav1 or SynGFP. SynCav1-containing lentivirus restored Cav-1 expression and significantly enhanced NMDA-mediated P-Src, P-ERK1/2, BDNF-mediated P-Akt, P-Src, P-ERK1/2, and Fsk-mediated P-ERK1/2 within 72 h after incubation with the lentivirus (n = 3–4 experiments, p < 0.05, unpaired t test) (Fig. 4A). Immunofluorescence confocal microscopy showed that the incubation with the SynCav1 lentivirus enhanced dendritic branching, length, area, and volume, as assessed by quantification of MAP2 and by the use of Autoneuron (n = 6 experiments, p < 0.05, unpaired t test) (Fig. 4B). These data thus show that reexpression of Cav-1 in neurons that are deficient in Cav-1 restores prosurvival and progrowth signaling in association with enhanced growth and arborization of dendrites.
FIGURE 4.
Neuron-targeted expression of Cav-1 in Cav-1 KO neurons restores prosurvival signaling and enhances growth of dendrites. A, primary neurons from Cav-1 KO mice were incubated with SynGFP or SynCav1 (2 × 109 viral particles) for 72 h and then treated with various agonists. SynCav1 significantly enhanced NMDA-mediated (10 μm, 10 min) P-ERK1/2 (n = 4; *, p < 0.05) expression, BDNF-promoted (50 ng/ml, 10 min) expression of P-Akt, P-Src, and P-ERK1/2 (n = 4; *, p < 0.05), and Fsk-promoted (10 μm, 10 min) expression of P-ERK1/2 (n = 4; *, p < 0.05). Bsl, basal; N, NMDA; B, BDNF; F, forskolin. B, SynCav1 expression in Cav-1 KO neurons significantly enhanced dendritic branching, length, and area 21 days post-treatment. *, p < 0.05. n = 5. Data are mean ± S.E. Neurons were stained for the dendritic marker MAP2 (red), Cav-1 (white), and DAPI (blue). Scale bar = 10 μm.
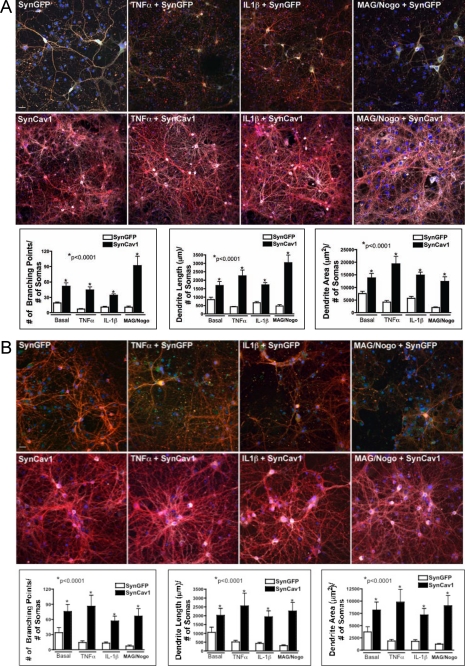
SynCav1 Promotes Dendritic Arborization in the Presence of Neuronal Growth Inhibitors
A major limitation to neuronal growth and repair in vivo is the presence of growth inhibitors, such as inflammatory cytokines (34) and myelin-associated glycoproteins, in the region surrounding the core of an injury (26). After injury (stroke or trauma), substantial activation of astrocytes and microglia occurs that is associated with the elaboration of inflammatory cytokines such as TNFα and IL-1β. In addition, the myelin-associated glycoproteins MAG and Nogo, which are present in the injured brain, act as growth inhibitors and limit neuronal sprouting and growth. We thus assessed whether expression of SynCav1 could promote growth in the presence of these inhibitors. WT and Cav-1 KO primary neurons (DIV3) were pretreated with TNFα, IL-1β, or MAG plus Nogo for 4 days. The neurons were then incubated with SynCav1 lentivirus and cultured for an additional 21 days. We found that even in the presence of the growth inhibitors, addition of the SynCav1 lentivirus increased neuronal branching, length, and area compared with control neurons incubated with SynGFP lentivirus (n = 4–7 experiments, p < 0.001, one-way ANOVA Bonferroni's multiple comparison test) (Fig. 5A) or to neurons from Cav-1 KO mice (n = 4–7 experiments, p < 0.001, one-way ANOVA Bonferroni's multiple comparison test) (Fig. 5B). Notably, we found that addition of the SynCav1 lentivirus 4 days after cytokine and MAG/Nogo exposure was able to augment neuronal growth.
FIGURE 5.
Neuron-targeted expression of Cav-1 enhances growth in primary neurons in the presence of inhibitory cytokines and myelin-associated glycoproteins. Primary neurons from wild-type (A) or Cav-1 KO (B) mice were incubated with TNFα (1 ng/ml), IL-1β (1 ng/ml), or MAG + Nogo (1 mg/ml) prior to incubation with SynGFP or SynCav1 for 21 days. Neurons were stained for β3-tubulin (red pixels) and Cav-1 (white pixels) followed by measurement of branching, length, and area of dendrites using Autoneuron. SynCav1 significantly enhanced dendritic arborization in neurons from both WT and Cav-1 KO mice in neurons pretreated with TNFα, IL-1β, and MAG/Nogo compared with neurons from SynGFP mice. One-way ANOVA Bonferroni's multiple comparison test; *, p < 0.0001; n = 4–7. Images were captured with an Olympus confocal microscope (FluoroView 1000). Optical sections spaced by 0.2–0.5 μm were obtained. Scale bar = 10 μm.
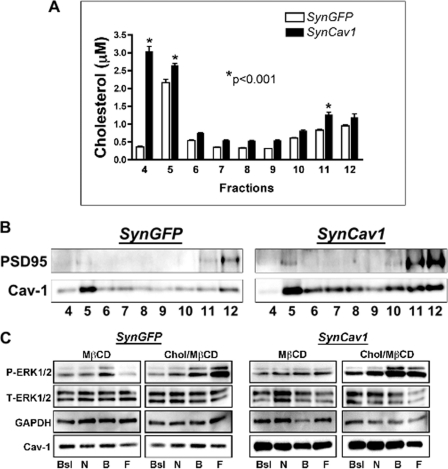
SynCav1 Increases Membrane Cholesterol
To confirm the effect from SynCav1 on membrane/lipid rafts, we performed sucrose density fractionation on neurons incubated with either SynCav1 or SynGFP followed by cholesterol measurements and immunoblot analysis. SynCav1 significantly increased cholesterol in buoyant membrane fractions 4 (6-fold) and 5 (approximately 20%) (n = 3 experiments, p < 0.001, one-way ANOVA Bonferroni's multiple comparison test) (Fig. 6A). Immunoblot analysis detected an increase in PSD-95 and Cav-1 in the buoyant membrane fractions of SynCav1-incubated neurons compared with SynGFP (Fig. 6B). To assess the role of cholesterol and membrane/lipid rafts on SynCav1-mediated prosurvival signaling, we pretreated neurons with the cholesterol-removing agent MβCD (30 min, 3 mm) or cholesterol-MβCD control (1:6 ration, 50 mg/ml) as described previously (35). Pretreatment with MβCD blunted NMDA (N), BDNF (B), and Fsk (F)-mediated activation of P-ERK1/2 in SynCav1 incubated neurons, whereas the cholesterol-loaded control SynCav1 neurons still exhibited an increase in NMDA, BDNF, and Fsk-mediated P-ERK1/2 compared with SynGFP (Fig. 6C).
FIGURE 6.
Neuron-targeted expression of Cav-1 in wild-type primary neurons enhances membrane cholesterol. A, primary neurons were incubated with SynGFP or SynCav1 (2 × 109 viral particles) for 72 h, and membrane/lipid rafts were purified by sucrose density fractionation. SynCav1 significantly increased cholesterol in buoyant fractions 4 and 5. One-way ANOVA Bonferroni's multiple comparison test; *, p < 0.001; n = 3. B, immunoblot analysis detected an increase in PSD-95 and Cav-1 in the buoyant fractions. C, SynCav1 neurons pretreated with MβCD (3 mm, 30 min) exhibited a loss in NMDA-mediated (10 μm, 10 min), BDNF-mediated (50 ng/ml, 10 min), and Fsk-mediated (10 μm, 10 min) P-ERK1/2 compared with cholesterol/MβCD-treated SynCav1 neurons. Bsl, basal; N, NMDA; B, BDNF; F, forskolin.
DISCUSSION
In this study, we tested the effect of neuron-targeted Cav-1 expression (SynCav1) on prosurvival and progrowth signaling in primary neurons in vitro. As far as we are aware, this is the first study to show that a single intervention (i.e. SynCav1) can enhance neuronal membrane/lipid raft formation, increase expression of neurotransmitter and neurotrophin receptors, elevate multiple neuronal pathways that converge to augment cAMP formation (i.e. glutamatergic, dopaminergic, serotonergic, and neurotrophin-mediated), and promote neuronal growth and arborization. Furthermore, we show that genetic knockdown of Cav-1, which blunts neurotransmitter and neurotrophin-mediated signaling, can be ablated by reexpression of Cav-1. Although Cav-1 is generally considered to be a “negative” regulator of cellular signaling (36), in neuronal systems Cav-1 can have both negative and positive effects (10). The current findings extend the notion that Cav-1 can be a positive regulator of neuronal growth-promoting pathways. Our results show that Cav-1- targeted expression in neurons increases the expression of receptors and postreceptor signaling components that promote neuronal growth and survival in addition to spatially organizing and scaffolding such molecules. One thereby achieves enhanced growth-promoting signal transduction along with increased efficacy of endogenous agonists and growth factors and of exogenous interventions that can facilitate recovery following neuronal injury or degeneration.
Lipid Rafts Promote Neuronal Growth
Growing evidence implicate membrane/lipid rafts as an essential component for promoting growth cone expansion, neurite outgrowth, axonal branching, and axonal guidance (6, 37, 38). Synapses and membrane/lipid rafts are traditionally considered distinct subcellular regions of the plasma membrane although they share certain characteristics that are essential to their function (e.g. enrichment in cholesterol, glycosphingolipids, sphingomyelin, and other saturated fatty acid-containing lipids (GM1 gangliosides, palmitic acid)) (39). Other evidence supports a role for free cholesterol and membrane/lipid rafts in formation of neuronal synapses and in the signaling and protection of neurons (7, 9, 12, 40, 41). Moreover, as an essential component of rafts, cholesterol and changes in the cellular content of cholesterol can affect Cav expression (42, 43). For example, BDNF, which is essential for synaptic function and development, stimulates cholesterol biosynthesis and increases membrane/lipid rafts and Cav expression in cortical and hippocampal neurons (44).
Our results show that neurons engineered to express SynCav1 have increased expression of the membrane/lipid raft marker CT-B and significantly enhance membrane cholesterol. Several previous studies that have investigated therapeutic approaches to promote axonal regeneration and synapse formation following brain or spinal cord injury have used CT-B as an indicator of axonal regeneration and de novo synapse formation (45–47). CT-B binds to GM1-gangliosides, sialic acid-containing glycosphingolipids essential for brain development, plasticity, and neurite outgrowth (48–50). Other data have shown that exogenous GM1-ganglioside evokes the release of BDNF and promotes neuronal survival (38) and that the lack of gangliosides inhibits nerve regeneration and induces axonal damage (51, 52). The latter results could help explain the loss of synaptic signaling components, signaling, and neuronal processes following siRNA-mediated knockdown of Cav-1.
SynCav1 Attenuates Elevated Basal P-ERK1/2 in Cav-1 KO Neurons
Our group has shown previously that reexpression of Cav-1 in Cav-1 KO neurons attenuates elevated basal levels of P-ERK and restores NMDA-mediated activation of P-Src and P-ERK1/2 (9). In addition to our group, other laboratories have demonstrated that elevated basal P-ERK1/2 occurs in a variety of tissues ranging from myocardium to lung to the vasculature (9, 53–56). Although mechanistically not completely understood, Cav-1 KO mice exhibit hypertrophy, neoplasia, increased cyclin D levels, elevated metalloproteinase secretion, and Smad-2 hyperactivation. This work again demonstrates high basal P-ERK in Cav-1 KO neurons and that this again is attenuated with reexpression of Cav-1 (e.g. SynCav1). The mechanisms that result in elevated basal P-ERK in Cav-1 KO neurons have yet to be determined. Considering that multiple signaling pathways converge upon P-ERK (GPCRs, receptor tyrosine kinases, cAMP), more work is needed to elucidate the role Cav-1 plays in regulating these various pathways.
Cav-1 Scaffolds Prosurvival and Progrowth Neuronal Signaling Components
Cav-1 and membrane/lipid rafts have been shown previously to localize neuronal signaling components that contribute to neurotransmission (9, 10, 14–16, 57). Cav-1 and membrane/lipid rafts can regulate estrogen receptor signaling (58), glutamate receptor neurotransmission (9, 43, 59), and neurotrophin receptor signaling (TrkB and p75) (14, 57). Although NMDAR subtypes are critical for neuroprotection against ischemic injury and for neurotransmission, their localization to extrasynaptic regions can facilitate neuronal cell death (33). Therefore, the subcellular localization of these signaling pathways helps determine neuronal cell fate. NMDAR-mediated activation of prosurvival kinases, which include CaMKII, Src, and ERK1/2, predominantly occurs in synaptic regions (33). A key finding in this study is that SynCav1-mediated enhanced expression of NMDAR subtypes promotes a prosurvival pathway that is dependent upon membrane cholesterol.
These results extend those that have shown that the loss of Cav-1, either through siRNA or transgenic models, can blunt neuroprotection (9) and metabotropic glutamate receptor-mediated long-term depression (43, 59) and accelerate a neurodegenerative phenotype (32, 60, 61). Our findings obtained with siRNA-mediated Cav-1 knockdown extend the role of Cav-1 in neuronal signaling to a variety of receptors in addition to glutamate. Interestingly, many of these receptors signal via regulating the formation of cAMP, a second messenger that regulates neuronal survival as well as growth of dendrites and axons (17, 19, 21). A more recent finding demonstrates that recruitment of TrkB receptors to neuronal lipid rafts, via adenosine A2A receptor activation, was required for BDNF-induced hippocampal long-term potentiation of CA1 synapses (35). This recruitment to lipid rafts by A2A receptor activation also enhanced P-TrkB, an effect that was mimicked by forskolin and blocked by protein kinase A or Src inhibition. These findings are akin to our data that show that transfection with SynCav1 enhances expression of the lipid raft marker CT-B, enhances membrane cholesterol, and augments BDNF-mediated P-TrkB and forskolin-stimulated cAMP production (Figs. 1 and 2). Although cAMP and cGMP promote, respectively, the growth of axons and dendrites (21), the precise role of Cav-1 in cGMP-mediated dendritic growth remains to be determined. Nevertheless, our data has implications for neuronal repair and emphasize the potentially beneficial effects of neuron-targeted Cav-1 in the enhancement of multiple signaling pathways that converge upon cAMP formation.
Loss of Cav-1 May Contribute to Neurodegeneration
Cav-1 KO mice have a CNS pathology similar to that exhibited in neurodegenerative diseases. Such features include altered glutamate receptor signaling (9, 43, 59), motor and behavioral abnormalities, increased ischemic cerebral injury, and impaired spatial memory and cholinergic function (60–62). Other recent evidence has demonstrated that the localization of synaptic signaling components in neuronal membrane/lipid rafts and synaptosomes is reduced in brains from aged WT and young Cav-1 KO mice and that Cav-1 KO mice develop a neuropathological phenotype similar to that of Alzheimer's disease (32).
In summary, the data in this study show that not only do membrane/lipid rafts and Cav-1 provide a key nexus for prosurvival and progrowth signaling components but also that an increase in expression of Cav-1 in neurons may be a novel means to preserve, restore, and enhance neuronal function following injury. Application of these results and this concept, in particular to augment the capacity of the brain to undergo repair in settings such as stroke and traumatic brain injury or during late stages of neurodegenerative diseases may have important therapeutic implications.
Acknowledgments
We thank the University of California, San Diego Cancer Center Digital Imaging Shared Resource for assistance. In particular, we thank James Feramisco, Ph.D. (Professor of Medicine, University of California, San Diego, La Jolla, CA) and Martin Marsala, M.D. (Professor of Anesthesiology, University of California, San Diego, La Jolla, CA) for use of the Stem Cell Core Facility confocal microscope.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 HL091071 (to H. H. P.) and RO1 GM085179 (to P. M. P.). This work was also supported by Veterans Affairs Career Development Award 2 (to B. P. H.) and a Merit Award from the Department of Veterans Affairs (to D. M. R.).
- Cav
- caveolin
- CT-B
- cholera toxin B
- MβCD
- methyl-β-cyclodextrin
- MES
- 4-morpholineethanesulfonic acid
- Syn
- synapsin
- ANOVA
- analysis or variance
- Fsk
- forskolin.
REFERENCES
- 1. Toescu E. C., Verkhratsky A., Landfield P. W. (2004) Trends Neurosci. 27, 614–620 [DOI] [PubMed] [Google Scholar]
- 2. Hattiangady B., Rao M. S., Shetty G. A., Shetty A. K. (2005) Exp. Neurol. 195, 353–371 [DOI] [PubMed] [Google Scholar]
- 3. Hotulainen P., Hoogenraad C. C. (2010) J. Cell Biol. 189, 619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huber A. B., Kolodkin A. L., Ginty D. D., Cloutier J. F. (2003) Annu. Rev. Neurosci. 26, 509–563 [DOI] [PubMed] [Google Scholar]
- 5. Calabrese B., Wilson M. S., Halpain S. (2006) Physiology 21, 38–47 [DOI] [PubMed] [Google Scholar]
- 6. Guirland C., Zheng J. Q. (2007) Adv. Exp. Med. Biol. 621, 144–155 [DOI] [PubMed] [Google Scholar]
- 7. Mauch D. H., Nägler K., Schumacher S., Göritz C., Müller E. C., Otto A., Pfrieger F. W. (2001) Science 294, 1354–1357 [DOI] [PubMed] [Google Scholar]
- 8. Allen J. A., Halverson-Tamboli R. A., Rasenick M. M. (2007) Nat. Rev. Neurosci. 8, 128–140 [DOI] [PubMed] [Google Scholar]
- 9. Head B. P., Patel H. H., Tsutsumi Y. M., Hu Y., Mejia T., Mora R. C., Insel P. A., Roth D. M., Drummond J. C., Patel P. M. (2008) FASEB J. 22, 828–840 [DOI] [PubMed] [Google Scholar]
- 10. Stern C. M., Mermelstein P. G. (2010) Cell. Mol. Life Sci. 67, 3785–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oshikawa J., Toya Y., Fujita T., Egawa M., Kawabe J., Umemura S., Ishikawa Y. (2003) Am. J. Physiol. Cell Physiol. 285, C567–574 [DOI] [PubMed] [Google Scholar]
- 12. Willmann R., Pun S., Stallmach L., Sadasivam G., Santos A. F., Caroni P., Fuhrer C. (2006) EMBO J. 25, 4050–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smart E. J., Graf G. A., McNiven M. A., Sessa W. C., Engelman J. A., Scherer P. E., Okamoto T., Lisanti M. P. (1999) Mol. Cell. Biol. 19, 7289–7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bilderback T. R., Gazula V. R., Lisanti M. P., Dobrowsky R. T. (1999) J. Biol. Chem. 274, 257–263 [DOI] [PubMed] [Google Scholar]
- 15. Hibbert A. P., Kramer B. M., Miller F. D., Kaplan D. R. (2006) Mol. Cell Neurosci. 32, 387–402 [DOI] [PubMed] [Google Scholar]
- 16. Björk K., Sjögren B., Svenningsson P. (2010) Exp. Cell Res. 316, 1351–1356 [DOI] [PubMed] [Google Scholar]
- 17. Neumann S., Bradke F., Tessier-Lavigne M., Basbaum A. I. (2002) Neuron 34, 885–893 [DOI] [PubMed] [Google Scholar]
- 18. Wayman G. A., Impey S., Marks D., Saneyoshi T., Grant W. F., Derkach V., Soderling T. R. (2006) Neuron 50, 897–909 [DOI] [PubMed] [Google Scholar]
- 19. MacDonald E., Van der Lee H., Pocock D., Cole C., Thomas N., VandenBerg P. M., Bourtchouladze R., Kleim J. A. (2007) Neurorehabil. Neural Repair 21, 486–496 [DOI] [PubMed] [Google Scholar]
- 20. Saneyoshi T., Wayman G., Fortin D., Davare M., Hoshi N., Nozaki N., Natsume T., Soderling T. R. (2008) Neuron 57, 94–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murray A. J., Tucker S. J., Shewan D. A. (2009) J. Neurosci. 29, 15434–15444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atkins C. M., Oliva A. A., Jr., Alonso O. F., Pearse D. D., Bramlett H. M., Dietrich W. D. (2007) Exp. Neurol. 208, 145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hicks R. R., Zhang L., Dhillon H. S., Prasad M. R., Seroogy K. B. (1998) Brain Res. Mol. Brain Res. 59, 264–268 [DOI] [PubMed] [Google Scholar]
- 24. Biegon A., Fry P. A., Paden C. M., Alexandrovich A., Tsenter J., Shohami E. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 5117–5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Atkins C. M., Falo M. C., Alonso O. F., Bramlett H. M., Dietrich W. D. (2009) Neurosci. Lett. 459, 52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carmichael S. T. (2008) Stroke 39, 1380–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lemkuil B. P., Head B. P., Pearn M. L., Patel H. H., Drummond J. C., Patel P. M. (2011) Anesthesiology 114, 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Head B. P., Patel H. H., Roth D. M., Lai N. C., Niesman I. R., Farquhar M. G., Insel P. A. (2005) J. Biol. Chem. 280, 31036–31044 [DOI] [PubMed] [Google Scholar]
- 29. Head B. P., Patel H. H., Roth D. M., Murray F., Swaney J. S., Niesman I. R., Farquhar M. G., Insel P. A. (2006) J. Biol. Chem. 281, 26391–26399 [DOI] [PubMed] [Google Scholar]
- 30. Yam P. Y., Li S., Wu J., Hu J., Zaia J. A., Yee J. K. (2002) Mol. Ther. 5, 479–484 [DOI] [PubMed] [Google Scholar]
- 31. Yee J. K., Friedmann T., Burns J. C. (1994) Methods Cell Biol. 43 Pt A, 99–112 [DOI] [PubMed] [Google Scholar]
- 32. Head B. P., Peart J. N., Panneerselvam M., Yokoyama T., Pearn M. L., Niesman I. R., Bonds J. A., Schilling J. M., Miyanohara A., Headrick J., Ali S. S., Roth D. M., Patel P. M., Patel H. H. (2010) PLoS ONE 5, e15697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hardingham G. E., Bading H. (2003) Trends Neurosci. 26, 81–89 [DOI] [PubMed] [Google Scholar]
- 34. Suzumura A., Takeuchi H., Zhang G., Kuno R., Mizuno T. (2006) Ann. N.Y. Acad. Sci. 1088, 219–229 [DOI] [PubMed] [Google Scholar]
- 35. Assaife-Lopes N., Sousa V. C., Pereira D. B., Ribeiro J. A., Chao M. V., Sebastião A. M. (2010) J. Neurosci. 30, 8468–8480 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36. Lisanti M. P., Scherer P. E., Tang Z., Sargiacomo M. (1994) Trends Cell Biol. 4, 231–235 [DOI] [PubMed] [Google Scholar]
- 37. Grider M. H., Park D., Spencer D. M., Shine H. D. (2009) J. Neurosci. Res. 87, 3033–3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lim S. T., Esfahani K., Avdoshina V., Mocchetti I. (2010) Neuropharmacology 60, 1160–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pike L. J. (2009) J. Lipid Res. 50, S323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hering H., Lin C. C., Sheng M. (2003) J. Neurosci. 23, 3262–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Renner M., Choquet D., Triller A. (2009) J. Neurosci. 29, 2926–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bist A., Fielding C. J., Fielding P. E. (2000) Biochemistry 39, 1966–1972 [DOI] [PubMed] [Google Scholar]
- 43. Francesconi A., Kumari R., Zukin R. S. (2009) J. Neurosci. 29, 3590–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suzuki S., Kiyosue K., Hazama S., Ogura A., Kashihara M., Hara T., Koshimizu H., Kojima M. (2007) J. Neurosci. 27, 6417–6427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alto L. T., Havton L. A., Conner J. M., Hollis Ii E. R., Blesch A., Tuszynski M. H. (2009) Nat. Neurosci. 12, 1106–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee H., McKeon R. J., Bellamkonda R. V. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 3340–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li S., Overman J. J., Katsman D., Kozlov S. V., Donnelly C. J., Twiss J. L., Giger R. J., Coppola G., Geschwind D. H., Carmichael S. T. (2010) Nat. Neurosci. 13, 1496–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Svennerholm L. (1956) Nature 177, 524–525 [DOI] [PubMed] [Google Scholar]
- 49. Suzuki K. (1965) J. Neurochem. 12, 629–638 [DOI] [PubMed] [Google Scholar]
- 50. Derry D. M., Wolfe L. S. (1967) Science 158, 1450–1452 [DOI] [PubMed] [Google Scholar]
- 51. Sparrow J. R., McGuinness C., Schwartz M., Grafstein B. (1984) J. Neurosci. Res. 12, 233–243 [DOI] [PubMed] [Google Scholar]
- 52. Yamashita T., Wu Y. P., Sandhoff R., Werth N., Mizukami H., Ellis J. M., Dupree J. L., Geyer R., Sandhoff K., Proia R. L. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2725–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cohen A. W., Park D. S., Woodman S. E., Williams T. M., Chandra M., Shirani J., Pereira de Souza A., Kitsis R. N., Russell R. G., Weiss L. M., Tang B., Jelicks L. A., Factor S. M., Shtutin V., Tanowitz H. B., Lisanti M. P. (2003) Am. J. Physiol. Cell Physiol. 284, C457–474 [DOI] [PubMed] [Google Scholar]
- 54. Williams T. M., Medina F., Badano I., Hazan R. B., Hutchinson J., Muller W. J., Chopra N. G., Scherer P. E., Pestell R. G., Lisanti M. P. (2004) J. Biol. Chem. 279, 51630–51646 [DOI] [PubMed] [Google Scholar]
- 55. Hassan G. S., Jasmin J. F., Schubert W., Frank P. G., Lisanti M. P. (2004) Biochemistry 43, 8312–8321 [DOI] [PubMed] [Google Scholar]
- 56. Sotgia F., Williams T. M., Schubert W., Medina F., Minetti C., Pestell R. G., Lisanti M. P. (2006) Am. J. Pathol. 168, 292–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Besshoh S., Bawa D., Teves L., Wallace M. C., Gurd J. W. (2005) J. Neurochem. 93, 186–194 [DOI] [PubMed] [Google Scholar]
- 58. Mermelstein P. G. (2009) J. Neuroendocrinol. 21, 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Takayasu Y., Takeuchi K., Kumari R., Bennett M. V., Zukin R. S., Francesconi A. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 21778–21783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Trushina E., Du Charme J., Parisi J., McMurray C. T. (2006) Behav. Brain Res. 172, 24–32 [DOI] [PubMed] [Google Scholar]
- 61. Jasmin J. F., Malhotra S., Singh Dhallu M., Mercier I., Rosenbaum D. M., Lisanti M. P. (2007) Circ. Res. 100, 721–729 [DOI] [PubMed] [Google Scholar]
- 62. Gioiosa L., Raggi C., Ricceri L., Jasmin J. F., Frank P. G., Capozza F., Lisanti M. P., Alleva E., Sargiacomo M., Laviola G. (2008) Behav. Brain Res. 188, 255–262 [DOI] [PubMed] [Google Scholar]